Clinical Impact of Polygenic Risk Score for Breast Cancer Risk Prediction in 382 Individuals with Hereditary Breast and Ovarian Cancer Syndrome
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
- (1)
- Availability of genotyping data;
- (2)
- Availability of personal medical information (e.g., cancer diagnosis);
- (3)
- Available information on family history (e.g., family members with a cancer diagnosis);
- (4)
- Age range of 18 to 69 years;
- (5)
- The presence of a PV for which a risk calculation using CanRisk was possible in case of a positive carrier status.
- (1)
- Available information on tumour pathology;
- (2)
- Available clinical data such as age of first onset of disease;
- (3)
- Unilaterality in women with BC.
2.2. Pedigree Collection and Initial Risk Assessment
- (1)
- Life status;
- (2)
- Year of birth and current age;
- (3)
- Cancer status.
2.3. Molecular Genetics
2.4. Variant Classification and Panel Sequencing
2.5. Targeted Sequencing for PRS Calculation
2.6. Statistical Testing
3. Results
3.1. Risk Calculations
3.2. Change in Prevention Management
- (1)
- Healthy women under the age of 50 with unremarkable predictive multigene panel diagnostic results;
- (2)
- Healthy women under the age of 50 with unremarkable targeted genetic testing in moderate-risk genes;
- (3)
- Ovarian cancer patients under the age of 50 with unremarkable multigene panel diagnostic results;
- (4)
- Relatives of index patients with a PV detected by the multigene panel diagnostic. We did not include this group in our analyses.
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Quante, A.S.; Strahwald, B.; Fischer, C.; Kiechle, M. Kiechle Individualized risk of breast cancer—How should it be calculated, evaluated and discussed? Gynakologe 2018, 51, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Deutsche Krebsgesellschaft. Deutsche Krebshilfe, and AWMF S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms, Version 4.4, Juni 2021. AWMF Registernummer: 032-045OL. 2021. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/ (accessed on 17 January 2023).
- Marmot, M.; Altman, D.G.; Cameron, D.A.; Dewar; Thompson, S.G.; Wilcox, M. The benefits and harms of breast cancer screening: An independent review. Lancet 2012, 380, 1778–1786. [Google Scholar] [CrossRef]
- Bleyer, A.; Welch, H.G. Effect of Three Decades of Screening Mammography on Breast-Cancer Incidence. N. Engl. J. Med. 2012, 367, 1998–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavaddat, N.; Michailidou, K.; Dennis, J.; Lush, M.; Fachal, L.; Lee, A.; Tyrer, J.P.; Chen, T.-H.; Wang, Q.; Bolla, M.K.; et al. Polygenic Risk Scores for Prediction of Breast Cancer and Breast Cancer Subtypes. Am. J. Hum. Genet. 2019, 104, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Sokolova, A.; Johnstone, K.J.; McCart Reed, A.E.; Simpson, P.T.; Lakhani, S.R. Hereditary breast cancer: Syndromes, tumour pathology and molecular testing. In Histopathology; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Quante, A.S.; Engel, C.; Kiechle, M.; Schmutzler, R.K.; Fischer, C. Changes in risk calculation for the intensified surveillance programme of the German Consortium for Breast and Ovarian Cancer. Gynakologe 2020, 53, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.-A.; Mooij, T.M.; Roos-Blom, M.-J.; Jervis, S.; Van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA—J. Am. Med. Assoc. 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [Green Version]
- Schon, K.; Tischkowitz, M. Clinical implications of germline mutations in breast cancer: TP53. Breast Cancer Res. Treat. 2018, 167, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Antoniou, A.C.; Casadei, S.; Heikkinen, T.; Barrowdale, D.; Pylkäs, K.; Roberts, J.; Lee, A.; Subramanian, D.; De Leeneer, K.; Fostira, F.; et al. Breast-Cancer Risk in Families with Mutations in PALB2. N. Engl. J. Med. 2014, 371, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Easton, D.F.; Pharoah, P.D.; Antoniou, A.C.; Tischkowitz, M.; Tavtigian, S.V.; Nathanson, K.L.; Devilee, P.; Meindl, A.; Couch, F.J.; Southey, M.; et al. Gene-Panel Sequencing and the Prediction of Breast-Cancer Risk. N. Engl. J. Med. 2015, 372, 2243–2257. [Google Scholar] [CrossRef] [Green Version]
- Mavaddat, N.; Pharoah, P.D.P.; Michailidou, K.; Tyrer, J.; Brook, M.N.; Bolla, M.K.; Wang, Q.; Dennis, J.; Dunning, A.M.; Shah, M.; et al. Prediction of breast cancer risk based on profiling with common genetic variants. J. Natl. Cancer Inst. 2015, 107, djv036. [Google Scholar] [CrossRef] [PubMed]
- Vachon, C.M.; Pankratz, V.S.; Scott, C.G.; Haeberle, L.; Ziv, E.; Jensen, M.R.; Brandt, K.R.; Whaley, D.H.; Olson, J.E.; Heusinger, K.; et al. The contributions of breast density and common genetic variation to breast cancer risk. J. Natl. Cancer Inst. 2015, 107, dju397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahcall, O.G. ICOGS collection provides a collaborative model. Nat. Genet. 2013, 45, 343. [Google Scholar] [CrossRef]
- Hall, P.; Easton, D. Breast cancer screening: Time to target women at risk. Br. J. Cancer 2013, 108, 2202–2204. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; McGuffog, L.; Barrowdale, D.; Lee, A.; Soucy, P.; Dennis, J.; Domchek, S.M.; Robson, M.; Spurdle, A.B.; Ramus, S.J.; et al. Evaluation of polygenic risk scores for breast and ovarian cancer risk prediction in BRCA1 and BRCA2 mutation carriers. J. Natl. Cancer Inst. 2017, 109, djw302. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, S.; Hughes, E.; Wagner, S.; Tshiaba, P.; Rosenthal, E.; Roa, B.B.; Kurian, A.W.; Domchek, S.M.; Garber, J.; Lancaster, J.; et al. Association of a Polygenic Risk Score with Breast Cancer among Women Carriers of High- And Moderate-Risk Breast Cancer Genes. JAMA Netw. Open 2020, 3, e208501. [Google Scholar] [CrossRef]
- Rouge-Bugat, M.-E.; Balleyguier, C.; Laurent, N.; Dautreppe, A.; Maillet, L.; Simon, P.; Fournet, P.; Scellier, C.; Menini, T.; Darmon, E.; et al. MyPeBS International randomized study comparing personalised, risk-stratified to standard breast cancer screening in women aged 40–70: Focus on recruitment strategy in France. Presse Médicale Open 2022, 3, 100022. [Google Scholar] [CrossRef]
- Louro, J.; Posso, M.; Boon, M.H.; Román, M.; Domingo, L.; Castells, X.; Sala, M. A systematic review and quality assessment of individualised breast cancer risk prediction models. Br. J. Cancer 2019, 121, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Mavaddat, N.; Wilcox, A.N.; Msc, A.P.C.; Carver, T.; Hartley, S.; de Villiers, C.B.; Izquierdo, A.; Simard, J.; Schmidt, M.K.; et al. BOADICEA: A comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet. Med. 2019, 21, 1708–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, P.P.; Brook, M.N.; Hurson, A.N.; Lee, A.; Mulder, C.V.; Coulson, P.; Schoemaker, M.J.; Jones, M.E.; Swerdlow, A.J.; Chatterjee, N.; et al. Comparative validation of the BOADICEA and Tyrer-Cuzick breast cancer risk models incorporating classical risk factors and polygenic risk in a population-based prospective cohort of women of European ancestry. Breast Cancer Res. 2021, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Eriksson, M.; Czene, K.; Lee, A.; Leslie, G.; Lush, M.; Wang, J.; Dennis, J.; Dorling, L.; Carvalho, S.; et al. Prospective validation of the BOADICEA multifactorial breast cancer risk prediction model in a large prospective cohort study. J. Med. Genet. 2022, 59, 1196–1205. [Google Scholar] [CrossRef]
- Lakeman, I.M.M.; Rodríguez-Girondo, M.; Lee, A.; Ruiter, R.; Stricker, B.H.; Wijnant, S.R.A.; Kavousi, M.; Antoniou, A.C.; Schmidt, M.K.; Uitterlinden, A.G.; et al. Validation of the BOADICEA model and a 313-variant polygenic risk score for breast cancer risk prediction in a Dutch prospective cohort. Genet. Med. 2020, 22, 1803–1811. [Google Scholar] [CrossRef]
- Shieh, Y.; Hu, D.; Ma, L.; Huntsman, S.; Gard, C.C.; Leung, J.W.T.; Tice, J.A.; Vachon, C.M.; Cummings, S.R.; Kerlikowske, K.; et al. Breast cancer risk prediction using a clinical risk model and polygenic risk score. Breast Cancer Res. Treat. 2016, 159, 513–525. [Google Scholar] [CrossRef] [Green Version]
- Dite, G.S.; MacInnis, R.J.; Bickerstaffe, A.; Dowty, J.G.; Allman, R.; Apicella, C.; Milne, R.L.; Tsimiklis, H.; Phillips, K.-A.; Giles, G.G.; et al. Breast cancer risk prediction using clinical models and 77 independent risk-associated SNPs for women aged under 50 years: Australian breast cancer family registry. Cancer Epidemiol. Biomark. Prev. 2016, 25, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Maas, P.; Barrdahl, M.; Joshi, A.D.; Auer, P.L.; Gaudet, M.M.; Milne, R.L.; Schumacher, F.R.; Anderson, W.F.; Check, D.; Chattopadhyay, S.; et al. Breast Cancer Risk From Modifiable and Nonmodifiable Risk Factors Among White Women in the United States. JAMA Oncol. 2016, 2, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rice, M.; Tworoger, S.S.; Rosner, B.A.; Eliassen, A.H.; Tamimi, R.M.; Joshi, A.D.; Lindstrom, S.; Qian, J.; Colditz, G.A.; et al. Addition of a polygenic risk score, mammographic density, and endogenous hormones to existing breast cancer risk prediction models: A nested case–control study. PLoS Med. 2018, 15, e1002644. [Google Scholar] [CrossRef] [Green Version]
- TCarver, T.; Hartley, S.; Lee, A.; Cunningham, A.P.; Archer, S.; de Villiers, C.B.; Roberts, J.; Ruston, R.; Walter, F.M.; Tischkowitz, M.; et al. Canrisk tool—A web interface for the prediction of breast and ovarian cancer risk and the likelihood of carrying genetic pathogenic variants. Cancer Epidemiol. Biomark. Prev. 2021, 30, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.; De Villiers, C.B.; Scheibl, F.; Carver, T.; Hartley, S.; Lee, A.; Cunningham, A.P.; Easton, D.F.; McIntosh, J.G.; Emery, J.; et al. Evaluating clinician acceptability of the prototype CanRisk tool for predicting risk of breast and ovarian cancer: A multi-methods study. PLoS ONE 2020, 15, e0229999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavaddat, N.; Ficorella, L.; Carver, T.; Lee, A.; Cunningham, A.P.; Lush, M.; Dennis, J.; Tischkowitz, M.; Downes, K.; Hu, D.; et al. Incorporating Alternative Polygenic Risk Scores into the BOADICEA Breast Cancer Risk Prediction Model. Cancer Epidemiol. Biomark. Prev. 2023, 32, 422–427. [Google Scholar] [CrossRef]
- CanRisk Knowledge Base: Risk Calculations. Available online: https://canrisk.atlassian.net/wiki/spaces/FAQS/pages/131203073/Why+has+the+program+calculated+mutation+carrier+probabilities+but+not+cancer+risks (accessed on 2 March 2023).
- Girdea, M.; Dumitriu, S.; Fiume, M.; Bowdin, S.; Boycott, K.M.; Chénier, S.; Chitayat, D.; Faghfoury, H.; Meyn, M.S.; Ray, P.N.; et al. PhenoTips: Patient phenotyping software for clinical and research use. Hum. Mutat. 2013, 34, 1057–1065. [Google Scholar] [CrossRef]
- Deutsche Krebsgesellschaft Checkliste zur Erfassung einer familiären Belastung für Brust- und Eierstockkrebs. Available online: https://www.krebsgesellschaft.de/zertdokumente.html (accessed on 22 February 2023).
- Rhiem, K.; Bücker-Nott, H.; Hellmich, M.; Fischer, H.; Ataseven, B.; Dittmer-Grabowski, C.; Latos, K.; Pelzer, V.; Seifert, M.; Schmidt, A.; et al. Benchmarking of a checklist for the identification of familial risk for breast and ovarian cancers in a prospective cohort. Breast J. 2019, 25, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Kast, K.; Rhiem, K.; Wappenschmidt, B.; Hahnen, E.; Hauke, J.; Bluemcke, B.; Zarghooni, V.; Herold, N.; Ditsch, N.; Kiechle, M.; et al. Prevalence of BRCA1/2 germline mutations in 21 401 families with breast and ovarian cancer. J. Med. Genet. 2016, 53, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Harter, P.; Hauke, J.; Heitz, F.; Reuss, A.; Kommoss, S.; Marmé, F.; Heimbach, A.; Prieske, K.; Richters, L.; Burges, A.; et al. Prevalence of deleterious germline variants in risk genes including BRCA1/2 in consecutive ovarian cancer patients (AGO-TR-1). PLoS ONE 2017, 12, e0186043. [Google Scholar] [CrossRef] [PubMed]
- Engel, C.; Rhiem, K.; Hahnen, E.; Loibl, S.; Weber, K.E.; Seiler, S.; Zachariae, S.; Hauke, J.; Wappenschmidt, B.; Waha, A.; et al. Prevalence of pathogenic BRCA1/2 germline mutations among 802 women with unilateral triple-negative breast cancer without family cancer history. BMC Cancer 2018, 18, 265. [Google Scholar] [CrossRef] [Green Version]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [Green Version]
- Landrum, M.J.; Lee, J.M.; Riley, G.R.; Jang, W.; Rubinstein, W.S.; Church, D.M.; Maglott, D.R. ClinVar: Public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014, 42, D980–D985. [Google Scholar] [CrossRef] [Green Version]
- Stenson, P.D.; Ball, E.V.; Mort, M.; Phillips, A.D.; Shiel, J.A.; Thomas, N.S.; Abeysinghe, S.; Krawczak, M.; Cooper, D.N. Human Gene Mutation Database (HGMD®): 2003 Update. Human. Mutat. 2003, 21, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Stenson, P.D.; Ball, E.V.; Mort, M.; Phillips, A.D.; Shiel, J.A.; Thomas, N.S.; Abeysinghe, S.; Krawczak, M.; Cooper, D.N. HerediCaRe: Documentation and IT Solution of a Specialized Registry for Hereditary Breast and Ovarian Cancer. Gesundheitswesen Suppl. 2021, 83, S12–S17. [Google Scholar] [CrossRef]
- Stenson, P.D.; Ball, E.V.; Mort, M.; Phillips, A.D.; Shiel, J.A.; Thomas, N.S.; Abeysinghe, S.; Krawczak, M.; Cooper, D.N. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Kircher, M.; Witten, D.M.; Jain, P.; O’roak, B.J.; Cooper, G.M.; Shendure, J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2019, 46, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Jaganathan, K.; Panagiotopoulou, S.K.; McRae, J.F.; Darbandi, S.F.; Knowles, D.; Li, Y.I.; Kosmicki, J.A.; Arbelaez, J.; Cui, W.; Schwartz, G.B.; et al. Predicting Splicing from Primary Sequence with Deep Learning. Cell 2019, 176, 535–548.e24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babraham Bioinformatics FastQC. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 15 June 2023).
- Bolger, A.M.; Lohse, M.; Usadel, B. Usadel Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Garrison, E.; Marth, G. Haplotype-Based Variant Detection from Short-read Sequencing. 2012. Available online: http://arxiv.org/abs/1207.3907 (accessed on 15 June 2023).
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [Green Version]
- CanRisk Knowledge Base: What Variants are Used in the PRS? Available online: https://canrisk.atlassian.net/wiki/spaces/FAQS/pages/35979266/What+variants+are+used+in+the+PRS (accessed on 22 June 2023).
- Rhiem, K.; Auber, B.; Briest, S.; Dikow, N.; Ditsch, N.; Dragicevic, N.; Grill, S.; Hahnen, E.; Horvath, J.; Jaeger, B.; et al. Consensus Recommendations of the German Consortium for Hereditary Breast and Ovarian Cancer. Breast Care 2022, 17, 199–207. [Google Scholar] [CrossRef]
- Bick, U.; Engel, C.; Krug, B.; Heindel, W.; Fallenberg, E.M.; Rhiem, K.; Maintz, D.; Golatta, M.; Speiser, D.; Rjosk-Dendorfer, D.; et al. High-risk breast cancer surveillance with MRI: 10-year experience from the German consortium for hereditary breast and ovarian cancer. Breast Cancer Res. Treat. 2019, 175, 217–228. [Google Scholar] [CrossRef]
- Waha, A.; Versmold, B.; Kast, K.; Kiechle, M.; Ditsch, N.; Meindl, A.; Niederacher, D.; Hahnen, E.; Arnold, N.; Mundhenke, C.; et al. Konsensusempfehlung des Deutschen Konsortiums Familiärer Brust- und Eierstockkrebs zum Umgang mit Ergebnissen der Multigenanalyse. Tumor Diagn. Und Ther. 2018, 39, 187–193. [Google Scholar] [CrossRef]
- IKNL Richtlijn Borstkanker—Screening Buiten het Bevolkingsonderzoek. 2017. Available online: https://richtlijnendatabase.nl/richtlijn/borstkanker/screening/screening_buiten_het_bob/screening_buiten_het_bevolkingsonderzoek.html (accessed on 31 May 2023).
- National Institute for Health and Care Excellence Familial Breast Cancer: Classification, Care and Managing Breast Cancer and Related Risks in People with a Family History of Breast Cancer Clinical Guideline Your Responsibility. 2013. Available online: www.nice.org.uk/guidance/cg164 (accessed on 2 March 2023).
- NCCN. Clinical Practice Guidelines in Oncology; Breast Cancer Screening and Diagnosis. 2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf (accessed on 31 May 2023).
- Lakeman, I.M.M.; Rodríguez-Girondo, M.D.M.; Lee, A.; Celosse, N.; Braspenning, M.E.; van Engelen, K.; van de Beek, I.; van der Hout, A.H.; García, E.B.G.; Mensenkamp, A.R.; et al. Clinical applicability of the Polygenic Risk Score for breast cancer risk prediction in familial cases Cancer genetics. J. Med. Genet. 2022, 60, 327–336. [Google Scholar] [CrossRef]
- Michailidou, K.; Lindstrom, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemacon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017, 551, 92–94. [Google Scholar] [CrossRef] [Green Version]
- Adedokun, B.; Du, Z.; Gao, G.; Ahearn, T.U.; Lunetta, K.L.; Zirpoli, G.; Figueroa, J.; John, E.M.; Bernstein, L.; Zheng, W.; et al. Cross-ancestry GWAS meta-analysis identifies six breast cancer loci in African and European ancestry women. Nat. Commun. 2021, 12, 4198. [Google Scholar] [CrossRef]
- Zhang, H.; kConFab Investigators; Ahearn, T.U.; Lecarpentier, J.; Barnes, D.; Beesley, J.; Qi, G.; Jiang, X.; O’mara, T.A.; Zhao, N.; et al. Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat. Genet. 2020, 52, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.; Keeney, E.; Taylor, J.C.; Wordsworth, S.; Martin, R.M. Can polygenic risk scores contribute to cost-effective cancer screening? A systematic review. Genet. Med. 2022, 24, 1604–1617. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.-K.; Tan, M.-M.; Mavaddat, N.; Tai, M.-C.; Mariapun, S.; Li, J.; Ho, P.-J.; Dennis, J.; Tyrer, J.P.; Bolla, M.K.; et al. European polygenic risk score for prediction of breast cancer shows similar performance in Asian women. Nat. Commun. 2020, 11, 3833. [Google Scholar] [CrossRef] [PubMed]
- Shieh, Y.; Fejerman, L.; Lott, P.C.; Marker, K.; Sawyer, S.D.; Hu, D.; Huntsman, S.; Torres, J.; Echeverry, M.; E Bohórquez, M.; et al. A Polygenic Risk Score for Breast Cancer in US Latinas and Latin American Women. J. Natl. Cancer Inst. 2020, 112, 590–598. [Google Scholar] [CrossRef] [PubMed]
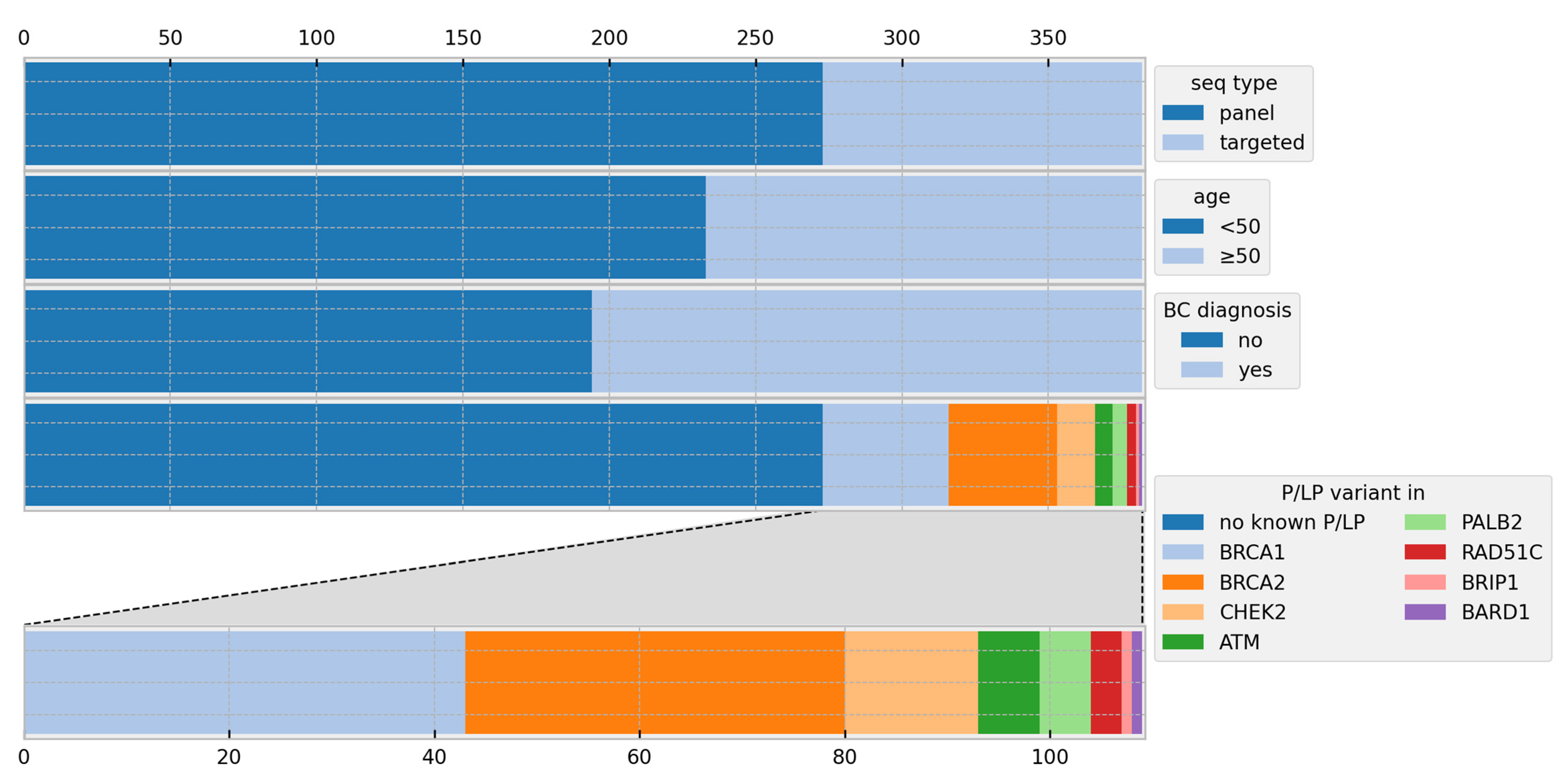
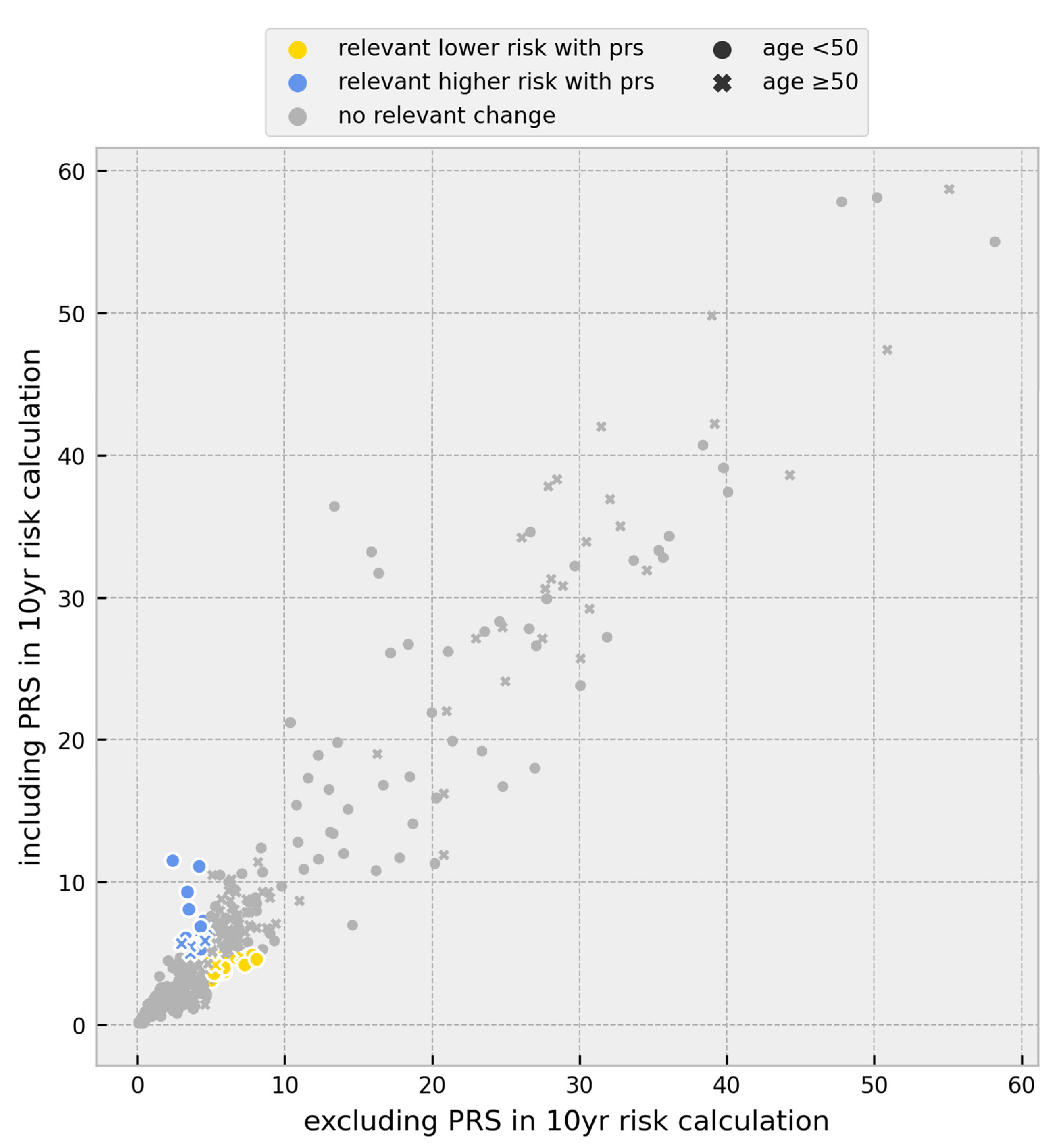
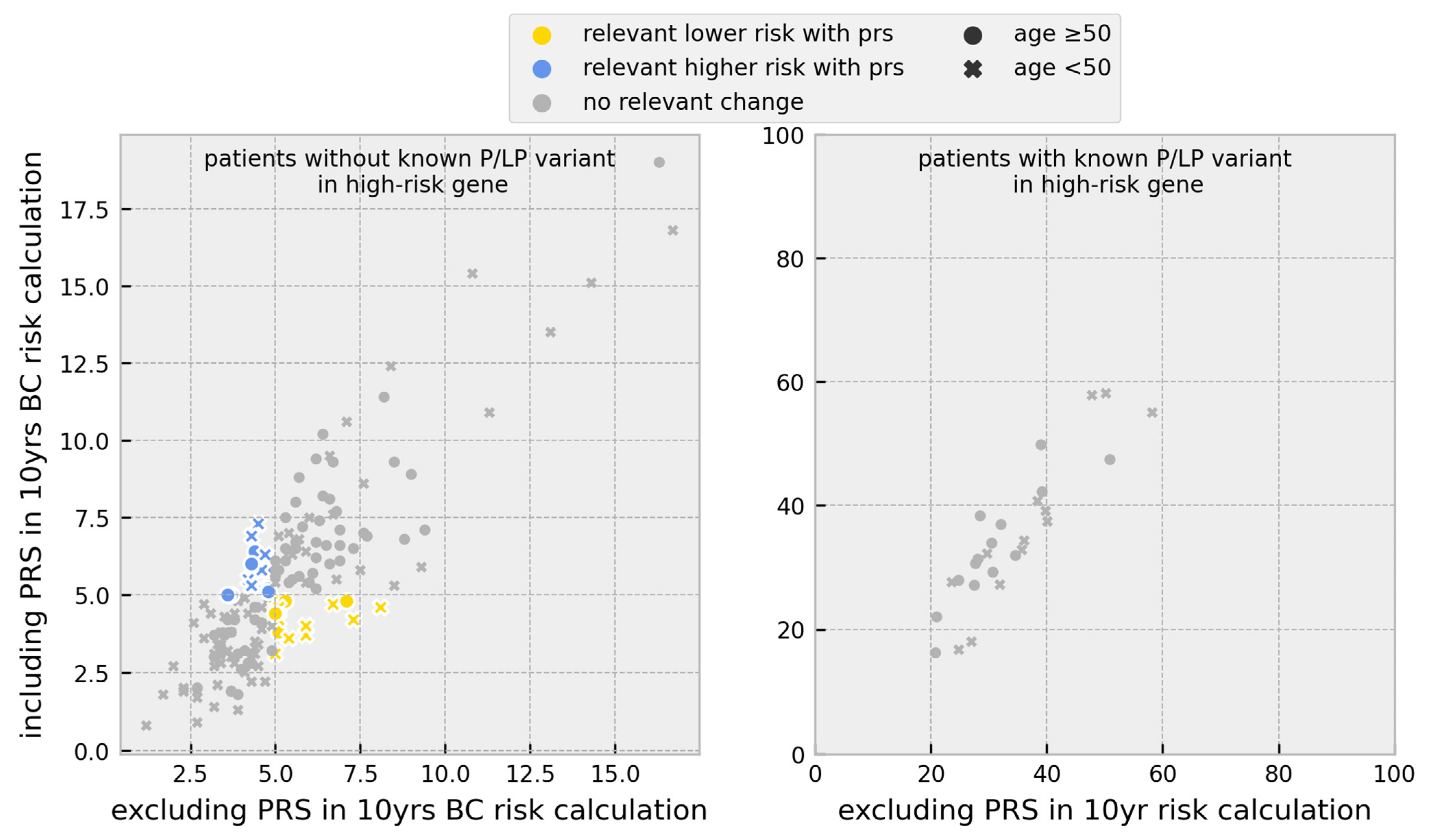
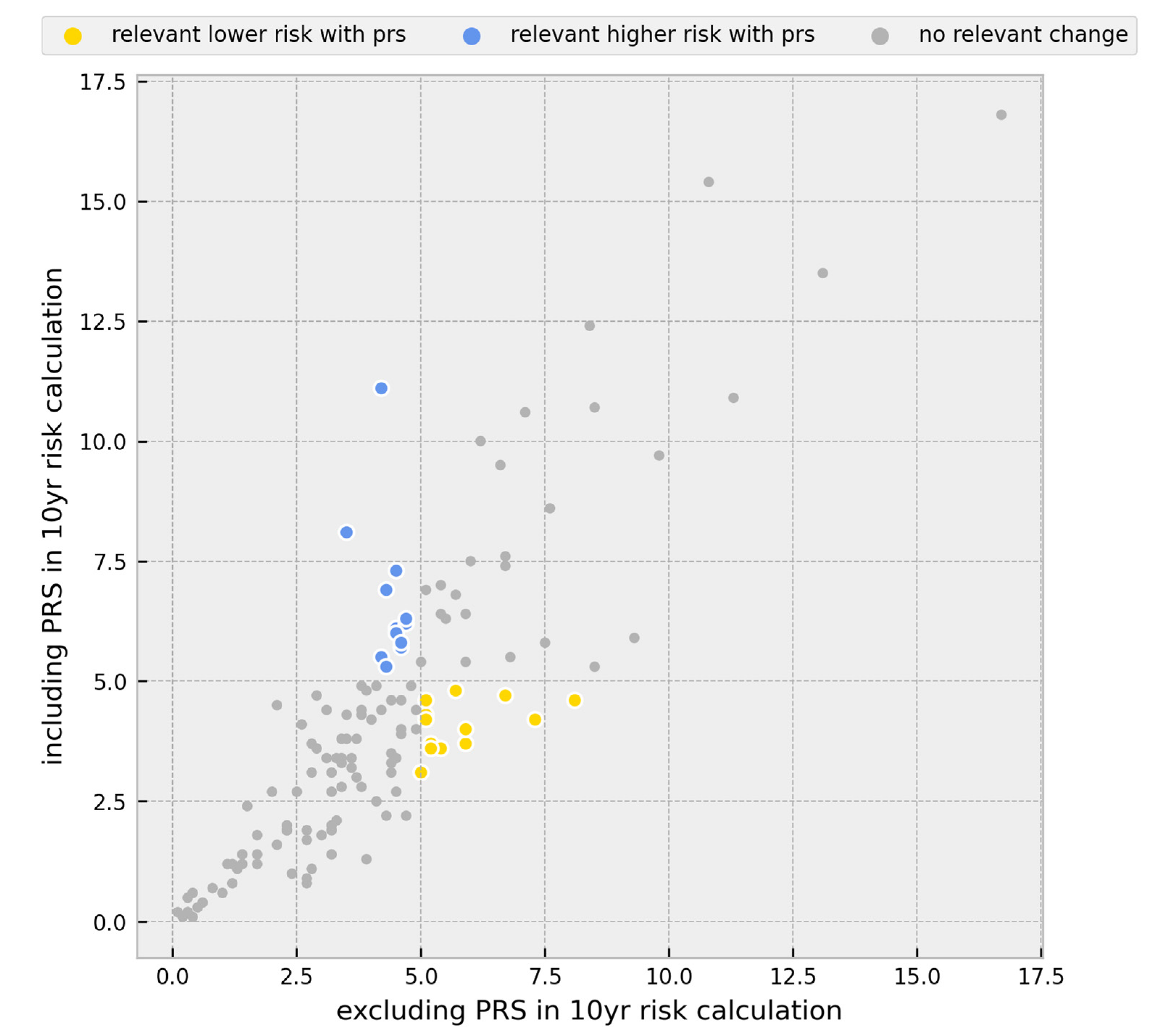
| Individuals | |
|---|---|
| n | 382 |
| Age | |
| Mean | 45 |
| Range | 18–69 |
| Healthy | 164 |
| Affected | 218 |
| Affected by BC | 186 |
| Affected by OC | 30 |
| Affected by BC + OC | 2 |
| Age of onset of disease BC | |
| Mean | 46 |
| Range | 27–67 |
| Age of onset of disease OC | |
| Mean | 54 |
| Range | 22–67 |
| Array | |
| Multigene panel | 273 |
| Performed for healthy probands | 67 |
| Detected PVs | 17 |
| Performed for affected probands | 206 |
| Detected PVs | 39 |
| Sanger + PRS only | 109 |
| Performed for healthy probands | 97 |
| Detected PVs | 44 |
| Performed for affected probands | 12 |
| Detected PVs | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiller, S.; Drukewitz, S.; Lehmann, K.; Hentschel, J.; Strehlow, V. Clinical Impact of Polygenic Risk Score for Breast Cancer Risk Prediction in 382 Individuals with Hereditary Breast and Ovarian Cancer Syndrome. Cancers 2023, 15, 3938. https://doi.org/10.3390/cancers15153938
Stiller S, Drukewitz S, Lehmann K, Hentschel J, Strehlow V. Clinical Impact of Polygenic Risk Score for Breast Cancer Risk Prediction in 382 Individuals with Hereditary Breast and Ovarian Cancer Syndrome. Cancers. 2023; 15(15):3938. https://doi.org/10.3390/cancers15153938
Chicago/Turabian StyleStiller, Sarah, Stephan Drukewitz, Kathleen Lehmann, Julia Hentschel, and Vincent Strehlow. 2023. "Clinical Impact of Polygenic Risk Score for Breast Cancer Risk Prediction in 382 Individuals with Hereditary Breast and Ovarian Cancer Syndrome" Cancers 15, no. 15: 3938. https://doi.org/10.3390/cancers15153938
APA StyleStiller, S., Drukewitz, S., Lehmann, K., Hentschel, J., & Strehlow, V. (2023). Clinical Impact of Polygenic Risk Score for Breast Cancer Risk Prediction in 382 Individuals with Hereditary Breast and Ovarian Cancer Syndrome. Cancers, 15(15), 3938. https://doi.org/10.3390/cancers15153938





