Validated Pretreatment Prediction Models for Response to Neoadjuvant Therapy in Patients with Rectal Cancer: A Systematic Review and Critical Appraisal
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reporting
2.2. Search Strategy
2.3. Eligibility Criteria
2.3.1. General Inclusion and Exclusion Criteria
2.3.2. Development and Validation Studies
2.4. Article Selection and Data Extraction
2.5. Risk of Bias Assessment
2.6. Data Synthesis
3. Results
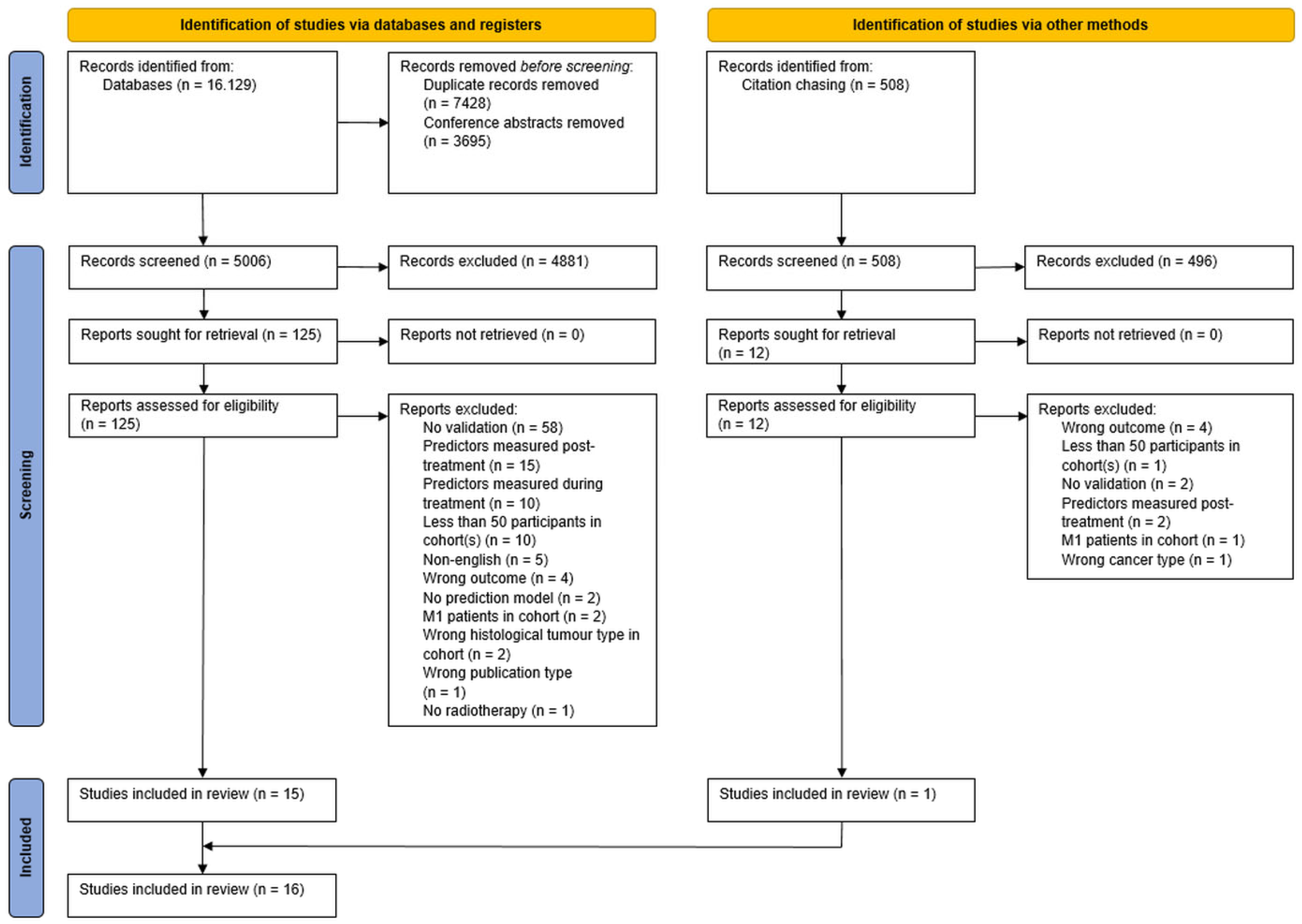
3.1. Search
3.2. Overview of Study Populations
3.3. PROBAST Risk of Bias Assessment
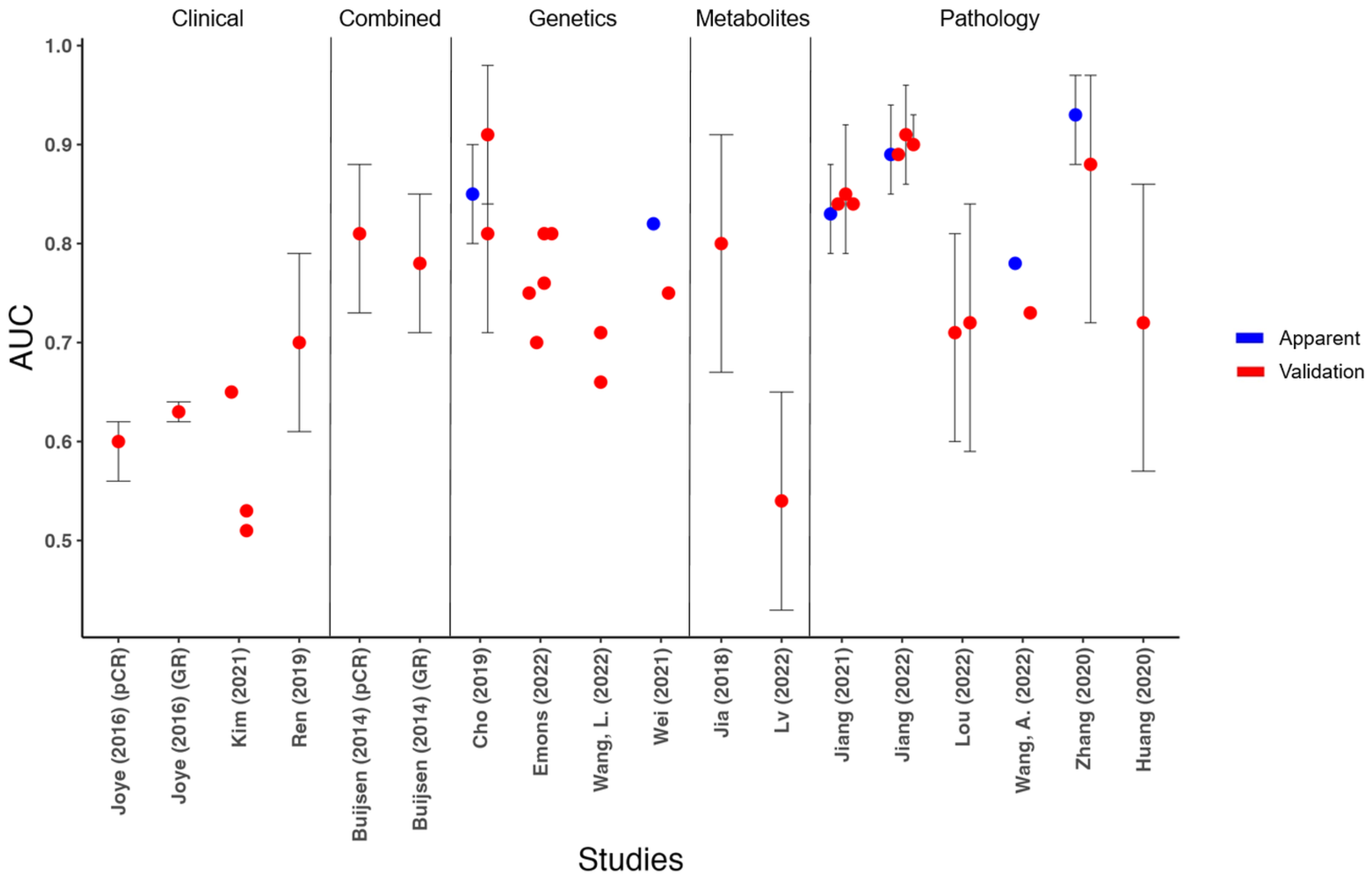
3.4. Model Results
3.4.1. General
3.4.2. Clinical Prediction Models
3.4.3. Combined Clinical, Serological and Imaging Prediction Model
3.4.4. Genetics Prediction Models
3.4.5. Metabolites Prediction Models
3.4.6. Pathology Prediction Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gao, Z.; Gu, J. Surgical treatment of locally recurrent rectal cancer: A narrative review. Ann. Transl. Med. 2021, 9, 1026. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.C.; Soucisse, M.; Michael, M.; Tie, J.; Ngan, S.Y.; Leong, T.; McCormick, J.; Warrier, S.K.; Heriot, A.G. Total Neoadjuvant Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Metaanalysis of Oncological and Operative Outcomes. Ann. Surg. Oncol. 2021, 28, 7476–7486. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, T.; Xiao, L.; Yang, S.; Liu, Q.; Gao, Y.; Chen, G.; Xiao, W. Total Neoadjuvant Therapy (TNT) versus Standard Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis. Oncologist 2021, 26, e1555–e1566. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, S.; Guo, Y.; Luo, Y.; Li, L. Total neoadjuvant therapy versus standard therapy in locally advanced rectal cancer: A systematic review and meta-analysis of 15 trials. PLoS ONE 2022, 17, e0276599. [Google Scholar] [CrossRef]
- Donnelly, M.; Ryan, O.K.; Ryan, É.J.; Creavin, B.; O’Reilly, M.; McDermott, R.; Kennelly, R.; Hanly, A.; Martin, S.T.; Winter, D.C. Total neoadjuvant therapy versus standard neoadjuvant treatment strategies for the management of locally advanced rectal cancer: Network meta-analysis of randomized clinical trials. Br. J. Surg. 2023, znad177. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, M.J.M.; Hilling, D.E.; Bastiaannet, E.; Meershoek-Klein Kranenbarg, E.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.O.; Renehan, A.G.; van de Velde, C.J.H. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018, 391, 2537–2545. [Google Scholar] [CrossRef] [Green Version]
- Birgisson, H.; Påhlman, L.; Gunnarsson, U.; Glimelius, B. Late adverse effects of radiation therapy for rectal cancer—A systematic overview. Acta Oncol. 2007, 46, 504–516. [Google Scholar] [CrossRef]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rödel, C.; Kuo, L.J.; Calvo, F.A.; García-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef]
- Maas, M.; Lambregts, D.M.; Nelemans, P.J.; Heijnen, L.A.; Martens, M.H.; Leijtens, J.W.; Sosef, M.; Hulsewé, K.W.; Hoff, C.; Breukink, S.O.; et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann. Surg. Oncol. 2015, 22, 3873–3880. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Aguilar, J.; Renfro, L.A.; Chow, O.S.; Shi, Q.; Carrero, X.W.; Lynn, P.B.; Thomas, C.R., Jr.; Chan, E.; Cataldo, P.A.; Marcet, J.E.; et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): Results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015, 16, 1537–1546. [Google Scholar] [CrossRef] [Green Version]
- Gerard, J.P.; Myint, A.S.; Barbet, N.; Dejean, C.; Thamphya, B.; Gal, J.; Montagne, L.; Vuong, T. Targeted Radiotherapy Using Contact X-ray Brachytherapy 50 kV. Cancers 2022, 14, 1313. [Google Scholar] [CrossRef] [PubMed]
- NCT05772923: Organ Preservation in Rectal Cancer: Contact X-ray Brachytherapy vs. Extending the Waiting Interval and Local Excision (OPAXX). Available online: ClinicalTrials.gov (accessed on 17 April 2023).
- Dossa, F.; Chesney, T.R.; Acuna, S.A.; Baxter, N.N. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Temmink, S.J.D.; Peeters, K.C.M.J.; Bahadoer, R.R.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Melenhorst, J.; Burger, J.W.A.; Wolthuis, A.; Renehan, A.G.; Figueiredo, N.L.; et al. Watch and wait after neoadjuvant treatment in rectal cancer: Comparison of outcomes in patients with and without a complete response at first reassessment in the International Watch &Wait Database (IWWD). Br. J. Surg. 2023, 110, 676–684. [Google Scholar] [CrossRef]
- Renehan, A.G.; Malcomson, L.; Emsley, R.; Gollins, S.; Maw, A.; Myint, A.S.; Rooney, P.S.; Susnerwala, S.; Blower, A.; Saunders, M.P.; et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): A propensity-score matched cohort analysis. Lancet Oncol. 2016, 17, 174–183. [Google Scholar] [CrossRef]
- Hupkens, B.J.P.; Martens, M.H.; Stoot, J.H.; Berbee, M.; Melenhorst, J.; Beets-Tan, R.G.; Beets, G.L.; Breukink, S.O. Quality of Life in Rectal Cancer Patients After Chemoradiation: Watch-and-Wait Policy Versus Standard Resection—A Matched-Controlled Study. Dis. Colon. Rectum. 2017, 60, 1032–1040. [Google Scholar] [CrossRef]
- Custers, P.A.; van der Sande, M.E.; Grotenhuis, B.A.; Peters, F.P.; van Kuijk, S.M.J.; Beets, G.L.; Breukink, S.O.; Consortium, D.W.-A.-W. Long-term Quality of Life and Functional Outcome of Patients With Rectal Cancer Following a Watch-and-Wait Approach. JAMA Surg. 2023, 158, e230146. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, M.J.M.; van der Sande, M.E.; Toebes, R.E.; Breukink, S.O.; Bröker, M.E.E.; Doornebosch, P.G.; Maliko, N.; Neijenhuis, P.A.; Marinelli, A.; Peters, F.P.; et al. Importance of patient reported and clinical outcomes for patients with locally advanced rectal cancer and their treating physicians. Do clinicians know what patients want? Eur. J. Surg. Oncol. 2020, 46, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Wrenn, S.M.; Cepeda-Benito, A.; Ramos-Valadez, D.I.; Cataldo, P.A. Patient Perceptions and Quality of Life After Colon and Rectal Surgery: What Do Patients Really Want? Dis. Colon. Rectum. 2018, 61, 971–978. [Google Scholar] [CrossRef]
- Aschele, C.; Glynne-Jones, R. Selecting a TNT Schedule in Locally Advanced Rectal Cancer: Can We Predict Who Actually Benefits? Cancers 2023, 15, 2567. [Google Scholar] [CrossRef] [PubMed]
- Couwenberg, A.M.; Varvoglis, D.N.; Grieb, B.C.; Marijnen, C.A.M.; Ciombor, K.K.; Guillem, J.G. New Opportunities for Minimizing Toxicity in Rectal Cancer Management. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389558. [Google Scholar] [CrossRef]
- Fokas, E.; Appelt, A.; Glynne-Jones, R.; Beets, G.; Perez, R.; Garcia-Aguilar, J.; Rullier, E.; Joshua Smith, J.; Marijnen, C.; Peters, F.P.; et al. International consensus recommendations on key outcome measures for organ preservation after (chemo)radiotherapy in patients with rectal cancer. Nat. Rev. Clin. Oncol. 2021, 18, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Bach, S.P. Can we Save the rectum by watchful waiting or TransAnal surgery following (chemo)Radiotherapy versus Total mesorectal excision for early REctal Cancer (STAR-TREC)? Protocol for the international, multicentre, rolling phase II/III partially randomized patient preference trial evaluating long-course concurrent chemoradiotherapy versus short-course radiotherapy organ preservation approaches. Color. Dis. 2022, 24, 639–651. [Google Scholar] [CrossRef]
- van den Berg, K.; Schaap, D.P.; Voogt, E.L.K.; Buffart, T.E.; Verheul, H.M.W.; de Groot, J.W.B.; Verhoef, C.; Melenhorst, J.; Roodhart, J.M.L.; de Wilt, J.H.W.; et al. Neoadjuvant FOLFOXIRI prior to chemoradiotherapy for high-risk (“ugly”) locally advanced rectal cancer: Study protocol of a single-arm, multicentre, open-label, phase II trial (MEND-IT). BMC Cancer 2022, 22, 957. [Google Scholar] [CrossRef] [PubMed]
- Bach, S.P.; Gilbert, A.; Brock, K.; Korsgen, S.; Geh, I.; Hill, J.; Gill, T.; Hainsworth, P.; Tutton, M.G.; Khan, J.; et al. Radical surgery versus organ preservation via short-course radiotherapy followed by transanal endoscopic microsurgery for early-stage rectal cancer (TREC): A randomised, open-label feasibility study. Lancet Gastroenterol. Hepatol. 2021, 6, 92–105. [Google Scholar] [CrossRef]
- Bökkerink, G.M.; de Graaf, E.J.; Punt, C.J.; Nagtegaal, I.D.; Rütten, H.; Nuyttens, J.J.; van Meerten, E.; Doornebosch, P.G.; Tanis, P.J.; Derksen, E.J.; et al. The CARTS study: Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery. BMC Surg. 2011, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar] [CrossRef]
- Gerard, J.-P.; Barbet, N.; Schiappa, R.; Magné, N.; Martel, I.; Mineur, L.; Deberne, M.; Zilli, T.; Dhadda, A.; Myint, A.S. Neoadjuvant chemoradiotherapy with radiation dose escalation with contact x-ray brachytherapy boost or external beam radiotherapy boost for organ preservation in early cT2–cT3 rectal adenocarcinoma (OPERA): A phase 3, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2023, 8, 356–367. [Google Scholar] [CrossRef]
- NCT04246684: Short RT Versus RCT, Followed by Chemo. and Organ Preservation for Interm and High-Risk Rectal Cancer Patients. Available online: ClinicalTrials.gov (accessed on 23 April 2023).
- Ryan, J.E.; Warrier, S.K.; Lynch, A.C.; Ramsay, R.G.; Phillips, W.A.; Heriot, A.G. Predicting pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: A systematic review. Color. Dis. 2016, 18, 234–246. [Google Scholar] [CrossRef]
- Fischer, J.; Eglinton, T.W.; Richards, S.J.; Frizelle, F.A. Predicting pathological response to chemoradiotherapy for rectal cancer: A systematic review. Expert Rev. Anticancer Ther. 2021, 21, 489–500. [Google Scholar] [CrossRef]
- Staal, F.C.R.; van der Reijd, D.J.; Taghavi, M.; Lambregts, D.M.J.; Beets-Tan, R.G.H.; Maas, M. Radiomics for the Prediction of Treatment Outcome and Survival in Patients With Colorectal Cancer: A Systematic Review. Clin. Color. Cancer 2021, 20, 52–71. [Google Scholar] [CrossRef]
- Jia, L.L.; Zheng, Q.Y.; Tian, J.H.; He, D.L.; Zhao, J.X.; Zhao, L.P.; Huang, G. Artificial intelligence with magnetic resonance imaging for prediction of pathological complete response to neoadjuvant chemoradiotherapy in rectal cancer: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 1026216. [Google Scholar] [CrossRef] [PubMed]
- Di Re, A.M.; Sun, Y.; Sundaresan, P.; Hau, E.; Toh, J.W.T.; Gee, H.; Or, M.; Haworth, A. MRI radiomics in the prediction of therapeutic response to neoadjuvant therapy for locoregionally advanced rectal cancer: A systematic review. Expert Rev. Anticancer Ther. 2021, 21, 425–449. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lee, D.; Young, C. Predictors for complete pathological response for stage II and III rectal cancer following neoadjuvant therapy—A systematic review and meta-analysis. Am. J. Surg. 2020, 220, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- The EndNote Team. EndNote, EndNote 20; Clarivate: Philadelphia, PA, USA, 2013. [Google Scholar]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef] [Green Version]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Moons, K.G.; de Groot, J.A.; Bouwmeester, W.; Vergouwe, Y.; Mallett, S.; Altman, D.G.; Reitsma, J.B.; Collins, G.S. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: The CHARMS checklist. PLoS Med. 2014, 11, e1001744. [Google Scholar] [CrossRef] [Green Version]
- Wolff, R.F.; Moons, K.G.M.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S. PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies. Ann. Intern. Med. 2019, 170, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Moons, K.G.M.; Wolff, R.F.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S. PROBAST: A Tool to Assess Risk of Bias and Applicability of Prediction Model Studies: Explanation and Elaboration. Ann. Intern. Med. 2019, 170, W1–W33. [Google Scholar] [CrossRef] [Green Version]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesth. 2022, 75, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Macheret, F.; Gabriel, R.A.; Ohno-Machado, L. A tutorial on calibration measurements and calibration models for clinical prediction models. J. Am. Med. Inform. Assoc. 2020, 27, 621–633. [Google Scholar] [CrossRef]
- Lv, J.; Jia, H.; Mo, M.; Yuan, J.; Wu, Z.; Zhang, S.; Zhe, F.; Gu, B.; Fan, B.; Li, C.; et al. Changes of serum metabolites levels during neoadjuvant chemoradiation and prediction of the pathological response in locally advanced rectal cancer. Metabolomics 2022, 18, 99. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.; Debucquoy, A.; Fieuws, S.; Wolthuis, A.; Sagaert, X.; D’Hoore, A.; Haustermans, K. Can clinical factors be used as a selection tool for an organ-preserving strategy in rectal cancer? Acta Oncol. 2016, 55, 1047–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Sohn, K.A.; Kwak, J.H.; Kim, M.J.; Ryoo, S.B.; Jeong, S.Y.; Park, K.J.; Kang, H.C.; Chie, E.K.; Jung, S.H.; et al. A Novel Scoring System for Response of Preoperative Chemoradiotherapy in Locally Advanced Rectal Cancer Using Early-Treatment Blood Features Derived From Machine Learning. Front. Oncol. 2021, 11, 790894. [Google Scholar] [CrossRef]
- Ren, D.L.; Li, J.; Yu, H.C.; Peng, S.Y.; Lin, W.D.; Wang, X.L.; Ghoorun, R.A.; Luo, Y.X. Nomograms for predicting pathological response to neoadjuvant treatments in patients with rectal cancer. World J. Gastroenterol. 2019, 25, 118–137. [Google Scholar] [CrossRef]
- Buijsen, J.; van Stiphout, R.G.; Menheere, P.P.; Lammering, G.; Lambin, P. Blood biomarkers are helpful in the prediction of response to chemoradiation in rectal cancer: A prospective, hypothesis driven study on patients with locally advanced rectal cancer. Radiother. Oncol. 2014, 111, 237–242. [Google Scholar] [CrossRef]
- Cho, E.; Park, I.J.; Yeom, S.S.; Hong, S.M.; Lee, J.B.; Kim, Y.W.; Kim, M.J.; Lim, H.M.; Lim, S.B.; Yu, C.S.; et al. A Multigene Model for Predicting Tumor Responsiveness After Preoperative Chemoradiotherapy for Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 834–842. [Google Scholar] [CrossRef]
- Emons, G.; Auslander, N.; Jo, P.; Kitz, J.; Azizian, A.; Hu, Y.; Hess, C.F.; Roedel, C.; Sax, U.; Salinas, G.; et al. Gene-expression profiles of pretreatment biopsies predict complete response of rectal cancer patients to preoperative chemoradiotherapy. Br. J. Cancer 2022, 127, 766–775. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Zhang, H.; Hong, L.; Wang, J.; Shao, L.; Chen, G.; Wu, J. Comprehensive analysis of transient receptor potential channels-related signature for prognosis, tumor immune microenvironment, and treatment response of colorectal cancer. Front. Immunol. 2022, 13, 1014834. [Google Scholar] [CrossRef]
- Wei, F.Z.; Mei, S.W.; Wang, Z.J.; Chen, J.N.; Shen, H.Y.; Zhao, F.Q.; Li, J.; Xiao, T.X.; Liu, Q. Development and Validation of a Nomogram and a Comprehensive Prognostic Analysis of an LncRNA-Associated Competitive Endogenous RNA Network Based on Immune-Related Genes for Locally Advanced Rectal Cancer With Neoadjuvant Therapy. Front. Oncol. 2021, 11, 697948. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Shen, X.; Guan, Y.; Xu, M.; Tu, J.; Mo, M.; Xie, L.; Yuan, J.; Zhang, Z.; Cai, S.; et al. Predicting the pathological response to neoadjuvant chemoradiation using untargeted metabolomics in locally advanced rectal cancer. Radiother. Oncol. 2018, 128, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, M.; Tan, J.; Feng, M.; Zheng, J.; Chen, D.; Liu, Z.; Yan, B.; Wang, G.; Xu, S.; et al. A Nomogram Based on a Collagen Feature Support Vector Machine for Predicting the Treatment Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer Patients. Ann. Surg. Oncol. 2021, 28, 6408–6421. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, S.; Wan, J.; Zheng, J.; Dong, X.; Liu, Z.; Wang, G.; Xu, S.; Xiao, W.; Gao, Y.; et al. Association of the collagen signature with pathological complete response in rectal cancer patients. Cancer Sci. 2022, 113, 2409–2424. [Google Scholar] [CrossRef]
- Lou, X.; Zhou, N.; Feng, L.; Li, Z.; Fang, Y.; Fan, X.; Ling, Y.; Liu, H.; Zou, X.; Wang, J.; et al. Deep Learning Model for Predicting the Pathological Complete Response to Neoadjuvant Chemoradiotherapy of Locally Advanced Rectal Cancer. Front. Oncol. 2022, 12, 807264. [Google Scholar] [CrossRef]
- Wang, A.; Ding, R.; Zhang, J.; Zhang, B.; Huang, X.; Zhou, H. Machine Learning of Histomorphological Features Predict Response to Neoadjuvant Therapy in Locally Advanced Rectal Cancer. J. Gastrointest. Surg. 2022, 27, 162–165. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, S.; Li, Z.; Liang, C.; Zhao, K.; Huang, Y.; Gao, Y.; Qu, J.; Li, Z.; Liu, Z. Predicting treatment response to neoadjuvant chemoradiotherapy in local advanced rectal cancer by biopsy digital pathology image features. Clin. Transl. Med. 2020, 10, e110. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.; Xiao, Y.; Peng, C.; Liu, T.; Lin, Z.; Yang, Q.; Zhang, T.; Liu, J.; Ma, H. 53BP1 expression and immunoscore are associated with the efficacy of neoadjuvant chemoradiotherapy for rectal cancer. Strahlenther. Onkol. 2020, 196, 465–473. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Mandard, A.M.; Dalibard, F.; Mandard, J.C.; Marnay, J.; Henry-Amar, M.; Petiot, J.F.; Roussel, A.; Jacob, J.H.; Segol, P.; Samama, G.; et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994, 73, 2680–2686. [Google Scholar] [CrossRef]
- Song, C.; Chung, J.H.; Kang, S.B.; Kim, D.W.; Oh, H.K.; Lee, H.S.; Kim, J.W.; Lee, K.W.; Kim, J.H.; Kim, J.S. Impact of Tumor Regression Grade as a Major Prognostic Factor in Locally Advanced Rectal Cancer after Neoadjuvant Chemoradiotherapy: A Proposal for a Modified Staging System. Cancers 2018, 10, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dworak, O.; Keilholz, L.; Hoffmann, A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int. J. Colorectal. Dis. 1997, 12, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Quirke, P.; Durdey, P.; Dixon, M.F.; Williams, N.S. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986, 2, 996–999. [Google Scholar] [CrossRef]
- Chang, H.J.; Park, C.K.; Kim, W.H.; Kim, Y.B.; Kim, Y.W.; Kim, H.G.; Bae, H.I.; Song, K.S.; Chang, M.S.; Chang, H.K.; et al. A Standardized Pathology Report for Colorectal Cancer. Korean J. Pathol. 2006, 40, 193–203. [Google Scholar]
- Pagès, F.; Kirilovsky, A.; Mlecnik, B.; Asslaber, M.; Tosolini, M.; Bindea, G.; Lagorce, C.; Wind, P.; Marliot, F.; Bruneval, P.; et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J. Clin. Oncol. 2009, 27, 5944–5951. [Google Scholar] [CrossRef] [PubMed]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Yashima-Abo, A.; Otsuka, K.; Nishizuka, S.S. Editorial Comment to “A nomogram Based on a Collagen Feature Support Vector Machine for Predicting the Treatment Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer Patients”. Ann. Surg. Oncol. 2021, 28, 5818–5819. [Google Scholar] [CrossRef]
- Chen, D.; Chen, G.; Jiang, W.; Fu, M.; Liu, W.; Sui, J.; Xu, S.; Liu, Z.; Zheng, X.; Chi, L.; et al. Association of the Collagen Signature in the Tumor Microenvironment With Lymph Node Metastasis in Early Gastric Cancer. JAMA Surg. 2019, 154, e185249. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, H.; Chen, W.; Zhao, Y.; Yan, B.; Chen, D.; Dong, X.; Cheng, J.; Lin, Z.; Zhuo, S.; et al. Association of collagen deep learning classifier with prognosis and chemotherapy benefits in stage II-III colon cancer. Bioeng. Transl. Med. 2023, 8, e10526. [Google Scholar] [CrossRef]
- Aeffner, F.; Zarella, M.D.; Buchbinder, N.; Bui, M.M.; Goodman, M.R.; Hartman, D.J.; Lujan, G.M.; Molani, M.A.; Parwani, A.V.; Lillard, K.; et al. Introduction to Digital Image Analysis in Whole-slide Imaging: A White Paper from the Digital Pathology Association. J. Pathol. Inform. 2019, 10, 9. [Google Scholar] [CrossRef]
- Davri, A.; Birbas, E.; Kanavos, T.; Ntritsos, G.; Giannakeas, N.; Tzallas, A.T.; Batistatou, A. Deep Learning on Histopathological Images for Colorectal Cancer Diagnosis: A Systematic Review. Diagnostics 2022, 12, 837. [Google Scholar] [CrossRef] [PubMed]
- Gold, A.; Choueiry, F.; Jin, N.; Mo, X.; Zhu, J. The Application of Metabolomics in Recent Colorectal Cancer Studies: A State-of-the-Art Review. Cancers 2022, 14, 725. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jia, H.; Gao, Y.; Zhang, H.; Fan, J.; Zhang, L.; Ren, F.; Yin, Y.; Cai, Y.; Zhu, J.; et al. Serum metabolic traits reveal therapeutic toxicities and responses of neoadjuvant chemoradiotherapy in patients with rectal cancer. Nat. Commun. 2022, 13, 7802. [Google Scholar] [CrossRef]
- El Sissy, C.; Kirilovsky, A.; Van den Eynde, M.; Muşină, A.M.; Anitei, M.G.; Romero, A.; Marliot, F.; Junca, A.; Doyen, J.; Mlecnik, B.; et al. A Diagnostic Biopsy-Adapted Immunoscore Predicts Response to Neoadjuvant Treatment and Selects Patients with Rectal Cancer Eligible for a Watch-and-Wait Strategy. Clin. Cancer Res. 2020, 26, 5198–5207. [Google Scholar] [CrossRef]
- Momma, T.; Okayama, H.; Kanke, Y.; Fukai, S.; Onozawa, H.; Fujita, S.; Sakamoto, W.; Saito, M.; Ohki, S.; Kono, K. Validation of Gene Expression-Based Predictive Biomarkers for Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Cancers 2021, 13, 4642. [Google Scholar] [CrossRef] [PubMed]
- Brettingham-Moore, K.H.; Duong, C.P.; Greenawalt, D.M.; Heriot, A.G.; Ellul, J.; Dow, C.A.; Murray, W.K.; Hicks, R.J.; Tjandra, J.; Chao, M.; et al. Pretreatment transcriptional profiling for predicting response to neoadjuvant chemoradiotherapy in rectal adenocarcinoma. Clin. Cancer Res. 2011, 17, 3039–3047. [Google Scholar] [CrossRef] [Green Version]
- Gim, J.; Cho, Y.B.; Hong, H.K.; Kim, H.C.; Yun, S.H.; Wu, H.G.; Jeong, S.Y.; Joung, J.G.; Park, T.; Park, W.Y.; et al. Predicting multi-class responses to preoperative chemoradiotherapy in rectal cancer patients. Radiat. Oncol. 2016, 11, 50. [Google Scholar] [CrossRef] [Green Version]
- Izzotti, A.; Ceccaroli, C.; Geretto, M.; Ruggieri, F.G.; Schenone, S.; Di Maria, E. Predicting Response to Neoadjuvant Therapy in Colorectal Cancer Patients the Role of Messenger-and Micro-RNA Profiling. Cancers 2020, 12, 1652. [Google Scholar] [CrossRef]
- de Jong, Y.; Ramspek, C.L.; Zoccali, C.; Jager, K.J.; Dekker, F.W.; van Diepen, M. Appraising prediction research: A guide and meta-review on bias and applicability assessment using the Prediction model Risk Of Bias ASsessment Tool (PROBAST). Nephrology 2021, 26, 939–947. [Google Scholar] [CrossRef]
- Venema, E.; Wessler, B.S.; Paulus, J.K.; Salah, R.; Raman, G.; Leung, L.Y.; Koethe, B.C.; Nelson, J.; Park, J.G.; van Klaveren, D.; et al. Large-scale validation of the prediction model risk of bias assessment Tool (PROBAST) using a short form: High risk of bias models show poorer discrimination. J. Clin. Epidemiol. 2021, 138, 32–39. [Google Scholar] [CrossRef]
- Riley, R.D.; Ensor, J.; Snell, K.I.E.; Harrell, F.E., Jr.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.; van Smeden, M. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef] [Green Version]
- Attia, A.M.; Farrag, A.; Attia, N.M.; Khalaf, L.M.; Hassan, H.M.; Ameen, M.G.; Aboeleuon, E.; El-Raheem, S.S.A.; Mahran, A.; Hefni, A.M. Signet ring cell component predicts the response to neoadjuvant chemoradiotherapy in rectal cancer. Long interim results of a single institution experience. Am. J. Cancer Res. 2022, 12, 1156–1168. [Google Scholar]
- McCawley, N.; Clancy, C.; O’Neill, B.D.; Deasy, J.; McNamara, D.A.; Burke, J.P. Mucinous Rectal Adenocarcinoma Is Associated with a Poor Response to Neoadjuvant Chemoradiotherapy: A Systematic Review and Meta-analysis. Dis. Colon. Rectum. 2016, 59, 1200–1208. [Google Scholar] [CrossRef]
- Lania, A.; Ferraù, F.; Rubino, M.; Modica, R.; Colao, A.; Faggiano, A. Neoadjuvant Therapy for Neuroendocrine Neoplasms: Recent Progresses and Future Approaches. Front. Endocrinol. 2021, 12, 651438. [Google Scholar] [CrossRef]
- Conte, B.; George, B.; Overman, M.; Estrella, J.; Jiang, Z.Q.; Mehrvarz Sarshekeh, A.; Ferrarotto, R.; Hoff, P.M.; Rashid, A.; Yao, J.C.; et al. High-Grade Neuroendocrine Colorectal Carcinomas: A Retrospective Study of 100 Patients. Clin. Colorectal. Cancer 2016, 15, e1–e7. [Google Scholar] [CrossRef] [Green Version]
- Kokaine, L.; Gardovskis, A.; Gardovskis, J. Evaluation and Predictive Factors of Complete Response in Rectal Cancer after Neoadjuvant Chemoradiation Therapy. Medicina 2021, 57, 1044. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wallace, M.; Livingstone, J.I.; Meyrick-Thomas, J. Complete clinical response after preoperative chemoradiation in rectal cancer: Is a “wait and see” policy justified? Dis. Colon. Rectum. 2008, 51, 10–19; discussion 19–20. [Google Scholar] [CrossRef]

| Study Type | TRIPOD Type | Development | Type of Validation | Explanation |
|---|---|---|---|---|
| Development studies | ||||
| Development only | 1a | Yes | No | Development and evaluation using the apparent performance. |
| Development and validation studies | ||||
| Development and validation using resampling | 1b | Yes | Internal | Development and evaluation using resampling methods (e.g., cross-validation or bootstrapping). The term “internal validation” commonly refers to resampling methods. |
| Development and validation using random split | 2a | Yes | Internal | Random split of data into a development and validation cohort. |
| Development and validation using nonrandom split | 2b | Yes | Intermediary | Nonrandom split (e.g., location or time) of data into a development and validation cohort. Type 2b studies are considered as intermediary between internal and external validation. |
| Development and validation using separate data | 3 | Yes | External | Development using one cohort and validation in a separate cohort (e.g., from a different study). |
| Validation studies | ||||
| Validation only | 4 | No | External | Validation of an existing (published) prediction model in a separate cohort. |
| Study, [Ref.] | Model Development (Yes/No) | Validation Cohort(s) (No. of Cohorts and TRIPOD Category) | Cohort Design | n | n with Outcome (%) | Stage | NAT | Surgery |
|---|---|---|---|---|---|---|---|---|
| Clinical | ||||||||
| Joye (2016), [47] | Yes | 1. 1b | R/S | 620 | pCR: 120 (19) GR: 170 (27) | cT1-4N0-2 | CRT | TME |
| Kim (2021), [48] | Yes | 1. 1b | R/S | 190 | 83 (43) | LARC ¥¥ | CRT | TME |
| 2. 2a | R/S | 41 | 19 (47) | LARC | CRT | TME | ||
| 3. 2a | R/S | 41 | 19 (47) | LARC | CRT | TME | ||
| Ren (2019), [49] | Yes | 1. 1b | R/S | 126 | 45 (36) | cT2-4N0-+ | CRT | TME |
| Clinical, serological and radiological | ||||||||
| Buijsen (2014), [50] | Yes | 1. 1b | P/S | 276 | pCR: 57 (21) GR: 130 (47) | cT3-4N0-2 | CRT | TME |
| Genetics | ||||||||
| Cho (2019), [51] | Yes | 1. 1b | R/S | 184 | 102 (55) | cT3-4N0-+ | CRT | TME (178) + LE (6) |
| Emons (2022), [52] | Yes | 1. 1b | P/M | 64 | 32 (50) | II-III | CRT | TME |
| 2. 1b/2b § | P/M | 161 | 32 (20) | II-III | CRT | TME | ||
| 3. 2b | P/M | 14 | 4 (28) | II-III | CRT | TME | ||
| 4. 3 | R/S | 38 | 8 (21) | cT3-4N0-2 | CRT | TME | ||
| 5. 3 | P/M | 24 | 4 (16) | II-III | CRT | TME | ||
| Wang, L. (2022), [53] | No | 1. 4 | R/S | 80 | 35 (44) | cT3-4N0-2 | CRT | TME |
| 2. 4 | R/S | 85 | 45 (53) | II-III | Unknown | Unknown | ||
| Wei (2021), [54] | Yes | 1. 1b | R/S | 59 | 27 (46) | cT3-4N0-2 | CRT | TME (52) + LE (7) |
| Metabolites | ||||||||
| Jia (2018), [55] | Yes | 1. 1b | P/S | 105 | 56 (53) | cT3-4N0-2 | CRT + Con. | TME |
| Lv (2022), [46] | Yes | 1. 1b | P/S | 106 | 56 (53) | cT2-4N0-2 | CRT + Con. | TME |
| Pathology | ||||||||
| Jiang (2021), [56] | Yes | 1. 1b | R/M | 299 | 163 (55) | cT3-4N0-+ | CRT | TME |
| 2. 2a | R/M | 129 | 70 (54) | cT3-4N0-+ | CRT | TME | ||
| 3. Cohort 1 + 2 ‡ | R/M | 428 | 233 (54) | cT3-4N0-+ | CRT | TME | ||
| Jiang (2022), [57] | Yes | 1. 1b | R/M | 353 | 76 (22) | cT3-4N0-+ | CRT | TME |
| 2. 3 | R/S | 163 | 37 (23) | cT3-4N0-+ | CRT | TME | ||
| 3. Cohort 1 + 2 ‡ | R/M | 516 | 113 (22) | cT3-4N0-+ | CRT | TME | ||
| Lou (2022), [58] | Yes | 1. – | R/M | 666 | 171 (26) | cT3-4N0-2 | CRT | TME |
| 2. 2a | R/M | 117 | 30 (26) | cT3-4N0-2 | CRT | TME | ||
| 3. 3 | R/M | 102 | 24 (24) | cT3-4N0-2 | CRT | TME | ||
| Wang, A. (2022), [59] | Yes | 1. - | R/S | 55 | 21 (38) | cT2-4N0-2 | Ind. + CRT | TME |
| 2. 2a | R/S | 14 | 5 (37) | cT2-4N0-2 | Ind. + CRT | TME | ||
| Zhang (2020), [60] | Yes | 1. - | R/S | 120 | 59 (49) | cT2-4N0-2 | CRT + Con. | Unknown |
| 2. 2a | R/S | 31 | 20 (65) | cT2-4N0-2 | CRT + Con. | Unknown | ||
| Huang (2020), [61] | No | 1. 4 | R/S | 55 | 34 (62) | cT3-4N0-+ | CRT | TME |
| Study, [Ref.] | Model with Final Predictors (No. of Predictors) | Method | Outcome | Apparent AUC | AUC of Validation Cohort(s) (No. of Cohorts and TRIPOD Category) |
|---|---|---|---|---|---|
| Clinical | |||||
| Joye (2016), [47] | - Model 1 (pCR): age, ASA score, CEA, cN stage, gender and Hb. (6) - Model 2 (GR): CEA, cT stage/MRF, cN stage and Hb. (4) | LR | pCR GR | pCR. Unknown GR. Unknown | 1. 1b. (pCR) 0.60 (range 0.56–0.62) 1. 1b. (GR) 0.63 (range 0.62–0.64) |
| Kim (2021), [48] | - Age, alcohol, ASA, BMI, diabetes, distance from anal verge, gender, hypertension, smoking and tumor grade. (10) | RR | GR | Unknown | 1. 1b. 0.65 (mean ± std: 0.02) 2. 2a. 0.53 (mean ± std 0.08) 3. 2a. 0.51 (mean ± std 0.08) |
| Ren (2019), [49] | - MRF and tumor length. (2) | LR | pCR | Unknown | 1. 1b. 0.70 (95% CI 0.61–0.79) |
| Clinical, serological and imaging | |||||
| Buijsen (2014), [50] | - CEA, cT stage, cN stage and tumor length, IL-6, IL-8, osteopontin, SUVmax. (8) | LR | pCR GR | pCR. Unknown GR. Unknown | 1. 1b. (pCR) 0.81 (95% CI 0.73–0.88) 1. 1b. (GR) 0.78 (95% CI 0.71–0.85) |
| Genetics | |||||
| Cho (2019), [51] | Radio-response prediction index - mRNAs. (8) | LR | GR | 0.85 (95% CI 0.80–0.90) | 1. 1b. 0.81 (95% CI 0.71–0.91)–0.91 (95% CI: 0.84–0.98) ¥¥ |
| Emons (2022), [52] | 21-transcript signature - Transcripts. (21) | SVM | pCR | Unknown | 1. 1b. 0.75 2. 1b/2b §. 0.81 3. 2b. 0.70 4. 3. 0.76 5. 3. 0.81 |
| Wang, L. (2022), [53] | Transient receptor potential channels (TRPC) score - TRPC-related genes. (8) | R package “glmnet” | GR | NA | 1. 4. 0.66 2. 4. 0.71 |
| Wei (2021), [54] | Response-Related Prediction Nomogram - IRDEGs, age and gender. (6) | R package “glmnet” | GR | 0.82 | 1. 1b. 0.75 |
| Metabolites | |||||
| Jia (2018), [55] | Metabolite panel - Metabolites. (15) | PLS | GR | Unknown | 1. 1b. 0.80 (95% CI 0.67– 0.91) |
| Lv (2022), [46] | Metabolite panel - Metabolites. (8) | RM-ANOVA, ML-PLS-DA and LR | GR | Unknown | 1. 1b. 0.54 (95% CI 0.43–0.65) |
| Pathology | |||||
| Jiang (2021), [56] | Collagen feature nomogram - CFs-SVM classifier, CEA, cT stage, and tumor differentiation. (6) | SVM and LR | GR | 0.83 (95% CI 0.79–0.88) | 1. 1b. 0.84 2. 2a. 0.85 (95% CI 0.79–0.92) 3. Cohort 1 + 2 ‡. 0.84 |
| Jiang (2022), [57] | Collagen feature nomogram - Collagen signature, CEA, cT stage, tumor differentiation, and tumor dimension (length). (8) | LR | pCR | 0.89 (95% CI 0.85–0.94) | 1. 1b. 0.89 2. 3. 0.91 (95% CI 0.86–0.96) 3. Cohort 1 + 2 ‡. 0.90 (95% CI 0.90–0.93) |
| Lou (2022), [58] | Deep pathological complete response model - WSIs. (NA) | MIL | pCR | Unknown | 2. 2a. 0.71 (95% CI 0.60–0.81) 3. 3. 0.72 (95% CI 0.59–0.84) |
| Wang, A. (2022), [59] | Digital-pathology-based deep learning model - WSIs. (NA) | CNN and GNN | GR | 0.78 | 2. 2a. 0.73 |
| Zhang (2020), [60] | Pathology signature - WSIs. (17) | SVM and LR | GR | 0.93 (95% CI 0.88–0.97) | 2. 2a. 0.88 (95% CI 0.72–0.97) |
| Huang (2020), [61] | Immunoscore - TILs. (4) | LR | GR | NA | 1. 4. 0.72 (95% CI 0.57–0.86) |
| Study | Type | ROB | Applicability | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participants | Predictors | Outcome | Analysis | Participants | Predictors | Outcome | ROB | Applicability | ||
| Clinical | ||||||||||
| Joye (2016), [47] | D | Low | Low | Low | High | Low | Low | Low | High | Low |
| Kim (2021), [48] | D | Low | Low | Low | High | Low | Unclear | Low | High | Unclear |
| Ren (2019), [49] | D | Low | Low | Low | High | Low | Low | Low | High | Low |
| Clinical, serological and radiological | ||||||||||
| Buijsen (2014), [50] | D | Low | Low | Low | High | Low | Low | Low | High | Low |
| Genetics | ||||||||||
| Cho (2019), [51] | D | Low | Low | Low | High | Low | Low | Low | High | Low |
| Emons (2022), [52] | D + V | Low | Low | Low | High | Low | Low | Low | High | Low |
| Wang, L. (2022), [53] | V | Low | High | Unclear | High | High | Low | Unclear | High | High |
| Wei (2021), [54] | D | Low | Low | Low | High | Low | Low | Low | High | Low |
| Metabolites | ||||||||||
| Jia (2018), [55] | D | Low | Unclear | Low | High | Low | Low | Low | High | Low |
| Lv (2022), [46] | D | Low | Unclear | Low | High | Low | Low | Low | High | Low |
| Pathology | ||||||||||
| Jiang (2021), [56] | D | Low | Low | Low | High | Low | Low | Low | High | Low |
| Jiang (2022), [57] | D + V | Low | Low | Low | High | Low | Low | Low | High | Low |
| Lou (2022), [58] | D + V | Low | Low | Low | High | Low | Low | Low | High | Low |
| Wang, A. (2022), [59] | D | Low | High | Unclear | High | Low | Low | Unclear | High | Unclear |
| Zhang (2020), [60] | D | Low | High | Low | High | Low | Low | Low | High | Low |
| Huang (2020), [61] | V | Low | Low | Low | High | Low | Low | Low | High | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, M.D.; Geubels, B.M.; Grotenhuis, B.A.; Marijnen, C.A.M.; Peters, F.P.; van der Mierden, S.; Maas, M.; Couwenberg, A.M. Validated Pretreatment Prediction Models for Response to Neoadjuvant Therapy in Patients with Rectal Cancer: A Systematic Review and Critical Appraisal. Cancers 2023, 15, 3945. https://doi.org/10.3390/cancers15153945
Tanaka MD, Geubels BM, Grotenhuis BA, Marijnen CAM, Peters FP, van der Mierden S, Maas M, Couwenberg AM. Validated Pretreatment Prediction Models for Response to Neoadjuvant Therapy in Patients with Rectal Cancer: A Systematic Review and Critical Appraisal. Cancers. 2023; 15(15):3945. https://doi.org/10.3390/cancers15153945
Chicago/Turabian StyleTanaka, Max D., Barbara M. Geubels, Brechtje A. Grotenhuis, Corrie A. M. Marijnen, Femke P. Peters, Stevie van der Mierden, Monique Maas, and Alice M. Couwenberg. 2023. "Validated Pretreatment Prediction Models for Response to Neoadjuvant Therapy in Patients with Rectal Cancer: A Systematic Review and Critical Appraisal" Cancers 15, no. 15: 3945. https://doi.org/10.3390/cancers15153945
APA StyleTanaka, M. D., Geubels, B. M., Grotenhuis, B. A., Marijnen, C. A. M., Peters, F. P., van der Mierden, S., Maas, M., & Couwenberg, A. M. (2023). Validated Pretreatment Prediction Models for Response to Neoadjuvant Therapy in Patients with Rectal Cancer: A Systematic Review and Critical Appraisal. Cancers, 15(15), 3945. https://doi.org/10.3390/cancers15153945








