The Role of Salvage Radical Prostatectomy in Patients with Radiation-Resistant Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
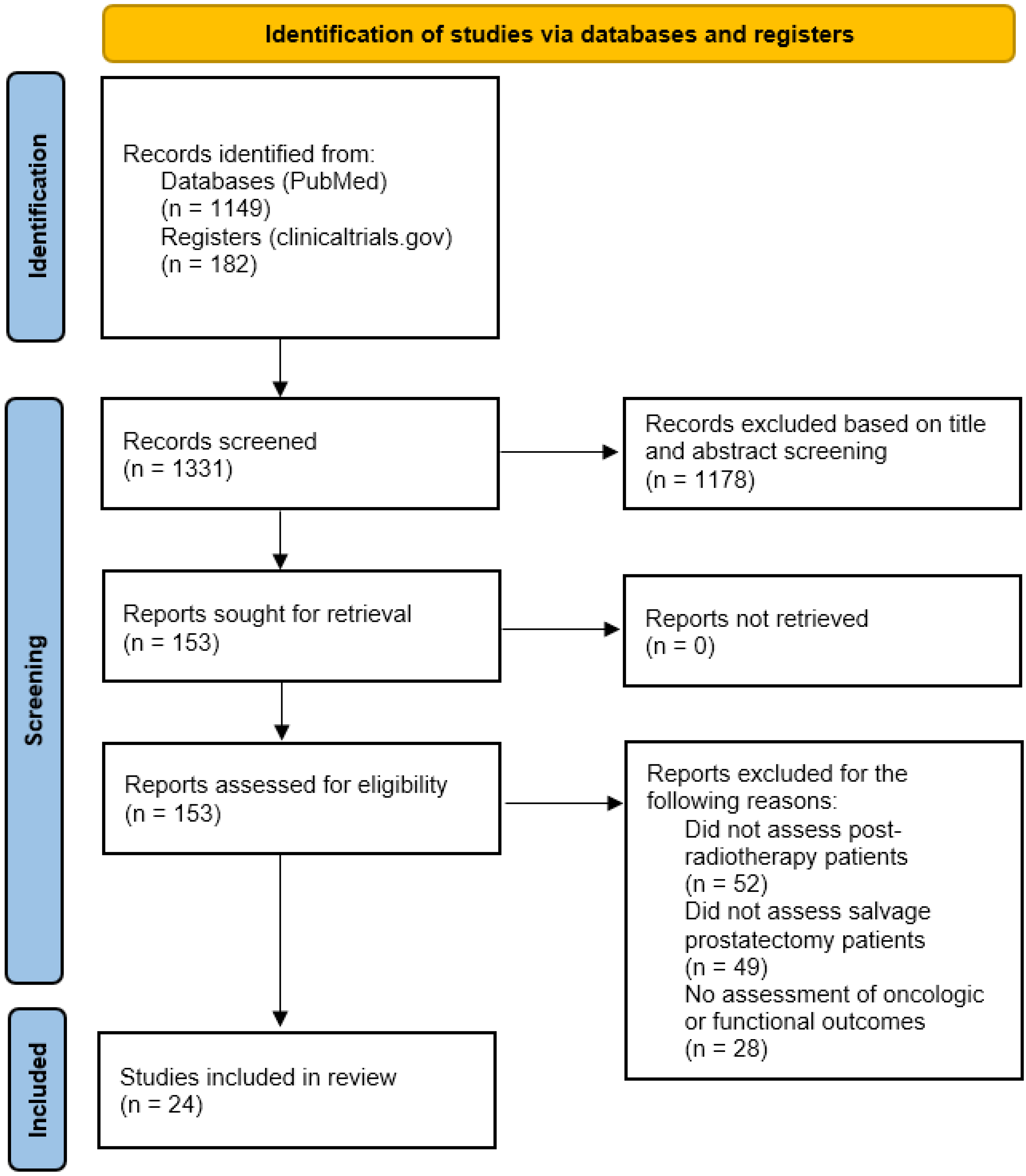
2. Methods
3. Results and Discussion
3.1. Oncologic Outcomes
| Authors (Year) | Study Type and Follow Up Period | N | BFS (95% CI) | PFS (95% CI) | MFS (95% CI) | CSS (95% CI) | OS (95% CI) | Factors Associated with Improved Oncologic Outcomes | Level of Evidence |
|---|---|---|---|---|---|---|---|---|---|
| Grubmuller et al. (2021) [35] | Systematic review, 10 years | 2232 | 31–37% | -- | 65–72% | 72–83% | -- |
| Level 2 |
| Mohler et al. (2019) [34] | Prospective, multicenter, 10 years | 41 | 33% | -- | -- | -- | 52% | -- | Level 2 |
| Chade et al. (2011) [27] | Retrospective, multicenter, 10 years | 404 | 37% (31–43) | -- | 77% (71–82) | 83% (76–88) | 77% (71–82) |
| Level 3 |
| Amling et al. (1999) [25] | Retrospective, single-center, 10 years | 108 | -- | 43% | -- | 70% | -- |
| Level 3 |
| Lerner et al. (1995) [23] | Retrospective, single-center, 10 years | 79 | 47.3% | -- | -- | 72% | 64% |
| Level 3 |
| Ward et al. (2005) [26] | Retrospective, single-center, 10 years | 199 | 48% | -- | -- | 65% | -- |
| Level 3 |
| Calleris et al. (2023) [38] | Retrospective, multicenter, 5 years | 1030 | 38–55% | -- | 75–90% | -- | 84–89% |
| Level 3 |
| Catarino et al. (2022) [36] | Prospective, single-center, 5 years | 29 | 50% | -- | -- | -- | -- |
| Level 2 |
| Gorin et al. (2011) [32] | Retrospective, single-center, 5 years | 24 | 39% | -- | -- | -- | 90% |
| Level 3 |
| Paparel et al. (2009) [31] | Retrospective, single-center, 5 years | 146 | 54% (44–63) | -- | -- | 89% | -- |
| Level 3 |
| Pisters et al. (2009) [30] | Retrospective, single-center, 5 years | 42 | 61% | -- | -- | -- | 95% | -- | Level 3 |
| Sanderson et al. (2006) [29] | Prospective, multicenter, 5 years | 51 | -- | 47% (39–55) | -- | -- | 85% (80–90) |
| Level 2 |
| Bianco et al. (2005) [28] | Prospective, single-center, 5 years | 100 | -- | 55% (46–64) | -- | -- | -- |
| Level 2 |
| Tefilli et al. (1998) [22] | Retrospective, single-center, 5 years | 27 | 44.4% | -- | -- | -- | -- | -- | Level 3 |
| Rogers et al. (1995) [21] | Retrospective, single-center, 5 years | 40 | -- | 55% (35–75) | -- | 95% | -- |
| Level 3 |
| Yuh et al. (2014) [33] | Prospective, single-center, 3 years | 51 | 57% | -- | -- | -- | -- |
| Level 2 |
| Gheiler et al. (1998) [24] | Retrospective, single-center, 3 years | 40 | 47.4% | -- | -- | -- | -- |
| Level 3 |
3.2. Functional Outcomes and Complications
| Authors (Year) | N | Incontinence at 1 Year, % | Anastomotic Stricture | Erectile Dysfunction at 1 Year, % | Rectal Injury | Venous Thromboembolism | Infection | Blood Transfusion | Hospital Length of Stay, Days | Level of Evidence |
|---|---|---|---|---|---|---|---|---|---|---|
| Calleris et al. (2023) [38] | 221 | 21% | -- | -- | -- | -- | -- | -- | -- | Level 3 |
| Catarino et al. (2022) [36] | 29 | 79% | 2 | 85% | 2 | 1 | 1 | 1 | -- | Level 2 |
| Gontero et al. (2019) [46] | 395 | 43% | 39 | 85% | -- | -- | -- | -- | -- | Level 3 |
| Mohler et al. (2019) [34] | 41 | 40% | 14 | 45% | 3 | -- | 5 | 13 | -- | Level 2 |
| Kenney et al. (2016) [45] | 39 | 90% | 10 | -- | 2 | 2 | 15 | -- | -- | Level 3 |
| Yuh et al. (2014) [33] | 51 | -- | 8 | -- | 1 | 2 | 4 | -- | -- | Level 2 |
| Gorin et al. (2011) [32] | 24 | 35% | 4 | 28% | 0 | -- | -- | 19 | -- | Level 3 |
| Eandi et al. (2010) [44] | 18 | 67% | 3 | 100% | 0 | -- | -- | -- | -- | Level 3 |
| Sanderson et al. (2006) [29] | 51 | 40% | 21 | -- | 1 | -- | -- | -- | -- | Level 2 |
| Bianco et al. (2005) [28] | 100 | 68% | -- | -- | 1 | -- | -- | -- | -- | Level 2 |
| Ward et al. (2005) [26] | 138 | 33% | 22 | -- | 9 | -- | 4 | 86 | -- | Level 3 |
| Stephenson et al. (2004) [42] | 100 | 61% | 30 | 84% | 7 | 1 | 1 | -- | -- | Level 2 |
| Amling et al. (1999) [25] | 108 | 50% | 23 | -- | 6 | 6 | -- | 46 | -- | Level 3 |
| Gheiler et al. (1998) [24] | 30 | 50% | -- | -- | -- | -- | -- | -- | 7.7 (2–30) | Level 3 |
| Lerner et al. (1995) [23] | 37 | 93.6% | 16 | -- | 5 | 5 | 4 | -- | 8 (2–44) | Level 3 |
| Rogers et al. (1995) [21] | 40 | 58% | 11 | -- | 6 | -- | 3 | 2 | 9.6 (6–16) | Level 3 |
3.3. Additional Considerations
3.3.1. Effect of Primary Therapy on Salvage Prostatectomy Outcomes
3.3.2. The Role of Lymph Node Dissection and Seminal Vesicle Biopsy
3.3.3. Novel Biomarkers
3.3.4. Comparison to Alternate Salvage Therapy
3.4. Future Directions and Ongoing Clinical Trials
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Tidd-Johnson, A.; Sebastian, S.A.; Co, E.L.; Afaq, M.; Kochhar, H.; Sheikh, M.; Mago, A.; Poudel, S.; Fernandez, J.A.; Rodriguez, I.D.; et al. Prostate cancer screening: Continued controversies and novel biomarker advancements. Curr. Urol. 2022, 16, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.T.; Barocas, D.; Carlsson, S.; Coakley, F.; Eggener, S.; Etzioni, R.; Fine, S.W.; Han, M.; Kim, S.K.; Kirkby, E.; et al. Early Detection of Prostate Cancer: AUA/SUO Guideline Part I: Prostate Cancer Screening. J. Urol. 2023, 210, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J. Urol. 2022, 208, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. NCCN Guidelines® Insights: Prostate Cancer, Version 1.2023. J. Natl. Compr. Canc. Netw. 2022, 20, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Metcalfe, C.; Davis, M.; Turner, E.L.; Martin, R.M.; Young, G.J.; Walsh, E.I.; Bryant, R.J.; et al. Fifteen-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. New Engl. J. Med. 2023, 388, 1547–1558. [Google Scholar] [CrossRef]
- Zhang-Yin, J.; Montravers, F.; Montagne, S.; Hennequin, C.; Renard-Penna, R. Diagnosis of early biochemical recurrence after radical prostatectomy or radiation therapy in patients with prostate cancer: State of the art. Diagn. Interv. Imaging 2022, 103, 191–199. [Google Scholar] [CrossRef]
- Pisansky, T.M.; Thompson, I.M.; Valicenti, R.K.; D’Amico, A.V.; Selvarajah, S. Adjuvant and Salvage Radiotherapy after Prostatectomy: ASTRO/AUA Guideline Amendment 2018–2019. J. Urol. 2019, 202, 533–538. [Google Scholar] [CrossRef]
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- Suardi, N.; Porter, C.R.; Reuther, A.M.; Walz, J.; Kodama, K.; Gibbons, R.P.; Correa, R.; Montorsi, F.; Graefen, M.; Huland, H.; et al. A nomogram predicting long-term biochemical recurrence after radical prostatectomy. Cancer 2008, 112, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J.; Gorin, M.A.; Rowe, S.P.; Carroll, P.R.; Pouliot, F.; Probst, S.; Saperstein, L.; Preston, M.A.; Alva, A.S.; Patnaik, A.; et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with (18)F-DCFPyL in Prostate Cancer Patients (OSPREY). J. Urol. 2021, 206, 52–61. [Google Scholar] [CrossRef]
- Morris, M.J.; Rowe, S.P.; Gorin, M.A.; Saperstein, L.; Pouliot, F.; Josephson, D.; Wong, J.Y.C.; Pantel, A.R.; Cho, S.Y.; Gage, K.L.; et al. Diagnostic Performance of (18)F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin. Cancer Res. 2021, 27, 3674–3682. [Google Scholar] [CrossRef]
- Ceci, F.; Castellucci, P.; Graziani, T.; Farolfi, A.; Fonti, C.; Lodi, F.; Fanti, S. (68)Ga-PSMA-11 PET/CT in recurrent prostate cancer: Efficacy in different clinical stages of PSA failure after radical therapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 31–39. [Google Scholar] [CrossRef]
- Westphalen, A.C.; Coakley, F.V.; Roach, M., 3rd; McCulloch, C.E.; Kurhanewicz, J. Locally recurrent prostate cancer after external beam radiation therapy: Diagnostic performance of 1.5-T endorectal MR imaging and MR spectroscopic imaging for detection. Radiology 2010, 256, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala, E.; Eberhardt, S.C.; Akin, O.; Moskowitz, C.S.; Onyebuchi, C.N.; Kuroiwa, K.; Ishill, N.; Zelefsky, M.J.; Eastham, J.A.; Hricak, H. Endorectal MR imaging before salvage prostatectomy: Tumor localization and staging. Radiology 2006, 238, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, C.; Plata, M.; Cataño, J.G.; Palau, M.; Aguirre, D.; Narvaez, J.; Trujillo, S.; Gómez, F.; Trujillo, C.G.; Caicedo, J.I.; et al. Diagnostic accuracy of multiparametric magnetic resonance imaging in detecting extracapsular extension in intermediate and high—Risk prostate cancer. Int. Braz J. Urol. 2018, 44, 688–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, J.D.; Gallagher, M.J.; Hammond, E.H.; Kaplan, R.S.; Schellhammer, P.F. Consensus statements on radiation therapy of prostate cancer: Guidelines for prostate re-biopsy after radiation and for radiation therapy with rising prostate-specific antigen levels after radical prostatectomy. American Society for Therapeutic Radiology and Oncology Consensus Panel. J. Clin. Oncol. 1999, 17, 1155. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II—2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Valle, L.F.; Lehrer, E.J.; Markovic, D.; Elashoff, D.; Levin-Epstein, R.; Karnes, R.J.; Reiter, R.E.; Rettig, M.; Calais, J.; Nickols, N.G.; et al. A Systematic Review and Meta-analysis of Local Salvage Therapies After Radiotherapy for Prostate Cancer (MASTER). Eur. Urol. 2021, 80, 280–292. [Google Scholar] [CrossRef]
- Kishan, A.U.; Chu, F.I.; King, C.R.; Seiferheld, W.; Spratt, D.E.; Tran, P.; Wang, X.; Pugh, S.E.; Sandler, K.A.; Bolla, M.; et al. Local Failure and Survival After Definitive Radiotherapy for Aggressive Prostate Cancer: An Individual Patient-level Meta-analysis of Six Randomized Trials. Eur. Urol. 2020, 77, 201–208. [Google Scholar] [CrossRef]
- Nguyen, P.L.; D’Amico, A.V.; Lee, A.K.; Suh, W.W. Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure: A systematic review of the literature. Cancer 2007, 110, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Page, M.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, 1–9. [Google Scholar]
- Rogers, E.; Ohori, M.; Kassabian, V.S.; Wheeler, T.M.; Scardino, P.T. Salvage radical prostatectomy: Outcome measured by serum prostate specific antigen levels. J. Urol. 1995, 153, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Tefilli, M.V.; Gheiler, E.L.; Tiguert, R.; Banerjee, M.; Forman, J.; Pontes, J.E.; Wood, D.P., Jr. Salvage surgery or salvage radiotherapy for locally recurrent prostate cancer. Urology 1998, 52, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Lerner, S.E.; Blute, M.L.; Zincke, H. Critical evaluation of salvage surgery for radio-recurrent/resistant prostate cancer. J. Urol. 1995, 154, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Gheiler, E.L.; Tefilli, M.V.; Tiguert, R.; Grignon, D.; Cher, M.L.; Sakr, W.; Pontes, J.E.; Wood, D.P., Jr. Predictors for maximal outcome in patients undergoing salvage surgery for radio-recurrent prostate cancer. Urology 1998, 51, 789–795. [Google Scholar] [CrossRef]
- Amling, C.L.; Lerner, S.E.; Martin, S.K.; Slezak, J.M.; Blute, M.L.; Zincke, H. Deoxyribonucleic acid ploidy and serum prostate specific antigen predict outcome following salvage prostatectomy for radiation refractory prostate cancer. J. Urol. 1999, 161, 857–862, discussion 862-853. [Google Scholar] [CrossRef]
- Ward, J.F.; Sebo, T.J.; Blute, M.L.; Zincke, H. Salvage surgery for radiorecurrent prostate cancer: Contemporary outcomes. J. Urol. 2005, 173, 1156–1160. [Google Scholar] [CrossRef]
- Chade, D.C.; Shariat, S.F.; Cronin, A.M.; Savage, C.J.; Karnes, R.J.; Blute, M.L.; Briganti, A.; Montorsi, F.; van der Poel, H.G.; Van Poppel, H.; et al. Salvage radical prostatectomy for radiation-recurrent prostate cancer: A multi-institutional collaboration. Eur. Urol. 2011, 60, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Bianco, F.J., Jr.; Scardino, P.T.; Stephenson, A.J.; Diblasio, C.J.; Fearn, P.A.; Eastham, J.A. Long-term oncologic results of salvage radical prostatectomy for locally recurrent prostate cancer after radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 448–453. [Google Scholar] [CrossRef]
- Sanderson, K.M.; Penson, D.F.; Cai, J.; Groshen, S.; Stein, J.P.; Lieskovsky, G.; Skinner, D.G. Salvage radical prostatectomy: Quality of life outcomes and long-term oncological control of radiorecurrent prostate cancer. J. Urol. 2006, 176, 2025–2031, discussion 2031-2022. [Google Scholar] [CrossRef]
- Pisters, L.L.; Leibovici, D.; Blute, M.; Zincke, H.; Sebo, T.J.; Slezak, J.M.; Izawa, J.; Ward, J.F.; Scott, S.M.; Madsen, L.; et al. Locally recurrent prostate cancer after initial radiation therapy: A comparison of salvage radical prostatectomy versus cryotherapy. J. Urol. 2009, 182, 517–525, discussion 525-517. [Google Scholar] [CrossRef]
- Paparel, P.; Cronin, A.M.; Savage, C.; Scardino, P.T.; Eastham, J.A. Oncologic outcome and patterns of recurrence after salvage radical prostatectomy. Eur. Urol. 2009, 55, 404–410. [Google Scholar] [CrossRef]
- Gorin, M.A.; Manoharan, M.; Shah, G.; Eldefrawy, A.; Soloway, M.S. Salvage open radical prostatectomy after failed radiation therapy: A single center experience. Cent. Eur. J. Urol. 2011, 64, 144–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuh, B.; Ruel, N.; Muldrew, S.; Mejia, R.; Novara, G.; Kawachi, M.; Wilson, T. Complications and outcomes of salvage robot-assisted radical prostatectomy: A single-institution experience. BJU Int. 2014, 113, 769–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohler, J.L.; Halabi, S.; Ryan, S.T.; Al-Daghmin, A.; Sokoloff, M.H.; Steinberg, G.D.; Sanford, B.L.; Eastham, J.A.; Walther, P.J.; Morris, M.J.; et al. Management of recurrent prostate cancer after radiotherapy: Long-term results from CALGB 9687 (Alliance), a prospective multi-institutional salvage prostatectomy series. Prostate Cancer Prostatic Dis. 2019, 22, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubmüller, B.; Jahrreiss, V.; Brönimann, S.; Quhal, F.; Mori, K.; Heidenreich, A.; Briganti, A.; Tilki, D.; Shariat, S.F. Salvage Radical Prostatectomy for Radio-Recurrent Prostate Cancer: An Updated Systematic Review of Oncologic, Histopathologic and Functional Outcomes and Predictors of Good Response. Curr. Oncol. 2021, 28, 2881–2892. [Google Scholar] [CrossRef]
- Catarino, R.; Otta-Oshiro, R.J.; Lista-Mateos, F.; García-Mediero, J.M.; Nunez-Mora, C. Outcomes of laparoscopic salvage radical prostatectomy after primary treatment of prostate cancer. Cent. Eur. J. Urol. 2022, 75, 59–64. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Calleris, G.; Marra, G.; Benfant, N.; Rajwa, P.; Ahmed, M.; Abreu, A.; Cacciamani, G.; Ghoreifi, A.; Ribeiro, L.; Westhofen, T.; et al. Salvage Radical Prostatectomy for Recurrent Prostate Cancer Following First-line Nonsurgical Treatment: Validation of the European Association of Urology Criteria in a Large, Multicenter, Contemporary Cohort. Eur. Urol. Focus 2023. [Google Scholar] [CrossRef]
- Bates, A.S.; Samavedi, S.; Kumar, A.; Mouraviev, V.; Rocco, B.; Coelho, R.; Palmer, K.; Patel, V.R. Salvage robot assisted radical prostatectomy: A propensity matched study of perioperative, oncological and functional outcomes. Eur. J. Surg. Oncol. 2015, 41, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.N.; Suy, S.; Wang, H.; Bhagat, A.; Woo, J.A.; Moures, R.A.; Kim, J.S.; Yung, T.M.; Lei, S.; Collins, B.T.; et al. Patient-reported urinary incontinence following stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer. Radiat. Oncol. 2014, 9, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, J.; Shamah, A.A.; Creed, T.M.; Pavlovic, R.; Matsui, H.; Kimura, M.; Molitoris, J.; Shukla, H.; Jackson, I.; Vujaskovic, Z. Radiation-induced erectile dysfunction: Recent advances and future directions. Adv. Radiat. Oncol. 2016, 1, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, A.J.; Scardino, P.T.; Bianco, F.J., Jr.; DiBlasio, C.J.; Fearn, P.A.; Eastham, J.A. Morbidity and functional outcomes of salvage radical prostatectomy for locally recurrent prostate cancer after radiation therapy. J. Urol. 2004, 172, 2239–2243. [Google Scholar] [CrossRef]
- Mason, J.B.; Hatch, L.; Dall, C.; Kowalczyk, K.J. Salvage Retzius-Sparing Radical Prostatectomy: A Review of Complications, Functional Outcomes, and Oncologic Outcomes. Curr. Oncol. 2022, 29, 9733–9743. [Google Scholar] [CrossRef]
- Eandi, J.A.; Link, B.A.; Nelson, R.A.; Josephson, D.Y.; Lau, C.; Kawachi, M.H.; Wilson, T.G. Robotic assisted laparoscopic salvage prostatectomy for radiation resistant prostate cancer. J. Urol. 2010, 183, 133–137, discussion 862-853. [Google Scholar] [CrossRef]
- Kenney, P.A.; Nawaf, C.B.; Mustafa, M.; Wen, S.; Wszolek, M.F.; Pettaway, C.A.; Ward, J.F.; Davis, J.W.; Pisters, L.L. Robotic-assisted laparoscopic versus open salvage radical prostatectomy following radiotherapy. Can J. Urol. 2016, 23, 8271–8277. [Google Scholar]
- Gontero, P.; Marra, G.; Alessio, P.; Filippini, C.; Oderda, M.; Munoz, F.; Linares, E.; Sanchez-Salas, R.; Challacombe, B.; Dasgupta, P.; et al. Salvage Radical Prostatectomy for Recurrent Prostate Cancer: Morbidity and Functional Outcomes from a Large Multicenter Series of Open versus Robotic Approaches. J. Urol. 2019, 202, 725–731. [Google Scholar] [CrossRef]
- Onol, F.F.; Bhat, S.; Moschovas, M.; Rogers, T.; Ganapathi, H.; Roof, S.; Rocco, B.; Patel, V. Comparison of outcomes of salvage robot-assisted laparoscopic prostatectomy for post-primary radiation vs focal therapy. BJU Int. 2020, 125, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, L.; Stonier, T.; Stroman, L.; Tourinho-Barbosa, R.; Alghazo, O.; Winkler, M.; Dasgupta, P.; Popert, R.; Cathelineau, X.; Sanchez-Salas, R.; et al. Is the Toxicity of Salvage Prostatectomy Related to the Primary Prostate Cancer Therapy Received? J. Urol. 2021, 205, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Würnschimmel, C.; Nocera, L.; Collà Ruvolo, C.; Tian, Z.; Shariat, S.F.; Saad, F.; Briganti, A.; Graefen, M.; Kluth, L.A.; et al. The effect of lymph node dissection on cancer-specific survival in salvage radical prostatectomy patients. Prostate 2021, 81, 339–346. [Google Scholar] [CrossRef]
- Quhal, F.; Rajwa, P.; Mori, K.; Laukhtina, E.; Grossmann, N.C.; Schuettfort, V.M.; König, F.; Aydh, A.; Motlagh, R.S.; Katayama, S.; et al. The role of lymph node dissection in salvage radical prostatectomy for patients with radiation recurrent prostate cancer. Prostate 2021, 81, 765–771. [Google Scholar] [CrossRef]
- Swanson, G.P.; Goldman, B.; Tangen, C.M.; Chin, J.; Messing, E.; Canby-Hagino, E.; Forman, J.D.; Thompson, I.M.; Crawford, E.D. The prognostic impact of seminal vesicle involvement found at prostatectomy and the effects of adjuvant radiation: Data from Southwest Oncology Group 8794. J. Urol. 2008, 180, 2453–2457. [Google Scholar] [CrossRef] [Green Version]
- Silberstein, J.L.; Vickers, A.J.; Power, N.E.; Fine, S.W.; Scardino, P.T.; Eastham, J.A.; Laudone, V.P. Reverse stage shift at a tertiary care center: Escalating risk in men undergoing radical prostatectomy. Cancer 2011, 117, 4855–4860. [Google Scholar] [CrossRef] [Green Version]
- Stock, R.G.; Lo, Y.C.; Gaildon, M.; Stone, N.N. Does prostate brachytherapy treat the seminal vesicles? A dose-volume histogram analysis of seminal vesicles in patients undergoing combined PD-103 prostate implantation and external beam irradiation. Int J. Radiat. Oncol. Biol. Phys. 1999, 45, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Meeks, J.J.; Walker, M.; Bernstein, M.; Eastham, J.A. Seminal vesicle involvement at salvage radical prostatectomy. BJU Int. 2013, 111, E342–E347. [Google Scholar] [CrossRef]
- Allepuz Losa, C.A.; Sanz Velez, J.I.; Gil Sanz, M.J.; Mas, L.P.; Rioja Sanz, L.A. Seminal vesicle biopsy in prostate cancer staging. J. Urol. 1995, 154, 1407–1411. [Google Scholar] [CrossRef]
- Panach-Navarrete, J.; García-Morata, F.; Hernández-Medina, J.A.; Martínez-Jabaloyas, J.M. When to biopsy seminal vesicles. Actas Urológicas Españolas (Engl. Ed.) 2015, 39, 203–209. [Google Scholar] [CrossRef]
- Santarpia, L.; Grandone, I.; Contaldo, F.; Pasanisi, F. Butyrylcholinesterase as a prognostic marker: A review of the literature. J. Cachexia Sarcopenia Muscle 2013, 4, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.Z.; Zhao, X.H.; Quan, P.; Li, S.B.; Pan, B.R. Alterations of serum cholinesterase in patients with gastric cancer. World J. Gastroenterol 2005, 11, 4604–4606. [Google Scholar] [CrossRef] [PubMed]
- Vartolomei, M.D.; D’Andrea, D.; Chade, D.C.; Soria, F.; Kimura, S.; Foerster, B.; Abufaraj, M.; Mathieu, R.; Moschini, M.; Rouprêt, M.; et al. Role of serum cholinesterase in patients treated with salvage radical prostatectomy. Urol. Oncol. 2019, 37, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Checcucci, E.; De Luca, S.; Piramide, F.; Garrou, D.; Mosca, A.; Galla, A.; Belli, G.; Russo, F.; Rescigno, P.; Poti, C.; et al. The real-time intraoperative guidance of the new HIFU Focal-One(®) platform allows to minimize the perioperative adverse events in salvage setting. J. Ultrasound 2022, 25, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Netherlands Cancer Institute. Tracer-Guided Surgery for Recurrent Prostate Cancer (Trace-II); ClinicalTrials.gov Identifier: NCT05555017; National Library of Medicine: Bethesda, MD, USA, 2022.
- VA Office of Research and Development. Veterans Affairs Seamless Phase II/III Randomized Trial of STAndard Systematic theRapy With or Without PET-Directed Local Therapy for OligoRecurrenT Prostate Cancer (VA STARPORT); ClinicalTrials.gov Identifier: NCT04787744; National Library of Medicine: Bethesda, MD, USA, 2021.
- University Health Network. Salvage Prostatectomy After Radiotherapy. ClinicalTrials.gov Identifier: NCT00791115; National Library of Medicine: Bethesda, MD, USA, 2023.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drobner, J.; Kaldany, A.; Shah, M.S.; Ghodoussipour, S. The Role of Salvage Radical Prostatectomy in Patients with Radiation-Resistant Prostate Cancer. Cancers 2023, 15, 3734. https://doi.org/10.3390/cancers15143734
Drobner J, Kaldany A, Shah MS, Ghodoussipour S. The Role of Salvage Radical Prostatectomy in Patients with Radiation-Resistant Prostate Cancer. Cancers. 2023; 15(14):3734. https://doi.org/10.3390/cancers15143734
Chicago/Turabian StyleDrobner, Jake, Alain Kaldany, Mihir S. Shah, and Saum Ghodoussipour. 2023. "The Role of Salvage Radical Prostatectomy in Patients with Radiation-Resistant Prostate Cancer" Cancers 15, no. 14: 3734. https://doi.org/10.3390/cancers15143734
APA StyleDrobner, J., Kaldany, A., Shah, M. S., & Ghodoussipour, S. (2023). The Role of Salvage Radical Prostatectomy in Patients with Radiation-Resistant Prostate Cancer. Cancers, 15(14), 3734. https://doi.org/10.3390/cancers15143734






