Influence of the Hypersensitivity to Low Dose Phenomenon on the Tumor Response to Hypofractionated Stereotactic Body Radiation Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Irradiation
2.3. Cell Survival
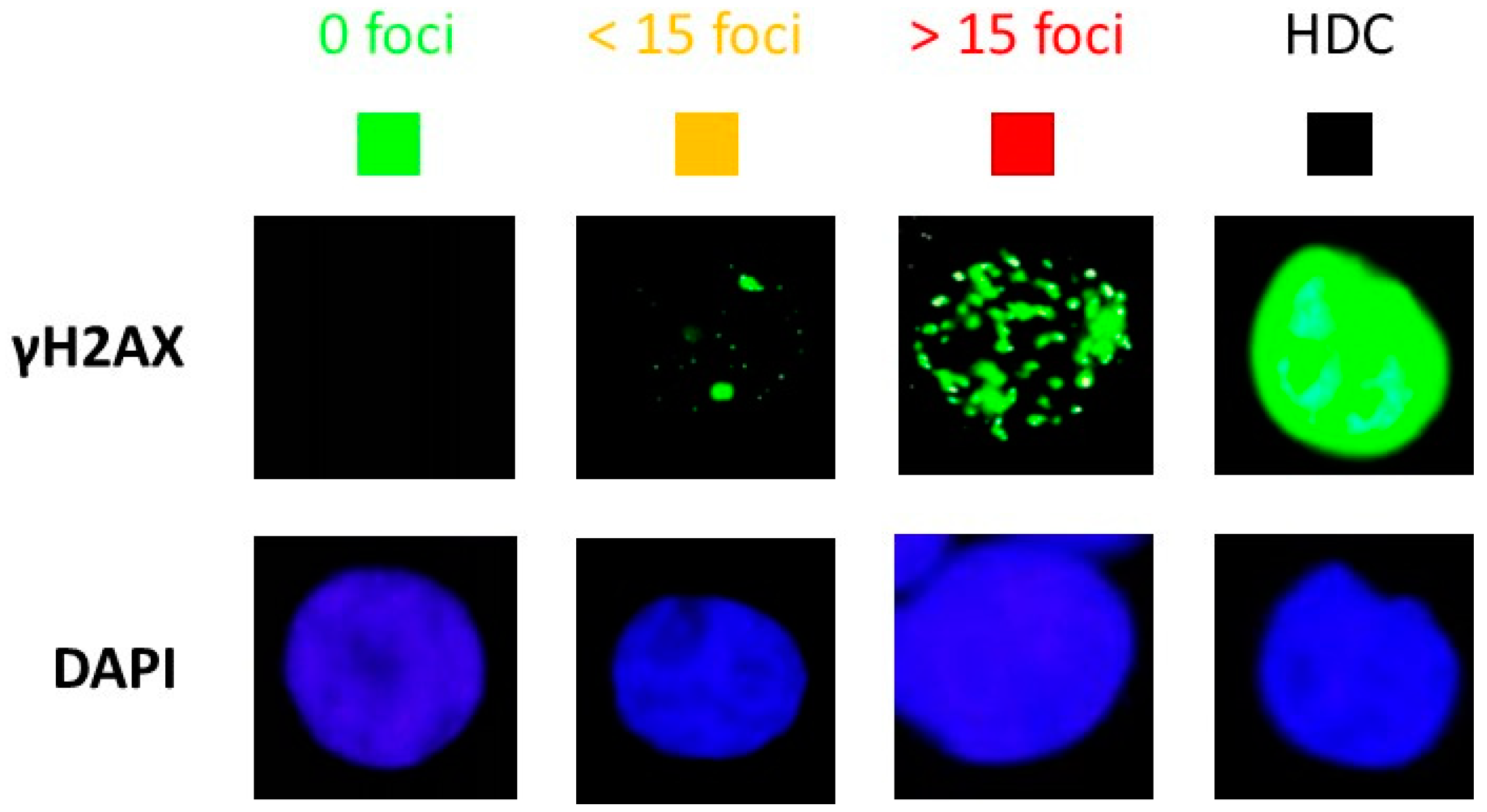
2.4. Immunofluorescence
2.5. Statistical Analysis
3. Results
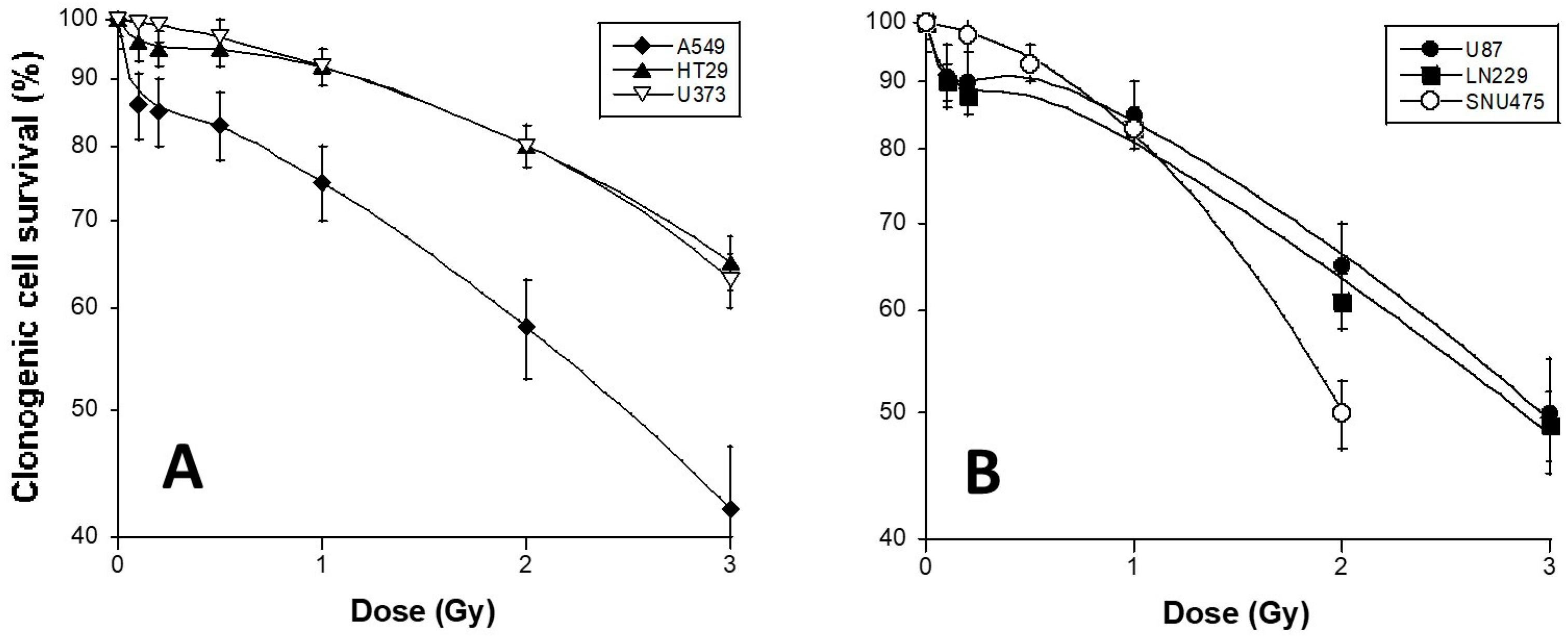
3.1. HRS Status of the 6 Tumor Cell Lines Used in This Study
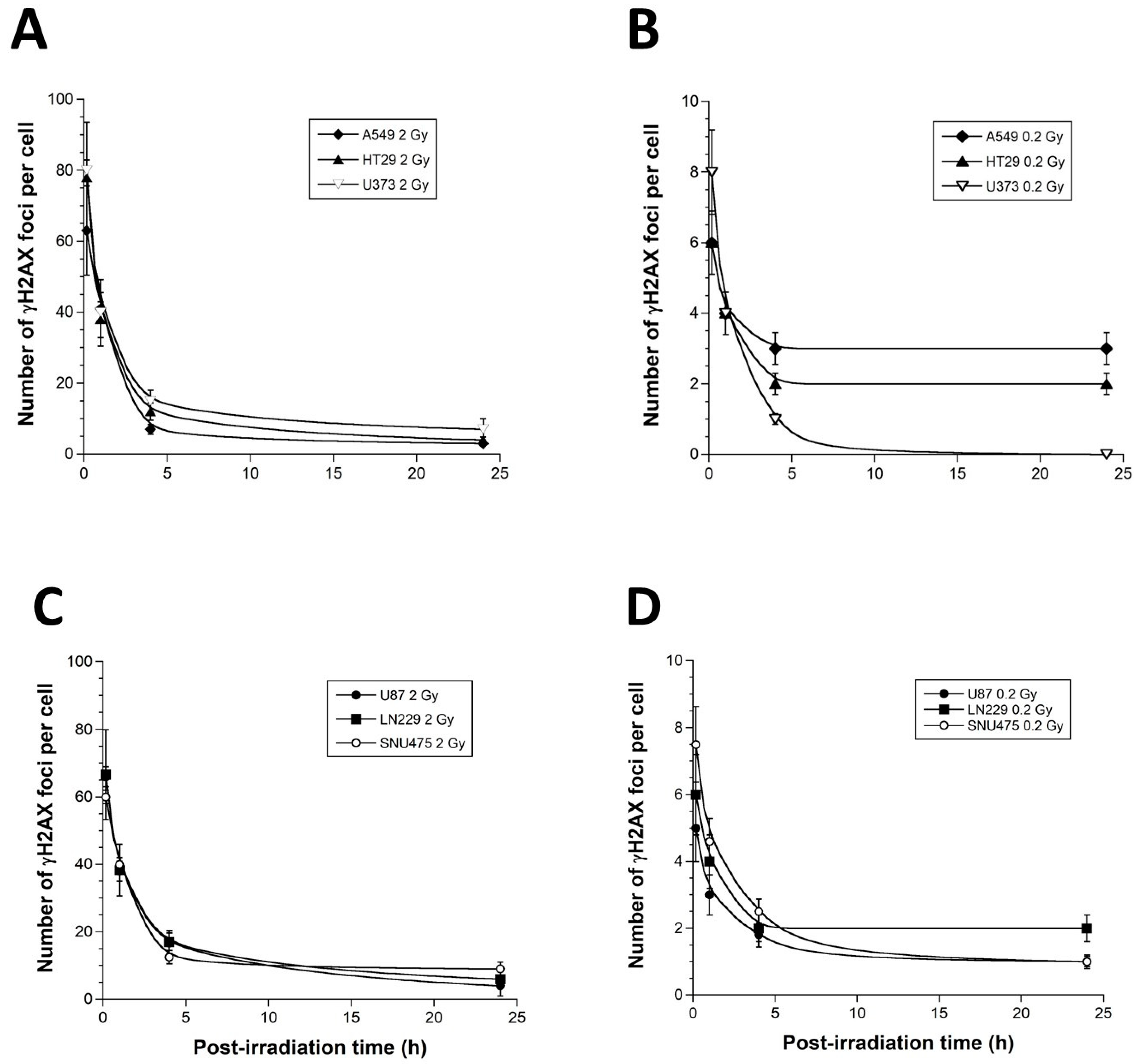
3.2. γH2AX Foci Kinetics of the 6 Tumor Cell Lines Used in This Study
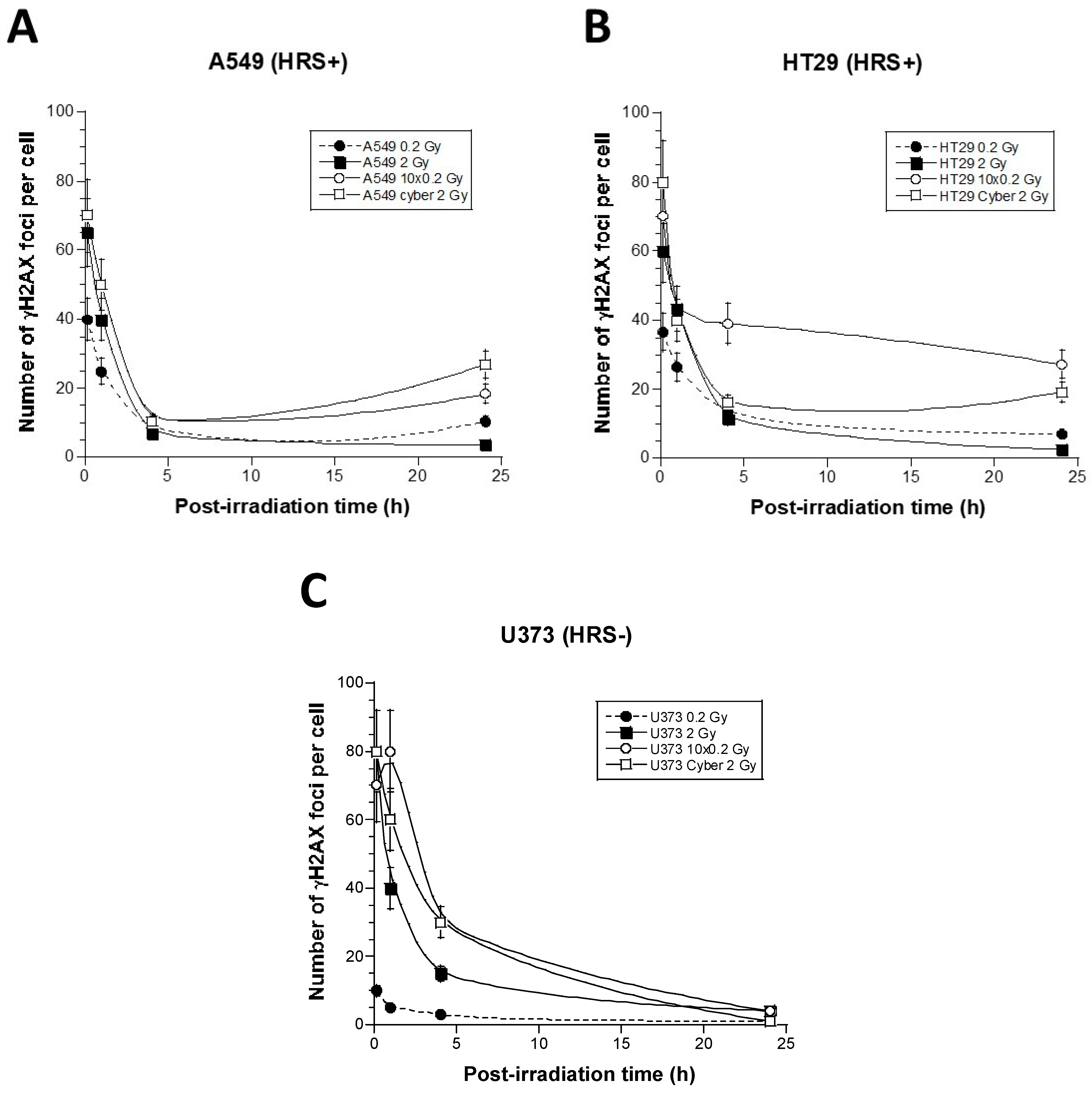
3.3. Molecular Response to SBRT of the 6 Tumor Cell Lines Used in This Study
3.4. Molecular Response to SBRT of the 13HNG and AG1521 Skin Fibroblast Cell Lines
3.5. Quantified Relationship between the HRS/IRR Ratio and the Percentage of HDC
4. Discussion
4.1. From Hypofractionated to Stereotactic RT Modalities
4.2. HRS Phenomenon and Stereotactic RT
4.3. Evidence That Each SBRT Session Results in a Repetition of Low Doses
4.4. The HRS Phenomenon in Stereotactic RT Modalities: Towards New Predictive Assays?
4.5. Towards a Biology-Guided Radiation Therapy (BGRT)?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bondiau, P.Y.; Benezery, K.; Beckendorf, V.; Peiffert, D.; Gerard, J.P.; Mirabel, X.; Noel, A.; Marchesi, V.; Lacornerie, T.; Dubus, F.; et al. CyberKnife robotic stereotactic radiotherapy: Technical aspects and medical indications. Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2007, 11, 338–344. [Google Scholar] [CrossRef]
- Kilby, W.; Dooley, J.R.; Kuduvalli, G.; Sayeh, S.; Maure, C.R. The CyberKnife® Robotic Radiosurgery System in 2010. Technol. Cancer Res. Treat. 2010, 9, 433–452. [Google Scholar] [CrossRef] [PubMed]
- De Jong, E.E.C.; Guckenberger, M.; Andratschke, N.; Dieckmann, K.; Hoogeman, M.S.; Milder, M.; Moller, D.S.; Nyeng, T.B.; Tanadini-Lang, S.; Lartigau, E.; et al. Variation in current prescription practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer: Recommendations for prescribing and recording according to the ACROP guideline and ICRU report 91. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2020, 142, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Wilke, L.; Andratschke, N.; Blanck, O.; Brunner, T.B.; Combs, S.E.; Grosu, A.L.; Moustakis, C.; Schmitt, D.; Baus, W.W.; Guckenberger, M. ICRU report 91 on prescribing, recording, and reporting of stereotactic treatments with small photon beams: Statement from the DEGRO/DGMP working group stereotactic radiotherapy and radiosurgery. Strahlenther. Onkol. Organ Dtsch. Rontgenges. 2019, 195, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Biston, M.C.; Dupuis, P.; Gassa, F.; Gregoire, V. Do all the linear accelerators comply with the ICRU 91’s constraints for stereotactic body radiation therapy treatments? Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2019, 23, 625–629. [Google Scholar] [CrossRef]
- ICRU. ICRU Report 91, Prescribing, Recording, and Reporting of Stereotactic Treatments with Small Photon Beams; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 2017. [Google Scholar]
- Calcerrada Diaz-Santos, N.; Blasco Amaro, J.A.; Cardiel, G.A.; Andradas Aragones, E. The safety and efficacy of robotic image-guided radiosurgery system treatment for intra- and extracranial lesions: A systematic review of the literature. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2008, 89, 245–253. [Google Scholar] [CrossRef]
- Gibbs, I.C.; Kamnerdsupaphon, P.; Ryu, M.R.; Dodd, R.; Kiernan, M.; Chang, S.D.; Adler, J.R., Jr. Image-guided robotic radiosurgery for spinal metastases. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2007, 82, 185–190. [Google Scholar] [CrossRef]
- Ehret, F.; Senger, C.; Kufeld, M.; Furweger, C.; Kord, M.; Haidenberger, A.; Windisch, P.; Ruess, D.; Kaul, D.; Ruge, M.; et al. Image-Guided Robotic Radiosurgery for the Management of Intramedullary Spinal Cord Metastases-A Multicenter Experience. Cancers 2021, 13, 297. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Beltramo, G.; Fariselli, L.; Fodor, C.; Santoro, L.; Vavassori, A.; Zerini, D.; Gherardi, F.; Ascione, C.; Bossi-Zanetti, I.; et al. Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 889–897. [Google Scholar] [CrossRef]
- Ihnat, P.; Skacelikova, E.; Tesar, M.; Penka, I. Stereotactic body radiotherapy using the CyberKnife((R)) system in the treatment of patients with liver metastases: State of the art. OncoTargets Ther. 2018, 11, 4685–4691. [Google Scholar] [CrossRef]
- Khadige, M.; Salleron, J.; Marchesi, V.; Oldrini, G.; Peiffert, D.; Beckendorf, V. Cyberknife((R)) stereotactic radiation therapy for stage I lung cancer and pulmonary metastases: Evaluation of local control at 24 months. J. Thorac. Dis. 2018, 10, 4976–4984. [Google Scholar] [CrossRef] [PubMed]
- Diamant, A.; Heng, V.J.; Chatterjee, A.; Faria, S.; Bahig, H.; Filion, E.; Doucet, R.; Khosrow-Khavar, F.; Naqa, I.E.; Seuntjens, J. Comparing local control and distant metastasis in NSCLC patients between CyberKnife and conventional SBRT. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2020, 144, 201–208. [Google Scholar] [CrossRef]
- Foray, N.; Bourguignon, M.; Hamada, N. Individual response to ionizing radiation. Mutat. Res. Rev. 2016, 770, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Joiner, M.C.; Marples, B.; Lambin, P.; Short, S.C.; Turesson, I. Low-dose hypersensitivity: Current status and possible mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 379–389. [Google Scholar] [CrossRef]
- Marples, B.; Collis, S.J. Low-dose hyper-radiosensitivity: Past, present, and future. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1310–1318. [Google Scholar] [CrossRef]
- Bodgi, L.; Foray, N. The nucleo-shuttling of the ATM protein as a basis for a novel theory of radiation response: Resolution of the linear-quadratic model. Int. J. Radiat. Biol. 2016, 92, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Martin, J.; Devic, C.; Diserbo, M.; Thariat, J.; Foray, N. Impact of dose-rate on the low-dose hyper-radiosensitivity and induced radioresistance (HRS/IRR) response. Int. J. Radiat. Biol. 2013, 89, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Rothkamm, K.; Lobrich, M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-ray doses. Proc. Natl. Acad. Sci. USA 2003, 100, 5057–5062. [Google Scholar] [CrossRef]
- Le Reun, E.; Bodgi, L.; Granzotto, A.; Sonzogni, L.; Ferlazzo, M.L.; Al-Choboq, J.; El-Nachef, L.; Restier-Verlet, J.; Berthel, E.; Devic, C.; et al. Quantitative correlations between radiosensitivity biomarkers show that the ATM protein kinase is strongly involved in the radiotoxicities observed after radiotherapy. Int. J. Mol. Sci. 2022, 23, 10434. [Google Scholar] [CrossRef]
- Granzotto, A.; Bencokova1, Z.; Vogin, G.; Devic, C.; Joubert, A.; Balosso, J.; Foray, N. DNA double-strand breaks repair and signaling of human gliomas and normal brain cells in response to radiation: Potential impact of the ATM- and BRCA1-dependent pathways. In Brain Tumors/Book 3; Abujamra, A.L., Ed.; Intechweb: Rijeka, Croatia, 2011. [Google Scholar]
- Foray, N.; Priestley, A.; Alsbeih, G.; Badie, C.; Capulas, E.P.; Arlett, C.F.; Malaise, E.P. Hypersensitivity of ataxia telangiectasia fibroblasts to ionizing radiation is associated with a repair deficiency of DNA double-strand breaks. Int. J. Radiat. Biol. 1997, 72, 271–283. [Google Scholar]
- Granzotto, A.; Benadjaoud, M.A.; Vogin, G.; Devic, C.; Ferlazzo, M.L.; Bodgi, L.; Pereira, S.; Sonzogni, L.; Forcheron, F.; Viau, M.; et al. Influence of Nucleoshuttling of the ATM Protein in the Healthy Tissues Response to Radiation Therapy: Toward a Molecular Classification of Human Radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 450–460. [Google Scholar] [CrossRef]
- Mengue, L.; Bertaut, A.; Ngo Mbus, L.; Dore, M.; Ayadi, M.; Clement-Colmou, K.; Claude, L.; Carrie, C.; Laude, C.; Tanguy, R.; et al. Brain metastases treated with hypofractionated stereotactic radiotherapy: 8 years experience after Cyberknife installation. Radiat. Oncol. 2020, 15, 82. [Google Scholar] [CrossRef]
- Ferlazzo, M.; Berthel, E.; Granzotto, A.; Devic, C.; Sonzogni, L.; Bachelet, J.T.; Pereira, S.; Bourguignon, M.; Sarasin, A.; Mezzina, M.; et al. Some mutations in the xeroderma pigmentosum D gene may lead to moderate but significant radiosensitivity associated with a delayed radiation-induced ATM nuclear localization. Int. J. Radiat. Biol. 2019, 96, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Al-Choboq, J.; Ferlazzo, M.L.; Sonzogni, L.; Granzotto, A.; El-Nachef, L.; Maalouf, M.; Berthel, E.; Foray, N. Usher Syndrome Belongs to the Genetic Diseases Associated with Radiosensitivity: Influence of the ATM Protein Kinase. Int. J. Mol. Sci. 2022, 23, 1570. [Google Scholar] [CrossRef] [PubMed]
- Marples, B.; Lambin, P.; Skov, K.A.; Joiner, M.C. Low dose hyper-radiosensitivity and increased radioresistance in mammalian cells. Int. J. Radiat. Biol. 1997, 71, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Y.; Chang, H.; Hu, T.; Wang, J.; Xie, Y.; Cheng, J. The Role and Mechanism of ATM-Mediated Autophagy in the Transition from Hyper-Radiosensitivity to Induced Radioresistance in Lung Cancer Under Low-Dose Radiation. Front. Cell Dev. Biol. 2021, 9, 650819. [Google Scholar] [CrossRef]
- Schoenherr, D.; Krueger, S.A.; Martin, L.; Marignol, L.; Wilson, G.D.; Marples, B. Determining if low dose hyper-radiosensitivity (HRS) can be exploited to provide a therapeutic advantage: A cell line study in four glioblastoma multiforme (GBM) cell lines. Int. J. Radiat. Biol. 2013, 89, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.A.; Seymour, C.B.; Joiner, M.C.; Mothersill, C.E. Radiation-induced adaptive response is not seen in cell lines showing a bystander effect but is seen in lines showing HRS/IRR response. Int. J. Radiat. Biol. 2009, 85, 87–95. [Google Scholar] [CrossRef]
- Cornforth, M.N.; Bedford, J.S. A quantitative comparison of potentially lethal damage repair and the rejoining of interphase chromosome breaks in low passage normal human fibroblasts. Radiat. Res. 1987, 111, 385–405. [Google Scholar] [CrossRef]
- Chavaudra, N.; Bourhis, J.; Foray, N. Quantified relationship between cellular radiosensitivity, DNA repair defects and chromatin relaxation: A study of 19 human tumour cell lines from different origin. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2004, 73, 373–382. [Google Scholar]
- Slonina, D.; Biesaga, B.; Urbanski, K.; Kojs, Z. Low-dose radiation response of primary keratinocytes and fibroblasts from patients with cervix cancer. Radiat. Res. 2007, 167, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Slonina, D.; Biesaga, B.; Urbanski, K.; Kojs, Z. The response of primary keratinocytes and fibroblasts from cancer patients to multiple low-dose irradiations. Radiat. Res. 2007, 168, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Slonina, D.; Biesaga, B.; Urbanski, K.; Kojs, Z. Comparison of chromosomal radiosensitivity of normal cells with and without HRS-like response and normal tissue reactions in patients with cervix cancer. Int. J. Radiat. Biol. 2008, 84, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Slonina, D.; Biesaga, B.; Urbanski, K.; Kojs, Z.; Waligorski, M. Evidence of low-dose hyper-radiosensitivity in normal cells of cervix cancer patients? Radiat. Prot. Dosim. 2006, 122, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Slonina, D.; Kowalczyk, A.; Janecka-Widla, A.; Kabat, D.; Szatkowski, W.; Biesaga, B. Low-Dose Hypersensitive Response for Residual pATM and gammaH2AX Foci in Normal Fibroblasts of Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 756–766. [Google Scholar] [CrossRef]
- Del Regato, J.A. Fractionation: A panoramic view. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 1329–1331. [Google Scholar] [CrossRef]
- Ellis, F. Fractionation in radiotherapy. In Modern Trends in Radiotherapy; Wood, D.A., Ed.; Buttersworth: London, UK, 1967; Volume 1, p. 34. [Google Scholar]
- Willers, H.; Beck-Bornholdt, H.P. Origins of radiotherapy and radiobiology: Separation of the influence of dose per fraction and overall treatment time on normal tissue damage by Reisner and Miescher in the 1930s. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1996, 38, 171–173. [Google Scholar] [CrossRef]
- Lasak, J.M.; Gorecki, J.P. The history of stereotactic radiosurgery and radiotherapy. Otolaryngol. Clin. N. Am. 2009, 42, 593–599. [Google Scholar] [CrossRef]
- Klement, R.J.; Sonke, J.J.; Allgauer, M.; Andratschke, N.; Appold, S.; Belderbos, J.; Belka, C.; Dieckmann, K.; Eich, H.T.; Flentje, M.; et al. Estimation of the alpha/beta ratio of non-small cell lung cancer treated with stereotactic body radiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2020, 142, 210–216. [Google Scholar] [CrossRef]
- Stuschke, M.; Pottgen, C. Altered fractionation schemes in radiotherapy. Front. Radiat. Ther. Oncol. 2010, 42, 150–156. [Google Scholar] [CrossRef]
- Nahum, A.E. The radiobiology of hypofractionation. Clin. Oncol. R. Coll. Radiol. 2015, 27, 260–269. [Google Scholar] [CrossRef]
- Brand, D.H.; Kirby, A.M.; Yarnold, J.R.; Somaiah, N. How Low Can You Go? The Radiobiology of Hypofractionation. Clin. Oncol. R. Coll. Radiol. 2022, 34, 280–287. [Google Scholar] [CrossRef]
- Thomas, C.; Charrier, J.; Massart, C.; Cherel, M.; Fertil, B.; Barbet, J.; Foray, N. Low-dose hyper-radiosensitivity of progressive and regressive cells isolated from a rat colon tumour: Impact of DNA repair. Int. J. Radiat. Biol. 2008, 84, 533–548. [Google Scholar] [PubMed]
- Berthel, E.; Foray, N.; Ferlazzo, M.L. The Nucleoshuttling of the ATM Protein: A Unified Model to Describe the Individual Response to High- and Low-Dose of Radiation? Cancers 2019, 11, 905. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.W. Stereotactic body radiotherapy: Current strategies and future development. J. Thorac. Dis. 2016, 8, S517–S527. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Crocetto, F.; Lucarelli, G.; Lievore, E.; Barone, B. Radiotherapy to the Primary Tumor: The First Step of a Tailored Therapy in Metastatic Prostate Cancer. Diagnostics 2022, 12, 1981. [Google Scholar] [CrossRef]


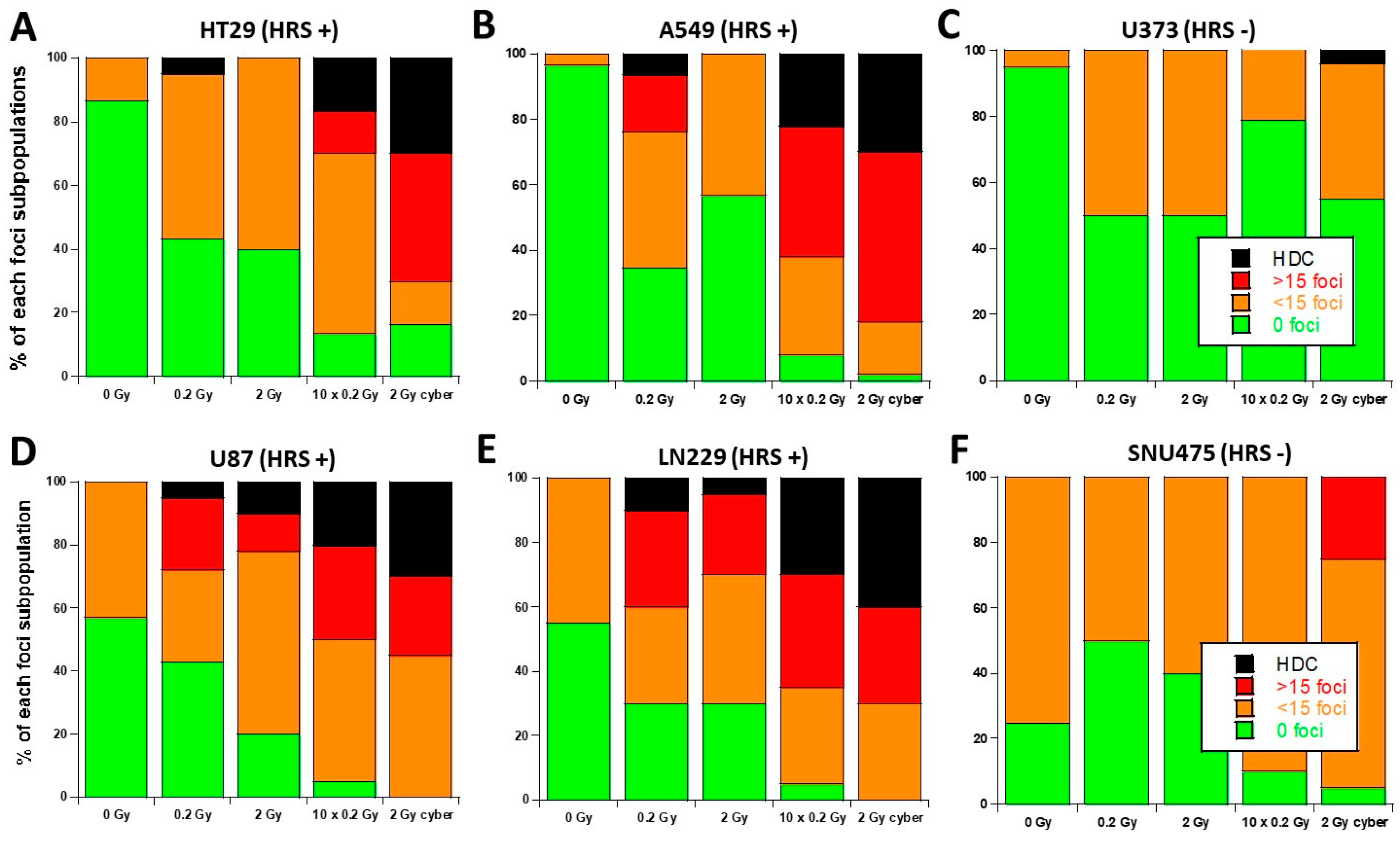
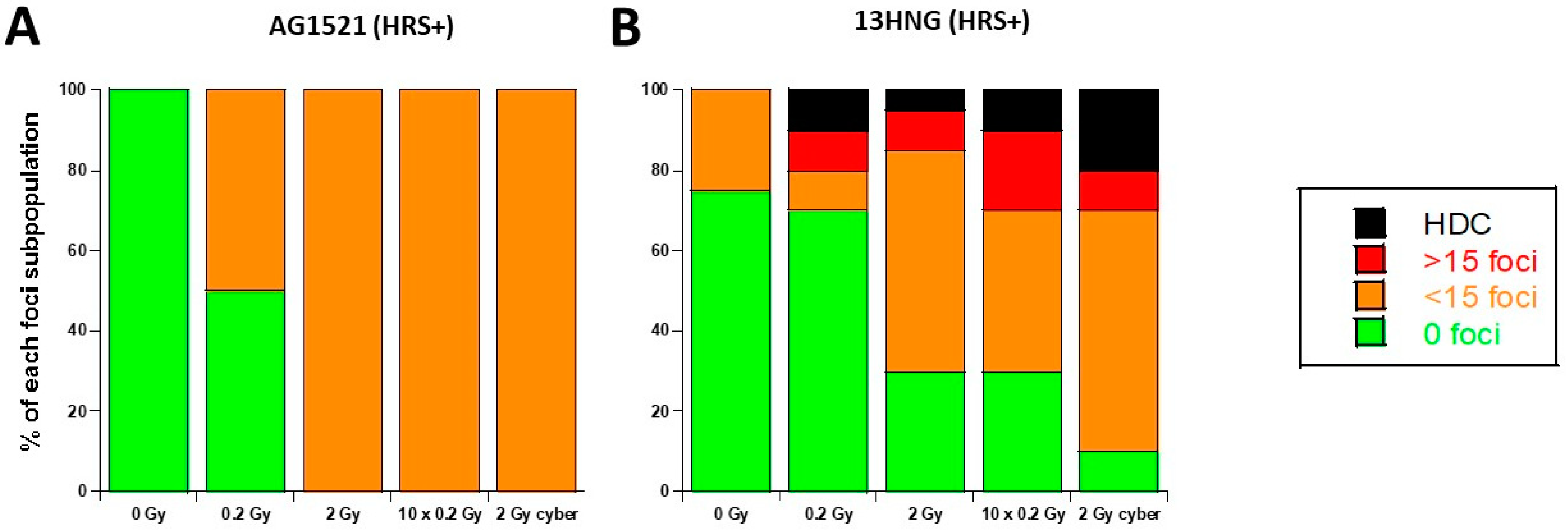

| Cell Line | Origin | HRS Status | |
|---|---|---|---|
| HT29 | Grade II colon adenocarcinoma isolated from a primary tumor from a white Caucasian female patient | #HTB-38, ATCC a | + |
| A549 | lung carcinoma from a white Caucasian male patient | #CCL-185, ATCC a | + |
| U373 MG | glioblastoma astrocytoma from a malignant tumor | #08061901, ECACC b | − |
| U87 MG | from malignant glioma from a male patient | #HTB-14, ATCC a | + |
| LN229 | from the right frontal parieto-occipital cortex of a white female patient with glioblastoma. | #CRL-2611, ATCC a | + |
| SNU475 | from an hepatocellular carcinoma | #CRL-2236 ATCC a | − |
| AG1521 | Skin (foreskin) fibroblast from a foreskin of a 3 day child | #AG01521, Coriell Institute Repository c | − |
| 13HNG | Skin fibroblast from a cancer patient showing adverse tissue after radiotherapy | COPERNIC Collection | + |
| Cell Lines | HRS Status | αres (Gy−1) | g | Dc (Gy) | HRS/IRR Ratio | β (Gy−2) | SF2 (%) |
|---|---|---|---|---|---|---|---|
| U373 | − | 0.05 | 0 | NA | 1 | 0.029 | 58 ± 6 |
| SNU475 | − | 0.05 | 0 | NA | 1 | 0.14 | 50 ± 4 |
| A549 | + | 0.273 | 8.11 | 0.17 | 4.5 | 0.0003 | 80 ± 4 |
| HT29 | + | 0.001 | 12.63 | 0.15 | 6 | 0.003 | 80 ± 6 |
| U87 | + | 0.14 | 12.05 | 0.13 | 3.75 | 0.03 | 65 ± 6 |
| LN229 | + | 0.19 | 7.03 | 0.15 | 7.5 | 0.016 | 61 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Reun, E.; Granzotto, A.; Pêtre, A.; Bodgi, L.; Beldjoudi, G.; Lacornerie, T.; Vallet, V.; Bouchet, A.; Al-Choboq, J.; Bourguignon, M.; et al. Influence of the Hypersensitivity to Low Dose Phenomenon on the Tumor Response to Hypofractionated Stereotactic Body Radiation Therapy. Cancers 2023, 15, 3979. https://doi.org/10.3390/cancers15153979
Le Reun E, Granzotto A, Pêtre A, Bodgi L, Beldjoudi G, Lacornerie T, Vallet V, Bouchet A, Al-Choboq J, Bourguignon M, et al. Influence of the Hypersensitivity to Low Dose Phenomenon on the Tumor Response to Hypofractionated Stereotactic Body Radiation Therapy. Cancers. 2023; 15(15):3979. https://doi.org/10.3390/cancers15153979
Chicago/Turabian StyleLe Reun, Eymeric, Adeline Granzotto, Adeline Pêtre, Larry Bodgi, Guillaume Beldjoudi, Thomas Lacornerie, Véronique Vallet, Audrey Bouchet, Joëlle Al-Choboq, Michel Bourguignon, and et al. 2023. "Influence of the Hypersensitivity to Low Dose Phenomenon on the Tumor Response to Hypofractionated Stereotactic Body Radiation Therapy" Cancers 15, no. 15: 3979. https://doi.org/10.3390/cancers15153979
APA StyleLe Reun, E., Granzotto, A., Pêtre, A., Bodgi, L., Beldjoudi, G., Lacornerie, T., Vallet, V., Bouchet, A., Al-Choboq, J., Bourguignon, M., Thariat, J., Bourhis, J., Lartigau, E., & Foray, N. (2023). Influence of the Hypersensitivity to Low Dose Phenomenon on the Tumor Response to Hypofractionated Stereotactic Body Radiation Therapy. Cancers, 15(15), 3979. https://doi.org/10.3390/cancers15153979









