New Era of Immune-Based Therapy in Intrahepatic Cholangiocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
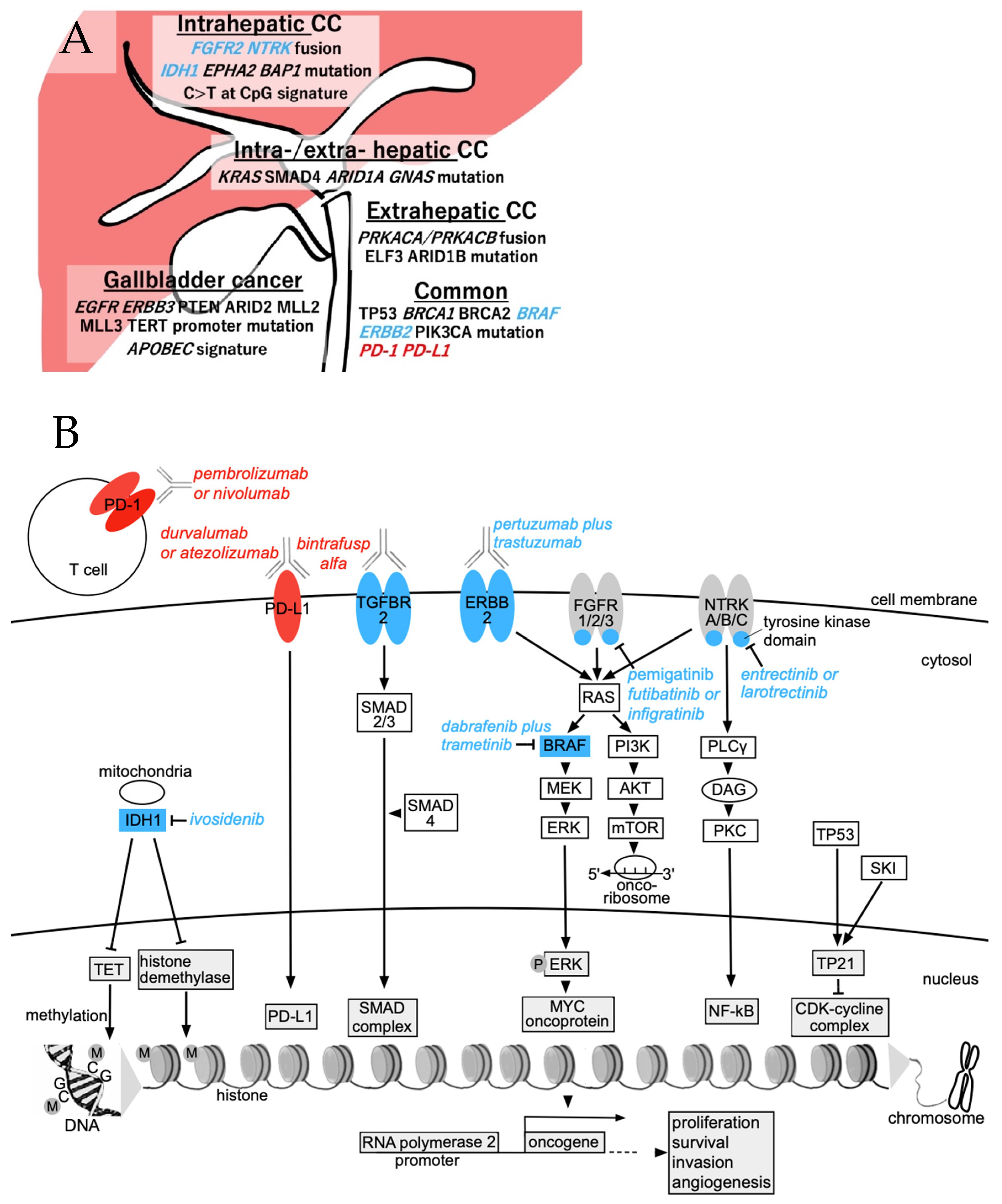
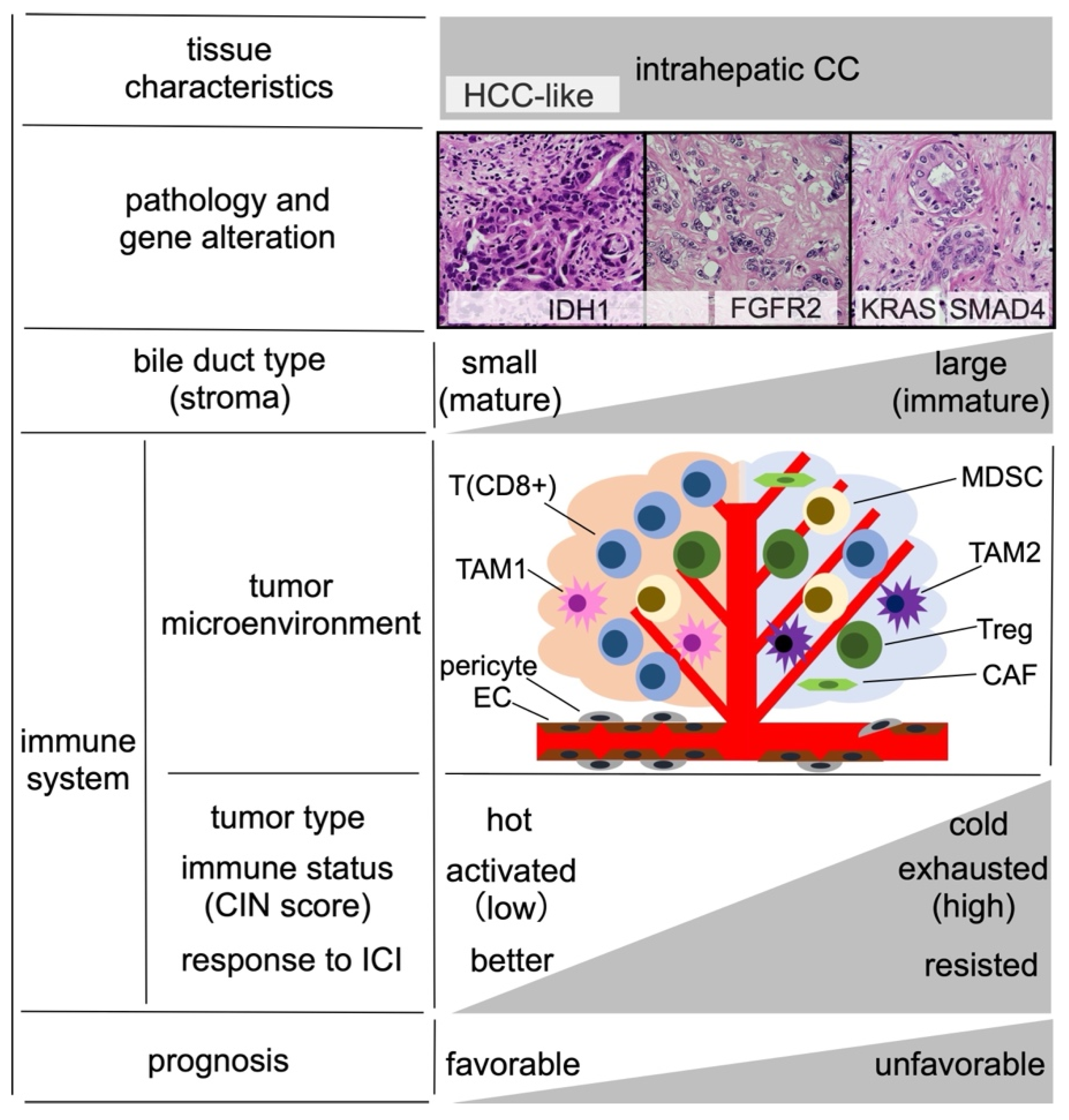
2. Pathogenesis and Immune Microenvironment of Intrahepatic CC
3. Immune-Based Therapy for BTC
3.1. Durvalumab
3.2. Pembrolizumab
3.3. Nivolumab
3.4. Standard Second-Line Therapies after First-Line Therapy Failure
4. Viable Targets and Ongoing Trials for Intrahepatic CC
5. Predicting ICI Resistance and Countermeasures for BTC
6. IrAEs in BTC Cases
6.1. Hepatotoxicity
6.2. Other irAEs
7. Other Perspectives
7.1. ICI Therapy during the Perioperative Period for BTC
7.2. Steatohepatitis and Primary Liver Cancer
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Sithithaworn, P.; Yongvanit, P.; Duenngai, K.; Kiatsopit, N.; Pairojkul, C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2014, 21, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanc, J.F.; Debaillon-Vesque, A.; Roth, G.; Barbare, J.C.; Baumann, A.S.; Boige, V.; Boudjema, K.; Bouattour, M.; Crehange, G.; Dauvois, B.; et al. Hepatocellular carcinoma: French Intergroup Clinical Practice Guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, AFEF, SIAD, SFR/FRI). Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101590. [Google Scholar] [CrossRef] [PubMed]
- European Association for The Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Blechacz, B.R.; Gores, G.J. Cholangiocarcinoma. Clin. Liver Dis. 2008, 12, 131–150. [Google Scholar] [CrossRef]
- Spolverato, G.; Kim, Y.; Alexandrescu, S.; Marques, H.P.; Lamelas, J.; Aldrighetti, L.; Clark Gamblin, T.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; et al. Management and Outcomes of Patients with Recurrent Intrahepatic Cholangiocarcinoma Following Previous Curative-Intent Surgical Resection. Ann. Surg. Oncol. 2016, 23, 235–243. [Google Scholar] [CrossRef]
- Kubo, S.; Shinkawa, H.; Asaoka, Y.; Ioka, T.; Igaki, H.; Izumi, N.; Itoi, T.; Unno, M.; Ohtsuka, M.; Okusaka, T.; et al. Liver Cancer Study Group of Japan Clinical Practice Guidelines for Intrahepatic Cholangiocarcinoma. Liver Cancer 2022, 11, 290–314. [Google Scholar] [CrossRef]
- Nagino, M.; Hirano, S.; Yoshitomi, H.; Aoki, T.; Uesaka, K.; Unno, M.; Ebata, T.; Konishi, M.; Sano, K.; Shimada, K.; et al. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition. J. Hepatobiliary Pancreat. Sci. 2021, 28, 26–54. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Furuse, J.; Okusaka, T.; Bridgewater, J.; Taketsuna, M.; Wasan, H.; Koshiji, M.; Valle, J. Lessons from the comparison of two randomized clinical trials using gemcitabine and cisplatin for advanced biliary tract cancer. Crit. Rev. Oncol. Hematol. 2011, 80, 31–39. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Ruth He, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evidence 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Uenishi, T.; Ariizumi, S.; Aoki, T.; Ebata, T.; Ohtsuka, M.; Tanaka, E.; Yoshida, H.; Imura, S.; Ueno, M.; Kokudo, N.; et al. Proposal of a new staging system for mass-forming intrahepatic cholangiocarcinoma: A multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J. Hepatobiliary Pancreat. Sci. 2014, 21, 499–508. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Kosaka, H.; Ueno, M.; Komeda, K.; Hokuto, D.; Iida, H.; Hirokawa, F.; Matsui, K.; Sekimoto, M.; Kaibori, M. The Impact of a Preoperative Staging System on Accurate Prediction of Prognosis in Intrahepatic Cholangiocarcinoma. Cancers 2022, 14, 1107. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef]
- Kuang, D.M.; Zhao, Q.; Peng, C.; Xu, J.; Zhang, J.P.; Wu, C.; Zheng, L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med. 2009, 206, 1327–1337. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef]
- Kawamura, E.; Matsubara, T.; Daikoku, A.; Deguchi, S.; Kinoshita, M.; Yuasa, H.; Urushima, H.; Odagiri, N.; Motoyama, H.; Kotani, K.; et al. Suppression of intrahepatic cholangiocarcinoma cell growth by SKI via upregulation of the CDK inhibitor p21. FEBS Open Bio 2022, 12, 2122–2135. [Google Scholar] [CrossRef]
- Scott, A.J.; Sharman, R.; Shroff, R.T. Precision Medicine in Biliary Tract Cancer. J. Clin. Oncol. 2022, 40, 2716–2734. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; Board, W.C.o.T.E. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, Y.; Kwon, S.M.; Rhee, H.; Yoo, J.E.; Chung, T.; Woo, H.G.; Park, Y.N. Molecular and radiopathologic spectrum between HCC and intrahepatic cholangiocarcinoma. Hepatology 2023, 77, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- De Guillebon, E.; Dardenne, A.; Saldmann, A.; Séguier, S.; Tran, T.; Paolini, L.; Lebbe, C.; Tartour, E. Beyond the concept of cold and hot tumors for the development of novel predictive biomarkers and the rational design of immunotherapy combination. Int. J. Cancer 2020, 147, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Goel, S.; Duda, D.G.; Fukumura, D.; Jain, R.K. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013, 73, 2943–2948. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef]
- Doki, Y.; Ueno, M.; Hsu, C.H.; Oh, D.Y.; Park, K.; Yamamoto, N.; Ioka, T.; Hara, H.; Hayama, M.; Nii, M.; et al. Tolerability and efficacy of durvalumab, either as monotherapy or in combination with tremelimumab, in patients from Asia with advanced biliary tract, esophageal, or head-and-neck cancer. Cancer Med. 2022, 11, 2550–2560. [Google Scholar] [CrossRef]
- Sahai, V.; Griffith, K.A.; Beg, M.S.; Shaib, W.L.; Mahalingam, D.; Zhen, D.B.; Deming, D.A.; Zalupski, M.M. A randomized phase 2 trial of nivolumab, gemcitabine, and cisplatin or nivolumab and ipilimumab in previously untreated advanced biliary cancer: BilT-01. Cancer 2022, 128, 3523–3530. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Elsayed, Z.; Elhalawani, H. Gemcitabine-based chemotherapy for advanced biliary tract carcinomas. Cochrane Database Syst. Rev. 2018, 4, Cd011746. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Recent advances of immunotherapy for biliary tract cancer. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 527–536. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Meric-Bernstam, F.; Swanton, C.; Hurwitz, H.; Spigel, D.R.; Sweeney, C.; Burris, H.; Bose, R.; Yoo, B.; Stein, A.; et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J. Clin. Oncol. 2018, 36, 536–542. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Morizane, C.; Okusaka, T.; Mizusawa, J.; Katayama, H.; Ueno, M.; Ikeda, M.; Ozaka, M.; Okano, N.; Sugimori, K.; Fukutomi, A.; et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann. Oncol. 2019, 30, 1950–1958. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAF. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Yoo, C.; Oh, D.Y.; Choi, H.J.; Kudo, M.; Ueno, M.; Kondo, S.; Chen, L.T.; Osada, M.; Helwig, C.; Dussault, I.; et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J. Immunother. Cancer 2020, 8, e000564. [Google Scholar] [CrossRef]
- Piha-Paul, S.A.; Oh, D.Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef]
- Ioka, T.; Kanai, M.; Kobayashi, S.; Sakai, D.; Eguchi, H.; Baba, H.; Seo, S.; Taketomi, A.; Takayama, T.; Yamaue, H.; et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401-MITSUBA). J. Hepatobiliary Pancreat. Sci. 2022, 30, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Lee, K.H.; Lee, D.W.; Yoon, J.; Kim, T.Y.; Bang, J.H.; Nam, A.R.; Oh, K.S.; Kim, J.M.; Lee, Y.; et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef]
- Shemesh, C.S.; Chan, P.; Marchand, M.; Gonçalves, A.; Vadhavkar, S.; Wu, B.; Li, C.; Jin, J.Y.; Hack, S.P.; Bruno, R. Early Decision Making in a Randomized Phase II Trial of Atezolizumab in Biliary Tract Cancer Using a Tumor Growth Inhibition-Survival Modeling Framework. Clin. Pharmacol. Ther. 2023. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Ueno, M.; Ikeda, M.; Morizane, C.; Kobayashi, S.; Ohno, I.; Kondo, S.; Okano, N.; Kimura, K.; Asada, S.; Namba, Y.; et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: A non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol. Hepatol. 2019, 4, 611–621. [Google Scholar] [CrossRef]
- Tanaka, S.; Umemoto, K.; Kubo, S.; Sato, Y.; Mimaki, S.; Tsuchihara, K.; Takemura, S.; Shinkawa, H.; Mori, A.; Ikeda, M. Nivolumab for treating patients with occupational cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2022, 29, 1153–1155. [Google Scholar] [CrossRef]
- Tanaka, S.; Kubo, S. Programmed death-1 inhibitor for occupational intrahepatic cholangiocarcinoma caused by chlorinated organic solvents. J. Hepatobiliary Pancreat. Sci. 2019, 26, 242–243. [Google Scholar] [CrossRef]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [Green Version]
- Churi, C.R.; Shroff, R.; Wang, Y.; Rashid, A.; Kang, H.C.; Weatherly, J.; Zuo, M.; Zinner, R.; Hong, D.; Meric-Bernstam, F.; et al. Mutation profiling in cholangiocarcinoma: Prognostic and therapeutic implications. PLoS ONE 2014, 9, e115383. [Google Scholar] [CrossRef] [Green Version]
- Perkhofer, L.; Berger, A.W.; Beutel, A.K.; Gallmeier, E.; Angermeier, S.; Fischer von Weikersthal, L.; Goetze, T.O.; Muche, R.; Seufferlein, T.; Ettrich, T.J. Nal-IRI with 5-fluorouracil (5-FU) and leucovorin or gemcitabine plus cisplatin in advanced biliary tract cancer—The NIFE trial (AIO-YMO HEP-0315) an open label, non-comparative, randomized, multicenter phase II study. BMC Cancer 2019, 19, 990. [Google Scholar] [CrossRef]
- Boerner, T.; Drill, E.; Pak, L.M.; Nguyen, B.; Sigel, C.S.; Doussot, A.; Shin, P.; Goldman, D.A.; Gonen, M.; Allen, P.J.; et al. Genetic Determinants of Outcome in Intrahepatic Cholangiocarcinoma. Hepatology 2021, 74, 1429–1444. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Droz Dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Woods, E.; Le, D.; Jakka, B.K.; Manne, A. Changing Landscape of Systemic Therapy in Biliary Tract Cancer. Cancers 2022, 14, 2137. [Google Scholar] [CrossRef]
- Tam, V.C.; Ramjeesingh, R.; Burkes, R.; Yoshida, E.M.; Doucette, S.; Lim, H.J. Emerging Systemic Therapies in Advanced Unresectable Biliary Tract Cancer: Review and Canadian Perspective. Curr. Oncol. 2022, 29, 7072–7085. [Google Scholar] [CrossRef]
- Ahn, D.H.; Uson Junior, P.L.S.; Masci, P.; Kosiorek, H.; Halfdanarson, T.R.; Mody, K.; Babiker, H.; DeLeon, T.; Sonbol, M.B.; Gores, G.; et al. A pilot study of Pan-FGFR inhibitor ponatinib in patients with FGFR-altered advanced cholangiocarcinoma. Investig. New Drugs 2022, 40, 134–141. [Google Scholar] [CrossRef]
- Zhou, S.L.; Xin, H.Y.; Sun, R.Q.; Zhou, Z.J.; Hu, Z.Q.; Luo, C.B.; Wang, P.C.; Li, J.; Fan, J.; Zhou, J. Association of KRAS Variant Subtypes With Survival and Recurrence in Patients With Surgically Treated Intrahepatic Cholangiocarcinoma. JAMA Surg. 2022, 157, 59–65. [Google Scholar] [CrossRef]
- Rizzo, A.; Brandi, G. Biochemical predictors of response to immune checkpoint inhibitors in unresectable hepatocellular carcinoma. Cancer Treat. Res. Commun. 2021, 27, 100328. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderaro, J.; Rousseau, B.; Amaddeo, G.; Mercey, M.; Charpy, C.; Costentin, C.; Luciani, A.; Zafrani, E.S.; Laurent, A.; Azoulay, D.; et al. Programmed death ligand 1 expression in hepatocellular carcinoma: Relationship with clinical and pathological features. Hepatology 2016, 64, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz de Galarreta, M.; Bresnahan, E.; Molina-Sánchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Dhainaut, M.; et al. β-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1124–1141. [Google Scholar] [CrossRef]
- Hayata, Y.; Nakagawa, H.; Kurosaki, S.; Kawamura, S.; Matsushita, Y.; Hayakawa, Y.; Suzuki, N.; Hata, M.; Tsuboi, M.; Kinoshita, H.; et al. Axin2. Gastroenterology 2021, 160, 2133–2148.e6. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Dong, Z.; Liao, B.; Shen, W.; Sui, C.; Yang, J. Expression of Programmed Death Ligand 1 Is Associated with the Prognosis of Intrahepatic Cholangiocarcinoma. Dig. Dis. Sci. 2020, 65, 480–488. [Google Scholar] [CrossRef]
- Macek Jilkova, Z.; Aspord, C.; Decaens, T. Predictive Factors for Response to PD-1/PD-L1 Checkpoint Inhibition in the Field of Hepatocellular Carcinoma: Current Status and Challenges. Cancers 2019, 11, 1554. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, X.; Ren, X.; Zhang, Y.; Huang, M.; Tan, L.; Dai, Z.; Lai, J.; Xie, W.; Chen, Z.; et al. Targeting tumour-intrinsic N7-methylguanosine tRNA modification inhibits MDSC recruitment and improves anti-PD-1 efficacy. Gut 2022, 72, 1555–1567. [Google Scholar] [CrossRef]
- Squibb, B.-M.; Eliquis, P.P.C. Package Insert; Bristol-Myers Squibb: Princeton, NJ, USA, 2019. [Google Scholar]
- AstraZeneca Pharmaceuticals; Wilmington, D. Imfinzi (Durvalumab) Package Insert; US Food and Drug Administration: Silver Spring, MD, USA, 2018. [Google Scholar]
- Dohme, M.S. Keytruda (pembrolizumab) [package insert]; US Food and Drug Administration: Silver Spring, MD, USA, 2019. [Google Scholar]
- De Martin, E.; Michot, J.-M.; Papouin, B.; Champiat, S.; Mateus, C.; Lambotte, O.; Roche, B.; Antonini, T.M.; Coilly, A.; Laghouati, S. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J. Hepatol. 2018, 68, 1181–1190. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Nakachi, K.; Konishi, M.; Ikeda, M.; Mizusawa, J.; Eba, J.; Okusaka, T.; Ishii, H.; Fukuda, H.; Furuse, J.; Group, H.a.P.O.G.o.t.J.C.O. A randomized Phase III trial of adjuvant S-1 therapy vs. observation alone in resected biliary tract cancer: Japan Clinical Oncology Group Study (JCOG1202, ASCOT). Jpn. J. Clin. Oncol. 2018, 48, 392–395. [Google Scholar] [CrossRef]
- Hui, Z.; Zhang, J.; Ren, Y.; Li, X.; Yan, C.; Yu, W.; Wang, T.; Xiao, S.; Chen, Y.; Zhang, R.; et al. Single-cell profiling of immune cells after neoadjuvant pembrolizumab and chemotherapy in IIIA non-small cell lung cancer (NSCLC). Cell Death Dis. 2022, 13, 607. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef]
- Rohaan, M.W.; Stahlie, E.H.A.; Franke, V.; Zijlker, L.P.; Wilgenhof, S.; van der Noort, V.; van Akkooi, A.C.J.; Haanen, J.B.A.G. Neoadjuvant nivolumab + T-VEC combination therapy for resectable early stage or metastatic (IIIB-IVM1a) melanoma with injectable disease: Study protocol of the NIVEC trial. BMC Cancer 2022, 22, 851. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Huang, Q.; Tan, S.; Xiong, X.; Gou, H. Rare DNA Mismatch Repair-Related Protein Loss in Patients with Intrahepatic Cholangiocarcinoma and Combined Hepatocellular-Cholangiocarcinoma and Their Response to Immunotherapy. Cancer Manag. Res. 2021, 13, 4283–4290. [Google Scholar] [CrossRef]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Breuer, D.A.; Pacheco, M.C.; Washington, M.K.; Montgomery, S.A.; Hasty, A.H.; Kennedy, A.J. CD8+ T cells regulate liver injury in obesity-related nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G211–G224. [Google Scholar] [CrossRef]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef]
- Welzel, T.M.; Graubard, B.I.; Zeuzem, S.; El-Serag, H.B.; Davila, J.A.; McGlynn, K.A. Metabolic syndrome increases the risk of primary liver cancer in the United States: A study in the SEER-Medicare database. Hepatology 2011, 54, 463–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Regimen | Mechanism | Trial | Phase | Author | Report Year | Patient Number | ORR (%) | Median PFS (Month) | Median OS (Month) | Treatment Line | Conditions | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GemCis | Cytotoxic anticancer | ABC-02 | III | Valle J | 2010 | 204 | 26 | 8 | 11.7 | 1st | [10] | |
| GemCis | Cytotoxic anticancer | BT22 | II | Furuse T | 2011 | 41 | 20 | 5.8 | 11.2 | 1st | [11] | |
| Pertuzumab + trastuzumab | Anti-ERBB(HER)2 | MyPathway | II | Hainsworth JD | 2018 | 7 | 29 | ND | ND | 2nd | Gene alteration | [31] |
| Larotrectinib | Anti-NTRK A/B/C | - | I/II | Drilon A | 2018 | 2 | 50 | ND | ND | 2nd | Gene alteration | [32] |
| GemCis | Cytotoxic anticancer | JCOG1113 | III | Morizane C | 2019 | 175 | 32.4 | 5.8 | 13.4 | 1st/2nd | [33] | |
| Gem + S-1 | Cytotoxic anticancer | JCOG1113 | III | Morizane C | 2019 | 179 | 29.8 | 6.8 | 15.1 | 1st/2nd | [33] | |
| Pemigatinib | Anti-FGFR1/2/3 | FIGHT-202 | II | Abou-Alfa GK | 2020 | 107 | 35.5 | 6.9 | 21.1 | 2nd | Gene alteration | [34] |
| Ivosidenib | Anti-IDH1 | ClarIDHy | III | Abou-Alfa GK | 2020 | 124 | 2.4 | 2.7 | 10.8 | 2nd | Gene alteration | [35] |
| Dabrafenib + trametinib | Anti-B-Raf + anti-MEK | ROAR | II | Subbiah V | 2020 | 43 | 47 | 9 | 14 | 2nd | Gene alteration | [36] |
| Entrectinib | Anti-NTRK A/B/C | STARTRK-1&2 | I/II | Doebele RC | 2020 | 1 | 100 | ND | ND | 2nd | Gene alteration | [37] |
| Bintrafusp alfa | Anti-PD-L1 + anti-TGFBR2 | NEOBIL | I | Yoo C | 2020 | 30 | 20 | 2.5 | 12.7 | 2nd | [38] | |
| Pembrolizumab | Anti-PD-1 | KEYNOTE-028 | Ib | Piha-Paul SA | 2020 | 24 | 13 | 1.8 | 5.7 | 2nd | MSI-high, PD-L1-positive | [39] |
| Pembrolizumab | Anti-PD-1 | KEYNOTE-158 | II | Piha-Paul SA | 2020 | 104 | 5.8 | 2 | 7.4 | 2nd | MSI-high | [39] |
| Folinic acid + fluorouracil + oxaliplatin | Cytotoxic anticancer | ABC-06 | III | Lamarca A | 2021 | 81 | 5 | ND | 6.2 | 2nd | [40] | |
| Infigratinib | Anti-FGFR1/2/3 | - | II | Javle M | 2021 | 122 | 23.1 | 6.7 | ND | 2nd | Gene alteration | [41] |
| Nivolumab | Anti-PD-1 | OPAL | II | ND | 2022 | 16 | ND | ND | ND | 2nd | * Interim analysis | ND |
| GemCis + S-1 | Cytotoxic anticancer | KHBO1401 | III | Ioka T | 2022 | 123 | 41.5 | 7.4 | 13.5 | 1st | [42] | |
| Durvalumab + GemCis | Cytotoxic anticancer + anti-PD-L1 | - | II | Oh DY | 2022 | 49 | 72 | 11 | 18.1 | 1st | [43] | |
| Durvalumab + GemCis | Cytotoxic anticancer + anti-PD-L1 | TOPAZ-1 | III | Oh DY | 2022 | 341 | 26.7 | 7.2 | 12.8 | 1st | Interim analysis | [12] |
| Futibatinib | Anti-FGFR1/2/3 | TAS-120 | II | Goyal L | 2023 | 103 | 42 | 9 | 21.7 | 2nd | Gene alteration | [44] |
| Pembrolizumab + GemCis | Anti-PD-1 + cytotoxic anticancer | KEYNOTE-966 | III | Kelley RK | 2023 | 533 | ND | 6.5 | 12.7 | 1st | [45] | |
| Atezolizumab + bevacizumab + GemCis | Cytotoxic anticancer + anti-PD-L1 + anti-VEGF | IMbrave151 | II | Shemesh CS | 2023 | 78 | ND | ND | ND | 1st | Interim analysis | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawamura, E.; Matsubara, T.; Kawada, N. New Era of Immune-Based Therapy in Intrahepatic Cholangiocarcinoma. Cancers 2023, 15, 3993. https://doi.org/10.3390/cancers15153993
Kawamura E, Matsubara T, Kawada N. New Era of Immune-Based Therapy in Intrahepatic Cholangiocarcinoma. Cancers. 2023; 15(15):3993. https://doi.org/10.3390/cancers15153993
Chicago/Turabian StyleKawamura, Etsushi, Tsutomu Matsubara, and Norifumi Kawada. 2023. "New Era of Immune-Based Therapy in Intrahepatic Cholangiocarcinoma" Cancers 15, no. 15: 3993. https://doi.org/10.3390/cancers15153993





