Genetic Profiling of Cell-Free DNA in Liquid Biopsies: A Complementary Tool for the Diagnosis of B-Cell Lymphomas and the Surveillance of Measurable Residual Disease
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
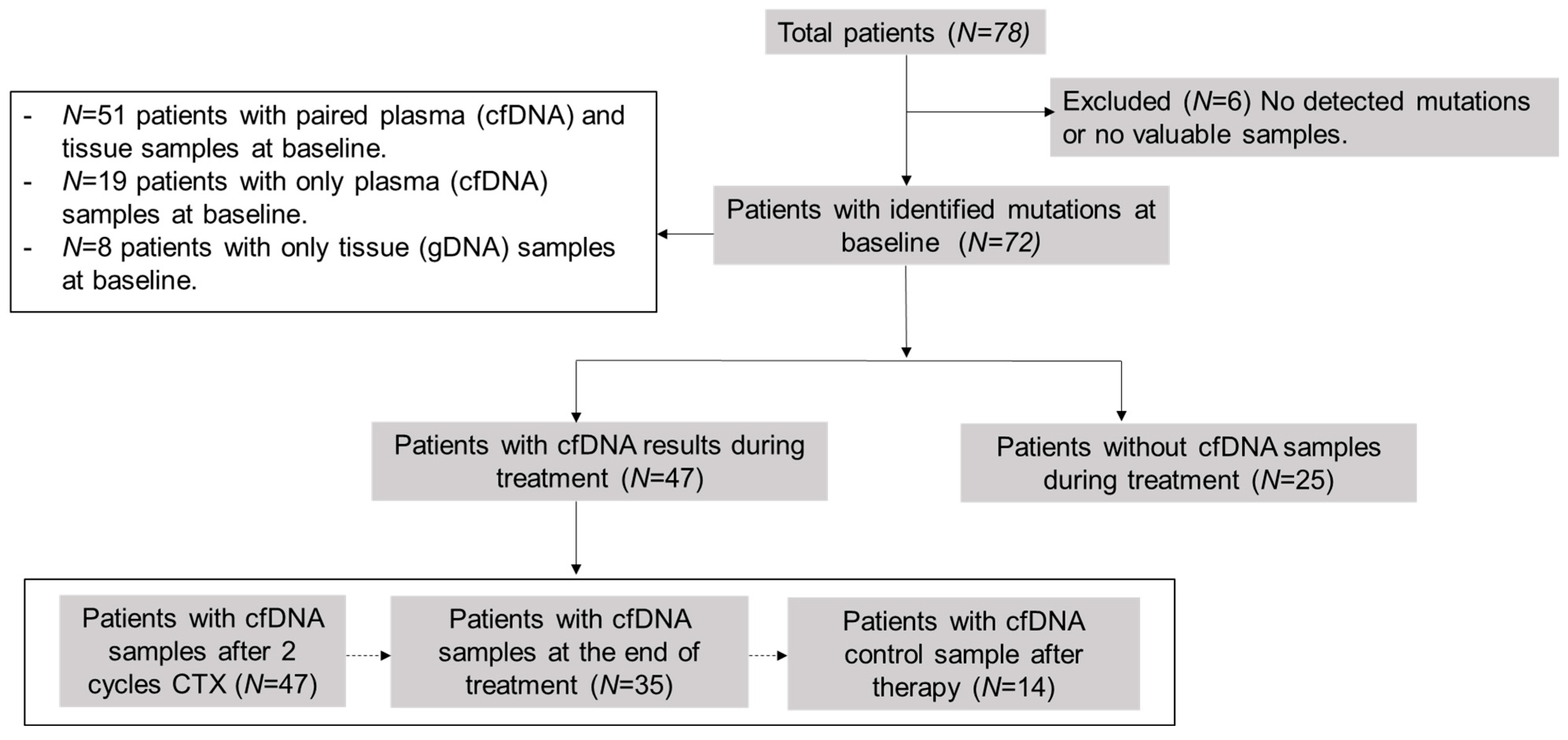
2.1. Patient Cohort and Sample Collection
2.2. DNA Purification and Quantification
2.3. Baseline Genotyping and LiqBio–MRD Biomarker Selection
2.4. LiqBio–MRD Methodology and Bioinformatic Pipeline
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
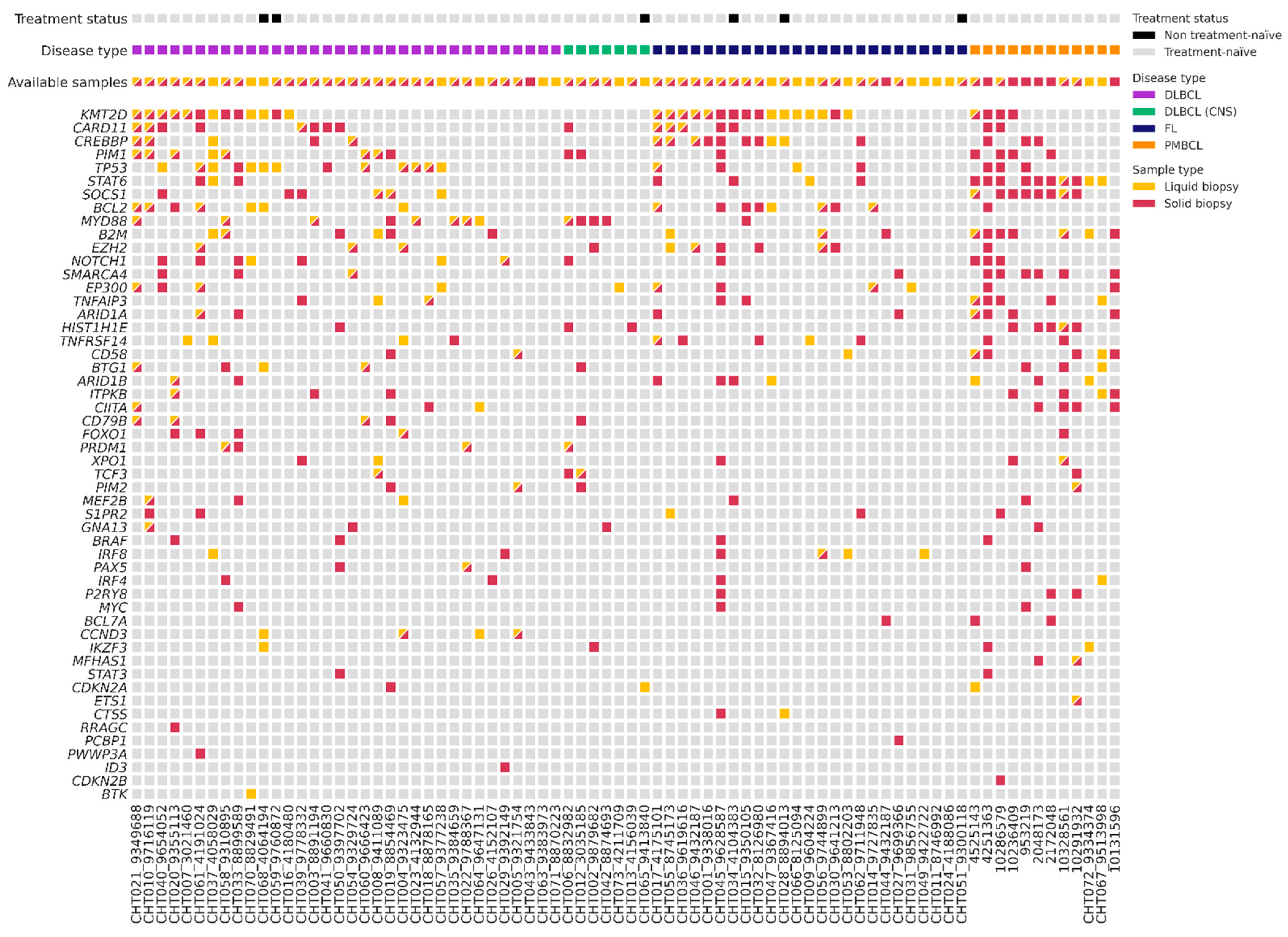
3.2. Baseline Genotyping of Plasma Samples (cfDNA) and Tissue Samples (gDNA)
3.3. Association between cfDNA Baseline Levels and Clinical Features
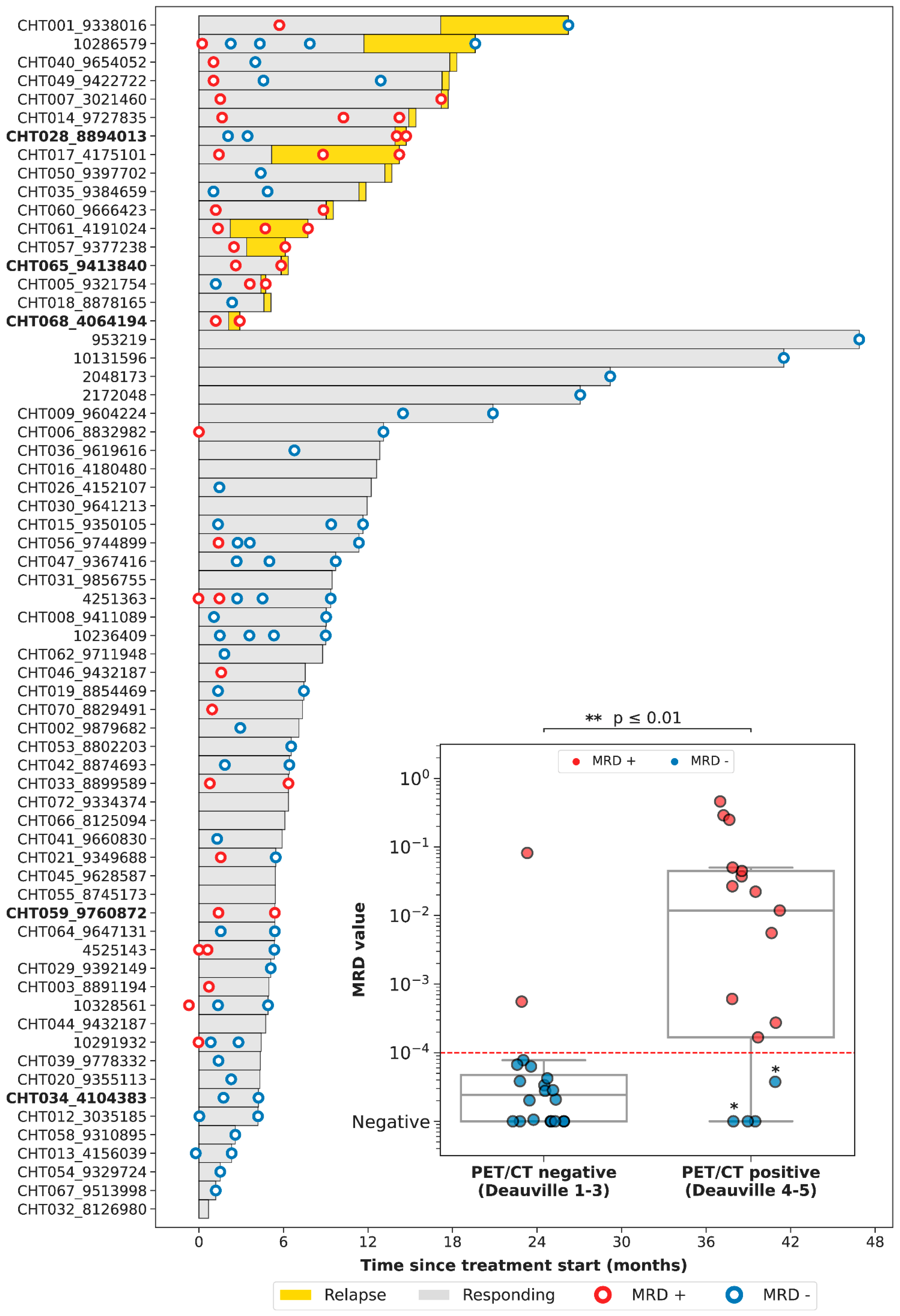
3.4. Correlation between PET/CT and LiqBio–MRD
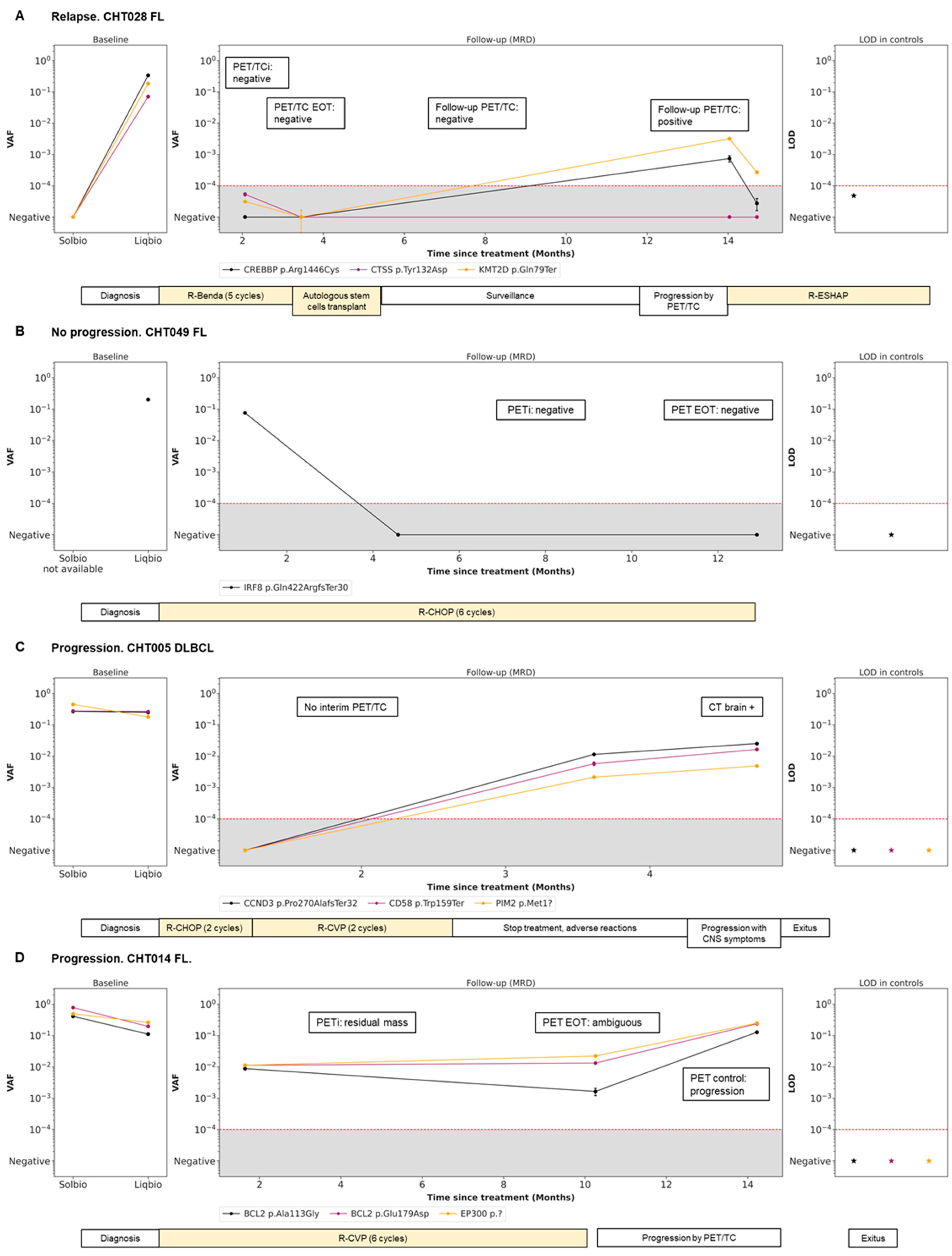
3.5. Dynamics during Follow-Up cfDNA (LiqBio–MRD): Representative Cases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, L.; Xu, H.; Wo, J.; Zhao, M.; Liu, Z.; Dong, T.; Xiao, S. Prognostic value of circulating tumor DNA in lymphoma: A meta-analysis. Clin. Exp. Med. 2021, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Heimann, P.; Dewispelaere, L. Indications of next-generation sequencing in non-Hodgkin’s lymphoma. Curr. Opin. Oncol. 2020, 32, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Shen, J.; Zhou, H.; Zhang, F.; Li, H.; Ma, Z.; Liu, T. Mutation profiling of circulating tumor DNA identifies distinct mutation patterns in non-Hodgkin lymphoma. Eur. J. Haematol. 2022, 108, 298–309. [Google Scholar] [CrossRef]
- Federico, M.; Bellei, M.; Marcheselli, L.; Luminari, S.; Lopez-Guillermo, A.; Vitolo, U.; Solal-Céligny, P. Follicular Lymphoma International Prognostic Index 2: A New Prognostic Index for Follicular Lymphoma Developed by the International Follicular Lymphoma Prognostic Factor Project. J. Clin. Oncol. 2009, 27, 4555–4562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastore, A.; Jurinovic, V.; Kridel, R.; Hoster, E.; Staiger, A.M.; Szczepanowski, M.; Weigert, O. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: A retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 2015, 16, 1111–1122. [Google Scholar] [CrossRef]
- Kurtz, D.M. Prognostication with circulating tumor DNA: Is it ready for prime time? Hematol. Am. Soc. Hematol. Educ. Program 2019, 2019, 47–52. [Google Scholar] [CrossRef]
- Reddy, A.; Zhang, J.; Davis, N.S.; Moffitt, A.B.; Love, C.L.; Waldrop, A.; Dave, S.S. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell 2017, 171, 481–494.e15. [Google Scholar] [CrossRef] [Green Version]
- Wright, G.W.; Huang, D.W.; Phelan, J.D.; Coulibaly, Z.A.; Roulland, S.; Young, R.M.; Wang, J.Q.; Staudt, L.M. A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell 2020, 37, 551–568.e14. [Google Scholar]
- Bastos-Oreiro, M.; Suárez-González, J.; Andrés-Zayas, C.; Carrión, N.C.; Moreno, S.; Carbonell, D.; Martínez-Laperche, C. Incorporation of next-generation sequencing in clinical practice using solid and liquid biopsy for patients with non-Hodgkin’s lymphoma. Sci. Rep. 2021, 11, 22815. [Google Scholar] [CrossRef]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Staudt, L.M. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Shipp, M.A. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, M.; Craig, A.F.M.; Borchmann, S.; Kurtz, D.M. Liquid biopsy in lymphoma: Molecular methods and clinical applications. Cancer Treat. Rev. 2020, 91, 102106. [Google Scholar] [CrossRef] [PubMed]
- Araf, S.; Wang, J.; Korfi, K.; Pangault, C.; Kotsiou, E.; Rio-Machin, A.; Okosun, J. Genomic profiling reveals spatial intra-tumor heterogeneity in follicular lymphoma. Leukemia 2018, 32, 1261–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, L.; Belloum, Y.; Wikman, H.; Pantel, K. Clinical relevance of blood-based ctDNA analysis: Mutation detection and beyond. Br. J. Cancer 2021, 124, 345–358. [Google Scholar] [CrossRef]
- Kurtz, D.M.; Esfahani, M.S.; Scherer, F.; Soo, J.; Jin, M.C.; Liu, C.L.; Alizadeh, A.A. Dynamic Risk Profiling Using Serial Tumor Biomarkers for Personalized Outcome Prediction. Cell 2019, 178, 699–713.e19. [Google Scholar] [CrossRef] [Green Version]
- Condoluci, A.; Rossi, D. The future of cell-free DNA testing to guide therapeutic decisions in B-cell lymphomas. Curr. Opin. Hematol. 2019, 26, 281–287. [Google Scholar] [CrossRef]
- Hou, Y.; Zi, J.; Liu, S.; Ge, Q.; Ge, Z. Mutational profiling of circulating tumor DNA and clinical characteristics in lymphoma: Based on next generation sequencing. Mol. Carcinog. 2022, 62, 200–209. [Google Scholar] [CrossRef]
- Lauer, E.M.; Mutter, J.; Scherer, F. Circulating tumor DNA in B-cell lymphoma: Technical advances, clinical applications, and perspectives for translational research. Leukemia 2022, 36, 2151–2164. [Google Scholar] [CrossRef]
- Jiménez-Ubieto, A.; Poza, M.; Martin-Muñoz, A.; Ruiz-Heredia, Y.; Dorado, S.; Figaredo, G.; Barrio, S. Real-life disease monitoring in follicular lymphoma patients using liquid biopsy ultra-deep sequencing and PET/CT. Leukemia 2023, 37, 659–669. [Google Scholar] [CrossRef]
- Fernández-Miranda, I.; Pedrosa, L.; Llanos, M.; Franco, F.F.; Gómez, S.; Martín-Acosta, P.; Sánchez-Beato, M. Monitoring of Circulating Tumor DNA Predicts Response to Treatment and Early Progression in Follicular Lymphoma: Results of a Prospective Pilot Study. Clin. Cancer Res. 2022, 29, 209–220. [Google Scholar] [CrossRef]
- Rivas-Delgado, A.; Nadeu, F.; Andrade-Campos, M.; López, C.; Enjuanes, A.; Mozas, P.; Bellosillo, B. Cell-Free DNA for Genomic Analysis in Primary Mediastinal Large B-Cell Lymphoma. Diagnostics 2022, 12, 1575. [Google Scholar] [CrossRef]
- Camus, V.; Viennot, M.; Lévêque, E.; Viailly, P.J.; Tonnelet, D.; Veresezan, E.L.; Jardin, F. Circulating tumor DNA in primary mediastinal large B-cell lymphoma versus classical Hodgkin lymphoma: A retrospective study. Leuk. Lymphoma 2022, 63, 834–844. [Google Scholar] [CrossRef]
- Huet, S.; Salles, G. Potential of Circulating Tumor DNA for the Management of Patients with Lymphoma. JCO Oncol. Pract. 2020, 16, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Hiemcke-Jiwa, L.S.; Minnema, M.C.; Radersma-van Loon, J.H.; Jiwa, N.M.; de Boer, M.; Leguit, R.J.; Huibers, M.M. The use of droplet digital PCR in liquid biopsies: A highly sensitive technique for MYD88 p.(L265P) detection in cerebrospinal fluid. Hematol. Oncol. 2018, 36, 429–435. [Google Scholar] [CrossRef]
- Hickmann, A.K. Molecular tumor analysis and liquid biopsy: A feasibility investigation analyzing circulating tumor DNA in patients with central nervous system lymphomas. BMC Cancer 2019, 12, 192. [Google Scholar]
- Decazes, P.; Camus, V.; Bohers, E.; Viailly, P.J.; Tilly, H.; Ruminy, P.; Jardin, F. Correlations between baseline 18F-FDG PET tumour parameters and circulating DNA in diffuse large B cell lymphoma and Hodgkin lymphoma. EJNMMI Res. 2020, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; World Health Organization Classification of Tumours, Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Eds.; International Agency for Research on Cancer: Lyon, France, 2017; 585p. [Google Scholar]
- Onecha, E.; Linares, M.; Rapado, I.; Ruiz-Heredia, Y.; Martinez-Sanchez, P.; Cedena, T.; Ayala, R. A novel deep targeted sequencing method for minimal residual disease monitoring in acute myeloid leukemia. Haematologica 2019, 104, 288. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Diop, F.; Spaccarotella, E.; Monti, S.; Zanni, M.; Rasi, S.; Gaidano, G. Diffuse large B-cell lymphoma genotyping on the liquid biopsy. Blood 2017, 129, 1947–1957. [Google Scholar] [CrossRef]
- Carbone, A.; Roulland, S.; Gloghini, A.; Younes, A.; von Keudell, G.; López-Guillermo, A.; Fitzgibbon, J. Follicular lymphoma. Nat. Rev. Dis. Prim. 2019, 5, 83. [Google Scholar] [CrossRef]
- Raman, L.; Van der Linden, M.; De Vriendt, C.; Van den Broeck, B.; Muylle, K.; Deeren, D.; Van Dorpe, J. Shallow-depth sequencing of cell-free DNA for Hodgkin and diffuse large B-cell lymphoma (differential) diagnosis: A standardized approach with underappreciated potential. Haematologica, 2020; ahead of print. [Google Scholar]
- Yoon, S.E.; Kim, Y.J.; Shim, J.H.; Park, D.; Cho, J.; Ko, Y.H.; Kim, S.J. Plasma Circulating Tumor DNA in Patients with Primary Central Nervous System Lymphoma. Cancer Res. Treat. 2022, 54, 597–612. [Google Scholar] [CrossRef]
- Kwok, M.; Wu, S.P.; Mo, C.; Summers, T.; Roschewski, M. Circulating Tumor DNA to Monitor Therapy for Aggressive B-Cell Lymphomas. Curr. Treat. Options Oncol. 2016, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Diaz, L.A., Jr. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas-Delgado, A.; Nadeu, F.; Enjuanes, A.; Casanueva-Eliceiry, S.; Mozas, P.; Magnano, L.; Lopez-Guillermo, A. Mutational Landscape and Tumor Burden Assessed by Cell-free DNA in Diffuse Large B-Cell Lymphoma in a Population-Based Study. Clin. Cancer Res. 2021, 27, 513–521. [Google Scholar] [CrossRef] [PubMed]
| FL | LBCL | PCNSL | Total Evaluable | |
|---|---|---|---|---|
| Sample size (n) | 25 (32%) | 46 (59%) | 7 (9%) | 78 |
| Age (median) | 62 (41–85) | 66 (19–89) | 53 (37–77) | 62 (19–89) |
| Sex | ||||
| - Male | 11 (44%) | 22 (48%) | 3 (43%) | 36 (46%) |
| - Female | 14 (66%) | 24 (52%) | 4 (47%) | 42 (54%) |
| Lines of treatment | ||||
| - Watch and Wait/unfit | 1 (4%) | 0 | 0 | 1 (1%) |
| - 1st line therapy | 21 (84%) | 44 (95%) | 6 (86%) | 71 (91%) |
| - >1st line therapy | 3 (12%) | 2 (4%) | 1 (14%) | 6 (8%) |
| Transformation | ⏀ | ⏀ | ||
| Yes | 8 (17%) | |||
| No | 36 (78%) | |||
| Ann Arbor stage | ⏀ | |||
| I–II | 6 (24%) | 21 (46%) | 27 (35%) | |
| III–IV | 19 (76%) | 23 (50%) | 42 (54%) | |
| ECOG | ||||
| 0–2 | 24 (96%) | 46 (100%) | 6 (86%) | 76 (97%) |
| >2 | 1 (4%) | 0 | 1 (14%) | 2 (3%) |
| Bulky | ⏀ | |||
| No | 17 (68%) | 21 (46%) | 38 (49%) | |
| Yes | 6 (24%) | 23 (50%) | 29 (37%) | |
| Extranodal involvement | ⏀ | |||
| Yes | 14 (56%) | 31 (67%) | 45 (58%) | |
| No | 11 (44%) | 15 (33%) | 26 (33%) | |
| Bone marrow involvement | ||||
| No | 10 (40%) | 37 (80%) | 7 (100%) | 54 (69%) |
| Yes | 14 (56%) | 6 (13%) | 0 | 20 (26%) |
| Hemoglobin (g/dL) | ||||
| ≤12 | 5 (20%) | 23 (50%) | 2 (29%) | 30 (38%) |
| >12 | 20 (80%) | 23 (50%) | 5 (71%) | 48 (62%) |
| LDH | ||||
| Elevated | 10 (40%) | 27 (59%) | 5 (71%) | 42 (54%) |
| Normal | 14 (56%) | 19 (41%) | 2 (29%) | 35 (45%) |
| β2-microglobulin | ||||
| Elevated | 14 (56%) | 28 (61%) | 3 (60%) | 45 (58%) |
| Normal | 10 (40%) | 16 (35%) | 2 (29%) | 28 (36%) |
| FLIPI/R-IPI/IELSG | ||||
| Low risk | 5 (20%) | 17 (37%) | 4 (57%) | 26 (33%) |
| Intermediate risk | 9 (36%) | 24 (52%) | 2 (29%) | 35 (45%) |
| High risk | 11 (44%) | 5 (11%) | 1 (14%) | 17 (19%) |
| Chemotherapy | ||||
| Rituximab | 1 (4%) | 0 | 0 | 1 (1%) |
| R-CHOP | 10 (40%) | 24 (52%) | 0 | 34 (44%) |
| R-COP | 0 | 1 (2%) | 0 | 1 (1%) |
| R-Benda | 10 (40%) | 0 | 0 | 10 (13%) |
| DA-EPOCH-R | 0 | 18 (39%) | 0 | 18 (23%) |
| MTX-based regime | 0 | 0 | 7 (100%) | 7 (9%) |
| Others | 2 (8%) | 2 (4%) | 0 | 4 (5%) |
| Radiotherapy | ||||
| Yes | 4 (16%) | 14 (30%) | 0 | 18 (23%) |
| No | 21 (84%) | 32 (70%) | 7 (100%) | 60 (77%) |
| CNS prophylaxis | ⏀ | |||
| Yes | 3 (12%) | 18 (39%) | 21 (27%) | |
| No | 22 (88%) | 28 (61%) | 50 (64%) | |
| Response | ||||
| Complete response | 20 (80%) | 24 (52%) | 5 (71%) | 49 (63%) |
| Partial response | 2 (8%) | 6 (13%) | 0 | 8 (10%) |
| Stable disease/progression | 2 (8%) | 9 (20%) | 1 (14%) | 12 (15%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figaredo, G.; Martín-Muñoz, A.; Barrio, S.; Parrilla, L.; Campos-Martín, Y.; Poza, M.; Rufián, L.; Algara, P.; De La Torre, M.; Jiménez Ubieto, A.; et al. Genetic Profiling of Cell-Free DNA in Liquid Biopsies: A Complementary Tool for the Diagnosis of B-Cell Lymphomas and the Surveillance of Measurable Residual Disease. Cancers 2023, 15, 4022. https://doi.org/10.3390/cancers15164022
Figaredo G, Martín-Muñoz A, Barrio S, Parrilla L, Campos-Martín Y, Poza M, Rufián L, Algara P, De La Torre M, Jiménez Ubieto A, et al. Genetic Profiling of Cell-Free DNA in Liquid Biopsies: A Complementary Tool for the Diagnosis of B-Cell Lymphomas and the Surveillance of Measurable Residual Disease. Cancers. 2023; 15(16):4022. https://doi.org/10.3390/cancers15164022
Chicago/Turabian StyleFigaredo, Gloria, Alejandro Martín-Muñoz, Santiago Barrio, Laura Parrilla, Yolanda Campos-Martín, María Poza, Laura Rufián, Patrocinio Algara, Marina De La Torre, Ana Jiménez Ubieto, and et al. 2023. "Genetic Profiling of Cell-Free DNA in Liquid Biopsies: A Complementary Tool for the Diagnosis of B-Cell Lymphomas and the Surveillance of Measurable Residual Disease" Cancers 15, no. 16: 4022. https://doi.org/10.3390/cancers15164022







