FLASH Radiotherapy and the Use of Radiation Dosimeters
Abstract
:Simple Summary
Abstract
1. Introduction
2. Monte Carlo Simulation
3. Radiation Dose Detectors
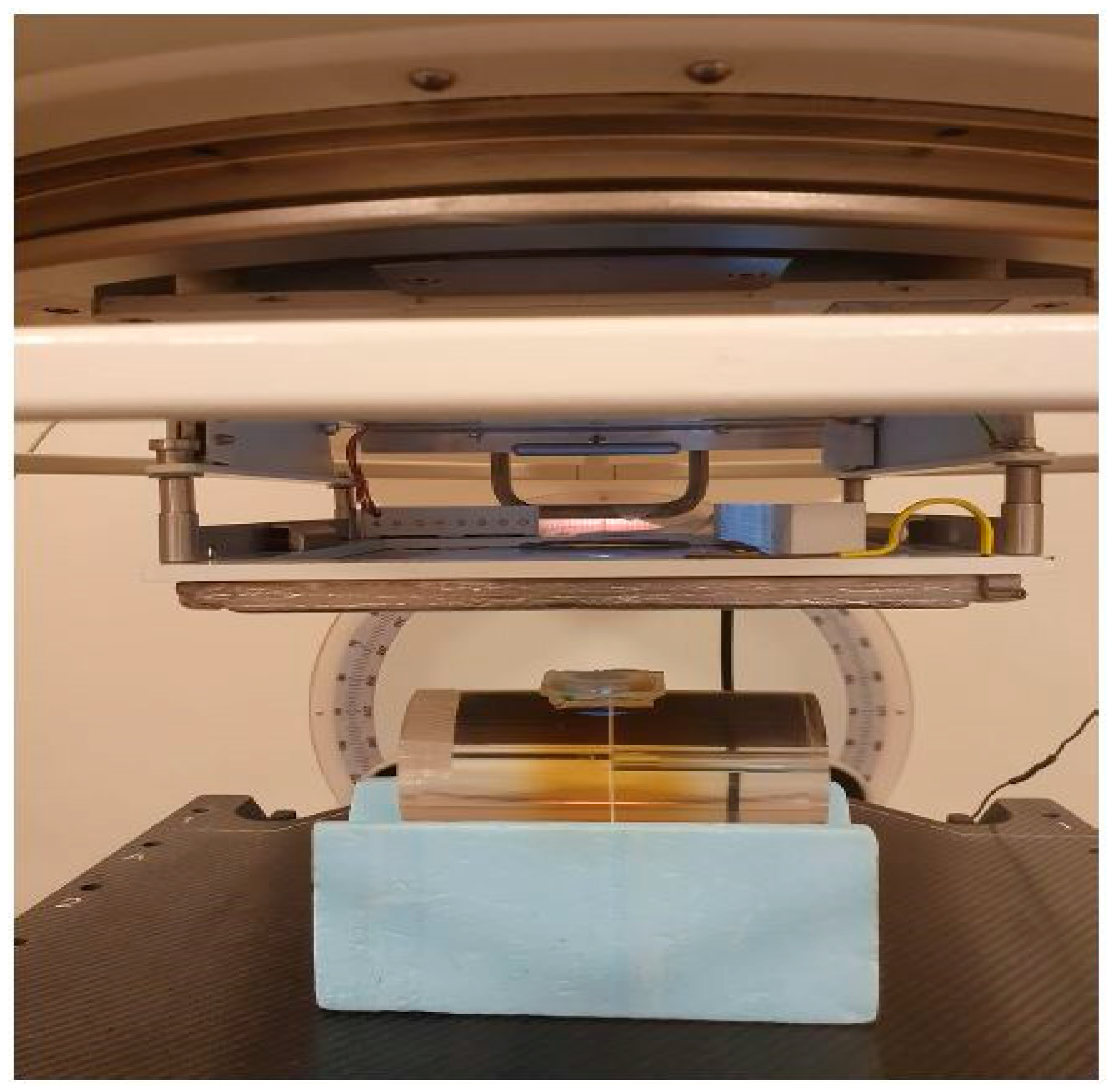
3.1. Diamond Detector
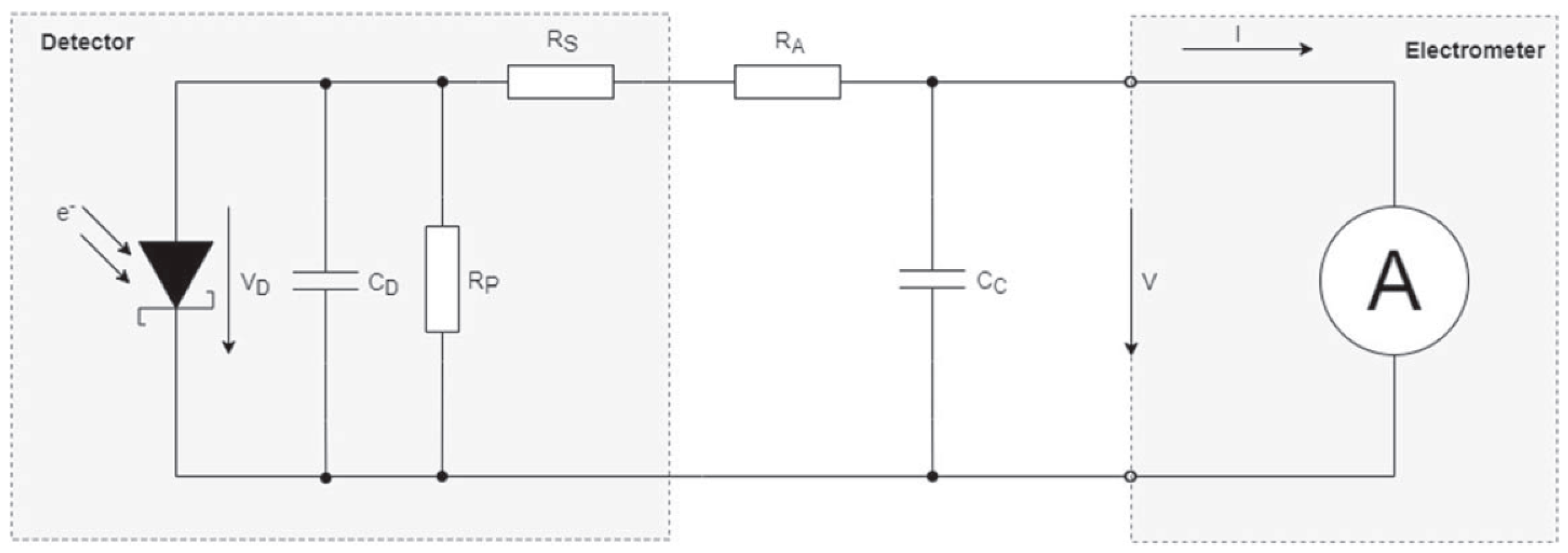
3.2. Ionization Chamber
3.3. Radiochromic Film
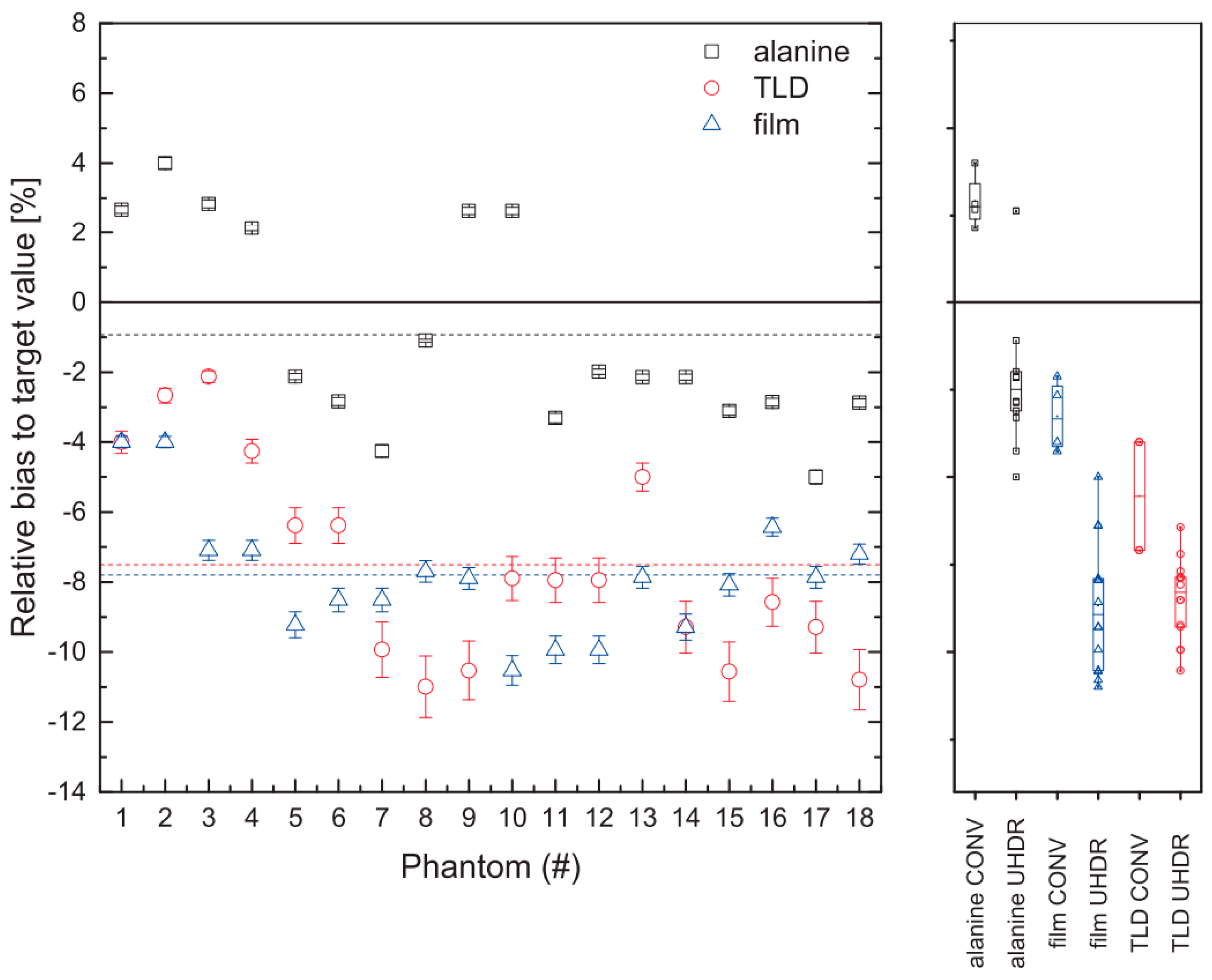
3.4. Alanine
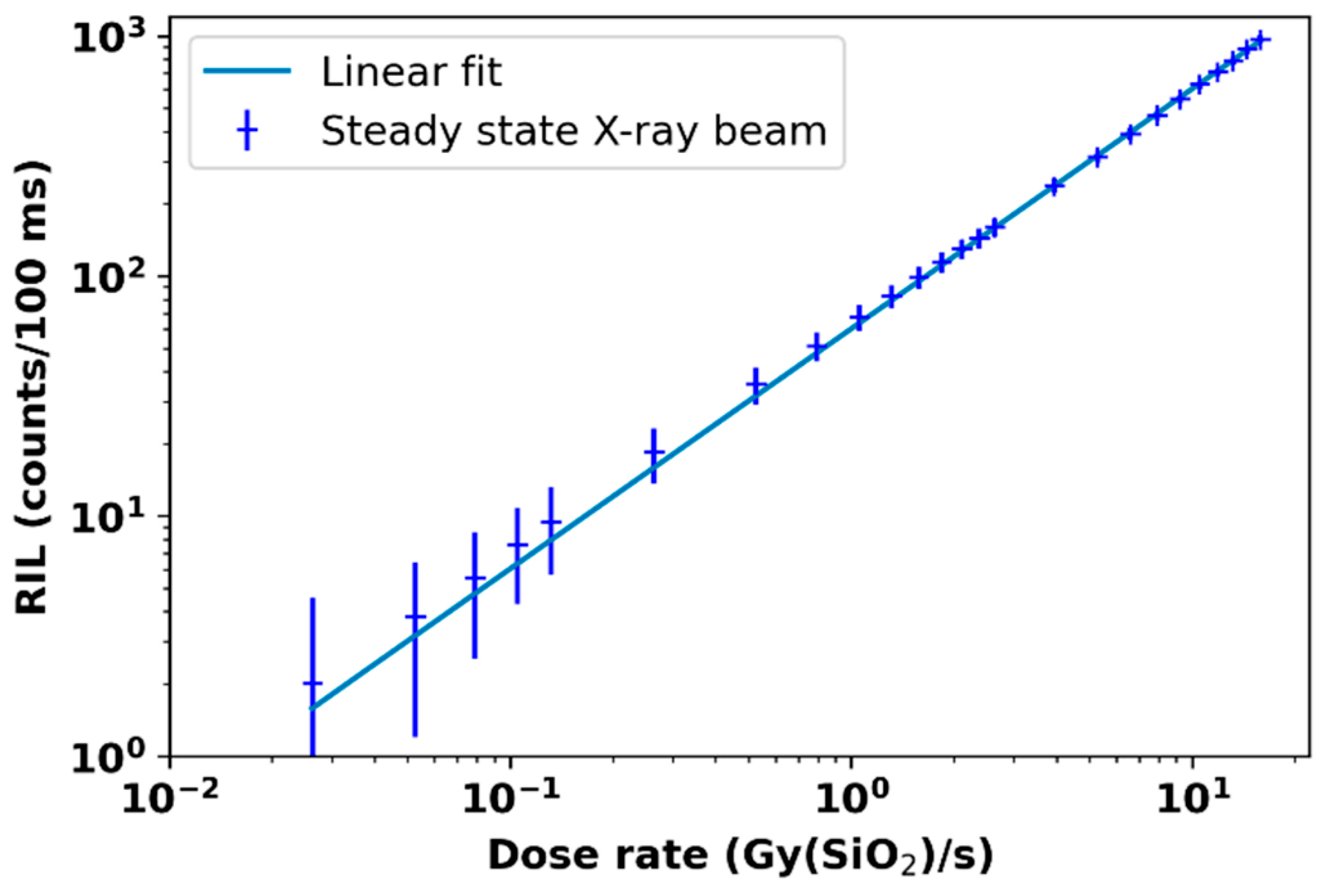
3.5. Radioluminescence, Cherenkov Radiation Dosimetry, and Others
4. Future Prospective
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lv, Y.; Lv, Y.; Wang, Z.; Lan, T.; Feng, X.; Chen, H.; Zhu, J.; Ma, X.; Du, J.; Hou, G.; et al. FLASH radiotherapy: A promising new method for radiotherapy (Review). Oncol. Lett. 2022, 24, 419. [Google Scholar] [CrossRef]
- Lin, B.; Gao, F.; Yang, Y.; Wu, D.; Zhang, Y.; Feng, G.; Dai, T.; Du, X. FLASH Radiotherapy: History and Future. Front. Oncol. 2021, 11, 644400. [Google Scholar] [CrossRef]
- Hughes, J.R.; Parsons, J.L. FLASH Radiotherapy: Current Knowledge and Future Insights Using Proton-Beam Therapy. Int. J. Mol. Sci. 2020, 21, 6492. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Huang, D.; Gao, F.; Yang, Y.; Wu, D.; Zhang, Y.; Feng, G.; Dai, T.; Du, X. Mechanisms of FLASH effect. Front. Oncol. 2022, 12, 995612. [Google Scholar] [CrossRef]
- Esplen, N.; Mendonca, M.S.; Bazalova-Carter, M. Physics and biology of ultrahigh dose-rate (FLASH) radiotherapy: A topical review. Phys. Med. Biol. 2020, 65, 23TR03. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.-F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Okoro, C.M.; Schüler, E.; Taniguchi, C.M. The Therapeutic Potential of FLASH-RT for Pancreatic Cancer. Cancers 2022, 14, 1167. [Google Scholar] [CrossRef]
- Matuszak, N.; Suchorska, W.M.; Milecki, P.; Kruszyna-Mochalska, M.; Misiarz, A.; Pracz, J.; Malicki, J. FLASH radiotherapy: An emerging approach in radiation therapy. Rep. Pract. Oncol. Radiother. 2022, 27, 343–351. [Google Scholar] [CrossRef]
- Rahman, M.; Trigilio, A.; Franciosini, G.; Moeckli, R.; Zhang, R.; Böhlen, T.T. FLASH radiotherapy treatment planning and models for electron beams. Radiother. Oncol. 2022, 175, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Subiel, A.; Romano, F. Recent developments in absolute dosimetry for FLASH radiotherapy. Br. J. Radiol. 2023, 96, 20220560. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Corde, S.; Laissue, J.A.; Bazalova-Carter, M. FLASH radiotherapy with photon beams. Med. Phys. 2022, 49, 2055–2067. [Google Scholar] [CrossRef]
- Almeida, A.; Togno, M.; Ballesteros-Zebadua, P.; Franco-Perez, J.; Geyer, R.; Schaefer, R.; Petit, B.; Grilj, V.; Meer, D.; Safai, S.; et al. Dosimetric and biologic intercomparison between electron and proton FLASH beams. bioRxiv 2023. preprint. [Google Scholar] [CrossRef]
- El Naqa, I.; Pogue, B.W.; Zhang, R.; Oraiqat, I.; Parodi, K. Image guidance for FLASH radiotherapy. Med. Phys. 2022, 49, 4109–4122. [Google Scholar] [CrossRef]
- Moon, E.J.; Petersson, K.; Olcina, M.M. The importance of hypoxia in radiotherapy for the immune response, metastatic potential and FLASH-RT. Int. J. Radiat. Biol. 2022, 98, 439–451. [Google Scholar] [CrossRef]
- Abolfath, R.; Grosshans, D.; Mohan, R. Oxygen depletion in FLASH ultra-high-dose-rate radiotherapy: A molecular dynamics simulation. Med. Phys. 2020, 47, 6551–6561. [Google Scholar] [CrossRef]
- Tinganelli, W.; Sokol, O.; Quartieri, M.; Puspitasari, A.; Dokic, I.; Abdollahi, A.; Durante, M.; Haberer, T.; Debus, J.; Boscolo, D.; et al. Ultra-High Dose Rate (FLASH) Carbon Ion Irradiation: Dosimetry and First Cell Experiments. Int. J. Radiat. Oncol. 2022, 112, 1012–1022. [Google Scholar] [CrossRef]
- Alanazi, A.; Meesungnoen, J.; Jay-Gerin, J.-P. A Computer Modeling Study of Water Radiolysis at High Dose Rates. Relevance to FLASH Radiotherapy. Radiat. Res. 2020, 195, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Lin, H.; Choi, J.I.; Simone, C.B.; Kang, M. A Novel Proton Pencil Beam Scanning FLASH RT Delivery Method Enables Optimal OAR Sparing and Ultra-High Dose Rate Delivery: A Comprehensive Dosimetry Study for Lung Tumors. Cancers 2021, 13, 5790. [Google Scholar] [CrossRef]
- Yang, G.; Lu, C.; Mei, Z.; Sun, X.; Han, J.; Qian, J.; Liang, Y.; Pan, Z.; Kong, D.; Xu, S.; et al. Association of Cancer Stem Cell Radio-Resistance Under Ultra-High Dose Rate FLASH Irradiation With Lysosome-Mediated Autophagy. Front. Cell Dev. Biol. 2021, 9, 672693. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Liang, Y.; Hu, Y.; Liu, C. Monte Carlo simulation of linac using PRIMO. Radiat. Oncol. 2022, 17, 185. [Google Scholar] [CrossRef] [PubMed]
- Kawrakow, I.; Rogers, D.W. The EGSnrc Code System; NRC Report PIRS-701; NRC: Ottawa, ON, Canda, 2000; Volume 17, p. 108.
- Walters, B.R.; Kawrakow, I.; Rogers, D.W. DOSXYZnrc Users Manual; NRC Report Pirs; NRC: Ottawa, ON, Canda, 2005; Volume 794, pp. 57–58.
- Gao, Y.; Liu, R.; Chang, C.; Charyyev, S.; Zhou, J.; Bradley, J.D.; Liu, T.; Yang, X. A potential revolution in cancer treatment: A topical review of FLASH radiotherapy. J. Appl. Clin. Med. Phys. 2022, 23, e13790. [Google Scholar] [CrossRef]
- Lazarus, G.L.; van Eeden, D.; du Plessis, F.C. Validation of Monte Carlo-based calculations for megavolt electron beams for IORT and FLASH-IORT. Heliyon 2022, 8, e10682. [Google Scholar] [CrossRef]
- Breitkreutz, D.Y.; Shumail, M.; Bush, K.K.; Tantawi, S.G.; Maxime, P.G.; Loo, B.W. Initial Steps Towards a Clinical FLASH Radiotherapy System: Pediatric Whole Brain Irradiation with 40 MeV Electrons at FLASH Dose Rates. Radiat. Res. 2020, 194, 594–599. [Google Scholar] [CrossRef]
- Rosenstrom, A.; Santana-Leitner, M.; Rokni, S.H.; Shumail, M.; Tantawi, S.; Dewji, S.; Loo, B.W. Monte Carlo simulation of shielding designs for a cabinet form factor preclinical MV-energy photon FLASH radiotherapy system. Med. Phys. 2023, 50, 3055–3065. [Google Scholar] [CrossRef] [PubMed]
- Sunbul, N.H.B.; Zhang, W.; Oraiqat, I.; Litzenberg, D.W.; Lam, K.L.; Cuneo, K.; Moran, J.M.; Carson, P.L.; Wang, X.; Clarke, S.D.; et al. A simulation study of ionizing radiation acoustic imaging (iRAI) as a real-time dosimetric technique for ultra-high dose rate radiotherapy (UHDR-RT). Med. Phys. 2021, 48, 6137–6151. [Google Scholar] [CrossRef]
- Brunbauer, F.; Lupberger, M.; Oliveri, E.; Resnati, F.; Ropelewski, L.; Streli, C.; Thuiner, P.; van Stenis, M. Radiation imaging with optically read out GEM-based detectors. J. Instrum. 2018, 13, T02006. [Google Scholar] [CrossRef] [Green Version]
- Esquivel, A.; Ferrero, A.; Mileto, A.; Baffour, F.; Horst, K.; Rajiah, P.S.; Inoue, A.; Leng, S.; McCollough, C.; Fletcher, J.G. Photon-Counting Detector CT: Key Points Radiologists Should Know. Korean J. Radiol. 2022, 23, 854–865. [Google Scholar] [CrossRef]
- Calin, M.R. Gas spherical ionization chamber. J. Radioanal. Nucl. Chem. 2011, 290, 361–366. [Google Scholar] [CrossRef]
- Yoosuf, A.M.; Jeevanandam, P.; Whitten, G.; Workman, G.; McGarry, C.K. Verification of high-dose-rate brachytherapy treatment planning dose distribution using liquid-filled ionization chamber array. J. Contemp. Brachytherapy 2018, 10, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.R.; Rahman, M.; Zhang, R.; Williams, B.B.; Gladstone, D.J.; Pogue, B.W.; Bruza, P. Dosimetry for FLASH Radiotherapy: A Review of Tools and the Role of Radioluminescence and Cherenkov Emission. Front. Phys. 2020, 8, 328. [Google Scholar] [CrossRef]
- Ravichandran, R.; Binukumar, J.P.; Al Amri, I.; Davis, C.A. Diamond detector in absorbed dose measurements in high-energy linear accelerator photon and electron beams. J. Appl. Clin. Med. Phys. 2016, 17, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Kranzer, R.; Schüller, A.; Bourgouin, A.; Hackel, T.; Poppinga, D.; Lapp, M.; Looe, H.K.; Poppe, B. Response of diamond detectors in ultra-high dose-per-pulse electron beams for dosimetry at FLASH radiotherapy. Phys. Med. Biol. 2022, 67, 075002. [Google Scholar] [CrossRef]
- Rinati, G.V.; Felici, G.; Galante, F.; Gasparini, A.; Kranzer, R.; Mariani, G.; Pacitti, M.; Prestopino, G.; Schüller, A.; Vanreusel, V.; et al. Application of a novel diamond detector for commissioning of FLASH radiotherapy electron beams. Med. Phys. 2022, 49, 5513–5522. [Google Scholar] [CrossRef]
- Marinelli, M.; Felici, G.; Galante, F.; Gasparini, A.; Giuliano, L.; Heinrich, S.; Pacitti, M.; Prestopino, G.; Vanreusel, V.; Verellen, D.; et al. Design, realization, and characterization of a novel diamond detector prototype for FLASH radiotherapy dosimetry. Med. Phys. 2022, 49, 1902–1910. [Google Scholar] [CrossRef]
- Polaczek-Grelik, K.; Kawa-Iwanicka, A.; Michalecki, Ł. Dosimetric accuracy of a cross-calibration coefficient for plane-parallel ionization chamber obtained in low-energy electron beams using various cylindrical dosimeters. Pol. J. Med. Phys. Eng. 2021, 27, 303–313. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, C.; Chen, C.; Tsai, P.; Kang, M.; Huang, S.; Lin, C.; Chang, F.; Chhabra, A.M.; Choi, J.I.; et al. A 2D strip ionization chamber array with high spatiotemporal resolution for proton pencil beam scanning FLASH radiotherapy. Med. Phys. 2022, 49, 5464–5475. [Google Scholar] [CrossRef]
- Di Martino, F.; Del Sarto, D.; Bisogni, M.G.; Capaccioli, S.; Galante, F.; Gasperini, A.; Linsalata, S.; Mariani, G.; Pacitti, M.; Paiar, F.; et al. A new solution for UHDP and UHDR (Flash) measurements: Theory and conceptual design of ALLS chamber. Phys. Medica 2022, 102, 9–18. [Google Scholar] [CrossRef]
- Di Martino, F.; Del Sarto, D.; Barone, S.; Bisogni, M.G.; Capaccioli, S.; Galante, F.; Gasparini, A.; Mariani, G.; Masturzo, L.; Montefiori, M.; et al. A new calculation method for the free electron fraction of an ionization chamber in the ultra-high-dose-per-pulse regimen. Phys. Medica 2022, 103, 175–180. [Google Scholar] [CrossRef]
- Petersson, K.; Jaccard, M.; Germond, J.-F.; Buchillier, T.; Bochud, F.; Bourhis, J.; Vozenin, M.-C.; Bailat, C. High dose-per-pulse electron beam dosimetry—A model to correct for the ion recombination in the Advanced Markus ionization chamber. Med. Phys. 2017, 44, 1157–1167. [Google Scholar] [CrossRef]
- Darafsheh, A.; Hao, Y.; Zhao, X.; Zwart, T.; Wagner, M.; Evans, T.; Reynoso, F.; Zhao, T. Spread-out Bragg peak proton FLASH irradiation using a clinical synchrocyclotron: Proof of concept and ion chamber characterization. Med. Phys. 2021, 48, 4472–4484. [Google Scholar] [CrossRef] [PubMed]
- Kranzer, R.; Poppinga, D.; Weidner, J.; Schüller, A.; Hackel, T.; Looe, H.K.; Poppe, B. Ion collection efficiency of ionization chambers in ultra-high dose-per-pulse electron beams. Med. Phys. 2021, 48, 819–830. [Google Scholar] [CrossRef]
- Gómez, F.; Gonzalez-Castaño, D.M.; Fernández, N.G.; Pardo-Montero, J.; Schüller, A.; Gasparini, A.; Vanreusel, V.; Verellen, D.; Felici, G.; Kranzer, R.; et al. Development of an ultra-thin parallel plate ionization chamber for dosimetry in FLASH radiotherapy. Med. Phys. 2022, 49, 4705–4714. [Google Scholar] [CrossRef]
- Cavallone, M.; Jorge, P.G.; Moeckli, R.; Bailat, C.; Flacco, A.; Prezado, Y.; Delorme, R. Determination of the ion collection efficiency of the Razor Nano Chamber for ultra-high dose-rate electron beams. Med. Phys. 2022, 49, 4731–4742. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lopez, A.; Lago-Martin, J.-D.; Vera-Sanchez, J.-A. Small fields measurements with radiochromic films. J. Med. Phys. 2015, 40, 61–67. [Google Scholar] [CrossRef]
- Aldweri, F.M.; Abuzayed, M.H.; Al-Ajaleen, M.S.; Rabaeh, K.A. Characterization of Thymol blue Radiochromic dosimeters for high dose applications. Results Phys. 2018, 8, 1001–1005. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Y.; Zhu, H.; Wang, J.; Xiao, D.; Zhou, Z.; Dai, T.; Zhang, Y.; Feng, G.; Li, J.; et al. First demonstration of the FLASH effect with ultrahigh dose rate high-energy X-rays. Radiother. Oncol. 2022, 166, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Poppinga, D.; Kranzer, R.; Farabolini, W.; Gilardi, A.; Corsini, R.; Wyrwoll, V.; Looe, H.K.; Delfs, B.; Gabrisch, L.; Poppe, B. VHEE beam dosimetry at CERN Linear Electron Accelerator for Research under ultra-high dose rate conditions. Biomed. Phys. Eng. Express 2020, 7, 015012. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.H.; Lee, M.; Lim, H.; Kang, S.K.; Lee, S.J.; Kim, H.C.; Lee, K.; Kim, S.H.; Lee, D.E.; Jang, K.W. Electron beam scattering device for FLASH preclinical studies with 6-MeV LINAC. Nucl. Eng. Technol. 2020, 53, 1289–1296. [Google Scholar] [CrossRef]
- Konradsson, E.; Arendt, M.L.; Jensen, K.B.; Børresen, B.; Hansen, A.E.; Bäck, S.; Kristensen, A.T.; Rosenschöld, P.M.A.; Ceberg, C.; Petersson, K. Establishment and Initial Experience of Clinical FLASH Radiotherapy in Canine Cancer Patients. Front. Oncol. 2021, 11, 658004. [Google Scholar] [CrossRef]
- Han, J.; Mei, Z.; Lu, C.; Qian, J.; Liang, Y.; Sun, X.; Pan, Z.; Kong, D.; Xu, S.; Liu, Z.; et al. Ultra-High Dose Rate FLASH Irradiation Induced Radio-Resistance of Normal Fibroblast Cells Can Be Enhanced by Hypoxia and Mitochondrial Dysfunction Resulting From Loss of Cytochrome C. Front. Cell Dev. Biol. 2021, 9, 672929. [Google Scholar] [CrossRef]
- Villoing, D.; Koumeir, C.; Bongrand, A.; Guertin, A.; Haddad, F.; Métivier, V.; Poirier, F.; Potiron, V.; Servagent, N.; Supiot, S.; et al. Technical note: Proton beam dosimetry at ultra-high dose rates (FLASH): Evaluation of GAFchromic™ (EBT3, EBT-XD) and OrthoChromic (OC-1) film performances. Med. Phys. 2022, 49, 2732–2745. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.; Subiel, A.; Lee, N.; Flynn, S.; Cotterill, J.; Shipley, D.; Romano, F.; Speth, J.; Lee, E.; Zhang, Y.; et al. Absolute dosimetry for FLASH proton pencil beam scanning radiotherapy. Sci. Rep. 2023, 13, 2054. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, M.F. Optical Passive Sensor Calibration for Satellite Remote Sensing and the Legacy of NOAA and NIST Cooperation. J. Res. Natl. Inst. Stand. Technol. 2014, 119, 277–295. [Google Scholar] [CrossRef]
- Desrosiers, M.F.; Publ, J.M.; Cooper, S.L. An absorbed-dose/dose-rate dependence for the alanine-EPR dosimetry system and its implications in high-dose ionizing radiation metrology. J. Res. Natl. Inst. Stand. Technol. 2008, 113, 79–95. [Google Scholar] [CrossRef]
- Gondré, M.; Jorge, P.G.; Vozenin, M.-C.; Bourhis, J.; Bochud, F.; Bailat, C.; Moeckli, R. Optimization of Alanine Measurements for Fast and Accurate Dosimetry in FLASH Radiation Therapy. Radiat. Res. 2020, 194, 573–579. [Google Scholar] [CrossRef]
- Soliman, Y.S.; Pellicioli, P.; Beshir, W.; Abdel-Fattah, A.A.; Fahim, R.A.; Krisch, M.; Bräuer-Krisch, E. A comparative dosimetry study of an alanine dosimeter with a PTW PinPoint chamber at ultra-high dose rates of synchrotron radiation. Phys. Medica 2020, 71, 161–167. [Google Scholar] [CrossRef]
- Bourgouin, A.; Hackel, T.; Marinelli, M.; Kranzer, R.; Schüller, A.; Kapsch, R.-P. Absorbed-dose-to-water measurement using alanine in ultra-high-pulse-dose-rate electron beams. Phys. Med. Biol. 2022, 67, 205011. [Google Scholar] [CrossRef]
- Jorge, P.G.; Jaccard, M.; Petersson, K.; Gondré, M.; Durán, M.T.; Desorgher, L.; Germond, J.-F.; Liger, P.; Vozenin, M.-C.; Bourhis, J.; et al. Dosimetric and preparation procedures for irradiating biological models with pulsed electron beam at ultra-high dose-rate. Radiother. Oncol. 2019, 139, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Jorge, P.G.; Melemenidis, S.; Grilj, V.; Buchillier, T.; Manjappa, R.; Viswanathan, V.; Gondré, M.; Vozenin, M.-C.; Germond, J.-F.; Bochud, F.; et al. Design and validation of a dosimetric comparison scheme tailored for ultra-high dose-rate electron beams to support multicenter FLASH preclinical studies. Radiother. Oncol. 2022, 175, 203–209. [Google Scholar] [CrossRef]
- Rickard, A.G.; Yoshikawa, H.; Palmer, G.M.; Liu, H.Q.; Dewhirst, M.W.; Nolan, M.W.; Zhang, X. Cherenkov emissions for studying tumor changes during radiation therapy: An exploratory study in domesticated dogs with naturally-occurring cancer. PLoS ONE 2020, 15, e0238106. [Google Scholar] [CrossRef]
- Rahman, M.; Ashraf, M.R.; Zhang, R.; Gladstone, D.J.; Cao, X.; Williams, B.B.; Hoopes, P.J.; Pogue, B.W.; Bruza, P. Spatial and temporal dosimetry of individual electron FLASH beam pulses using radioluminescence imaging. Phys. Med. Biol. 2021, 66, 135009. [Google Scholar] [CrossRef] [PubMed]
- Vidalot, J.; Campanella, C.; Dachicourt, J.; Marcandella, C.; Duhamel, O.; Morana, A.; Poujols, D.; Assaillit, G.; Gaillardin, M.; Boukenter, A.; et al. Monitoring of Ultra-High Dose Rate Pulsed X-ray Facilities with Radioluminescent Nitrogen-Doped Optical Fiber. Sensors 2022, 22, 3192. [Google Scholar] [CrossRef]
- Romano, F.; Bailat, C.; Jorge, P.G.; Lerch, M.L.F.; Darafsheh, A. Ultra-high dose rate dosimetry: Challenges and opportunities for FLASH radiation therapy. Med. Phys. 2022, 49, 4912–4932. [Google Scholar] [CrossRef]
- Poirier, Y.; Xu, J.; Mossahebi, S.; Therriault-Proulx, F.; Sawant, A. Technical note: Characterization and practical applications of a novel plastic scintillator for online dosimetry for an ultrahigh dose rate (FLASH). Med. Phys. 2022, 49, 4682–4692. [Google Scholar] [CrossRef]
- Vanreusel, V.; Gasparini, A.; Galante, F.; Mariani, G.; Pacitti, M.; Cociorb, M.; Giammanco, A.; Reniers, B.; Reulens, N.; Shonde, T.B.; et al. Point scintillator dosimetry in ultra-high dose rate electron “FLASH” radiation therapy: A first characterization. Phys. Medica 2022, 103, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Szpala, S.; Huang, V.; Zhao, Y.; Kyle, A.; Minchinton, A.; Karan, T.; Kohli, K. Dosimetry with a clinical linac adapted to FLASH electron beams. J. Appl. Clin. Med. Phys. 2021, 22, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Lee, J.; Kim, J. Recent developments of optically stimulated luminescence materials and techniques for radiation dosimetry and clinical applications. J. Med. Phys. 2008, 33, 85–99. [Google Scholar] [CrossRef]
- Hart, A.; Cecchi, D.; Giguère, C.; Larose, F.; Therriault-Proulx, F.; Esplen, N.; Beaulieu, L.; Bazalova-Carter, M. Lead-doped scintillator dosimeters for detection of ultrahigh dose-rate X-rays. Phys. Med. Biol. 2022, 67, 105007. [Google Scholar] [CrossRef]
- Abouzahr, F.; Cesar, J.P.; Crespo, P.; Gajda, M.; Hu, Z.; Kaye, W.; Klein, K.; Kuo, A.S.; Majewski, S.; Mawlawi, O.; et al. The first PET glimpse of a proton FLASH beam. Phys. Med. Biol. 2023, 68, 125001. [Google Scholar] [CrossRef]
- Jeong, D.-H.; Lee, M.; Lim, H.; Kang, S.-K.; Lee, K.; Lee, S.-J.; Kim, H.; Han, W.-K.; Kang, T.-W.; Jang, K.-W. Optical Filter-Embedded Fiber-Optic Radiation Sensor for Ultra-High Dose Rate Electron Beam Dosimetry. Sensors 2021, 21, 5840. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Kozelka, J.; Hildreth, J.; Schönfeld, A.; Sloop, A.M.; Ashraf, M.R.; Bruza, P.; Gladstone, D.J.; Pogue, B.W.; Simon, W.E.; et al. Characterization of a diode dosimeter for UHDR FLASH radiotherapy. Med. Phys. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- De Kruijff, R.M. FLASH radiotherapy: Ultra-high dose rates to spare healthy tissue. Int. J. Radiat. Biol. 2020, 96, 419–423. [Google Scholar] [CrossRef]
- Diffenderfer, E.S.; Sørensen, B.S.; Mazal, A.; Carlson, D.J. The current status of preclinical proton FLASH radiation and future directions. Med. Phys. 2022, 49, 2039–2054. [Google Scholar] [CrossRef]
- Marcu, L.G.; Bezak, E.; Peukert, D.D.; Wilson, P. Translational Research in FLASH Radiotherapy—From Radiobiological Mechanisms to In Vivo Results. Biomedicines 2021, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Petersson, K. Collaborators FLASH radiotherapy: What, how and why? Res. Outreach 2020, 2020, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.L.; Ruda, H.E. Flash Radiotherapy: Innovative Cancer Treatment. Encyclopedia 2023, 3, 808–823. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddique, S.; Ruda, H.E.; Chow, J.C.L. FLASH Radiotherapy and the Use of Radiation Dosimeters. Cancers 2023, 15, 3883. https://doi.org/10.3390/cancers15153883
Siddique S, Ruda HE, Chow JCL. FLASH Radiotherapy and the Use of Radiation Dosimeters. Cancers. 2023; 15(15):3883. https://doi.org/10.3390/cancers15153883
Chicago/Turabian StyleSiddique, Sarkar, Harry E. Ruda, and James C. L. Chow. 2023. "FLASH Radiotherapy and the Use of Radiation Dosimeters" Cancers 15, no. 15: 3883. https://doi.org/10.3390/cancers15153883






