Beyond the Frontline: A Triple-Line Approach of Thoracic Surgeons in Lung Cancer Management—State of the Art
Abstract
:Simple Summary
Abstract
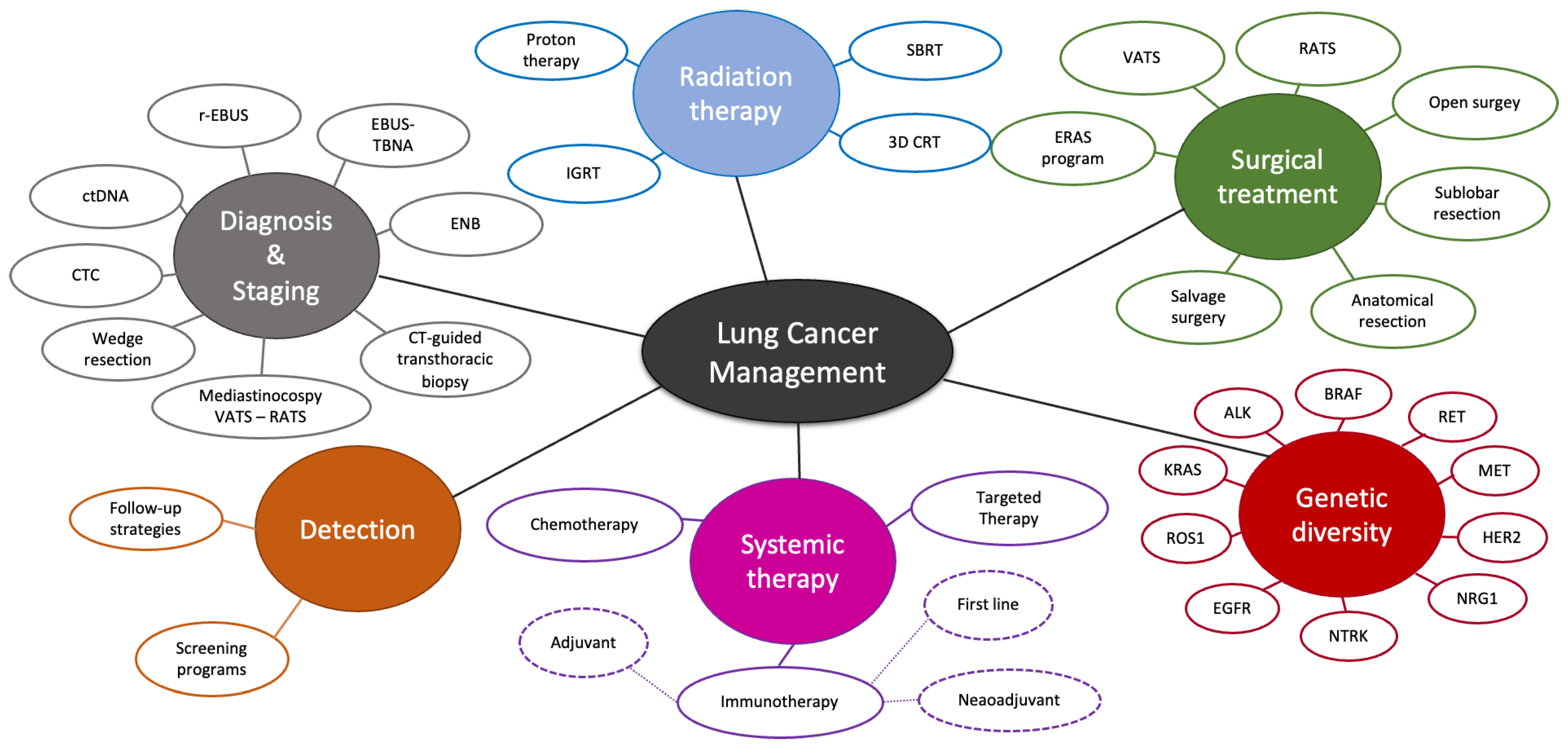
1. Introduction: State of the Art of Lung Cancer in 2023
2. Advances in Thoracic Surgery—The Evolving Landscape of First-Line Surgical Approaches
2.1. Progress in Minimally Invasive Thoracic Surgical Procedures
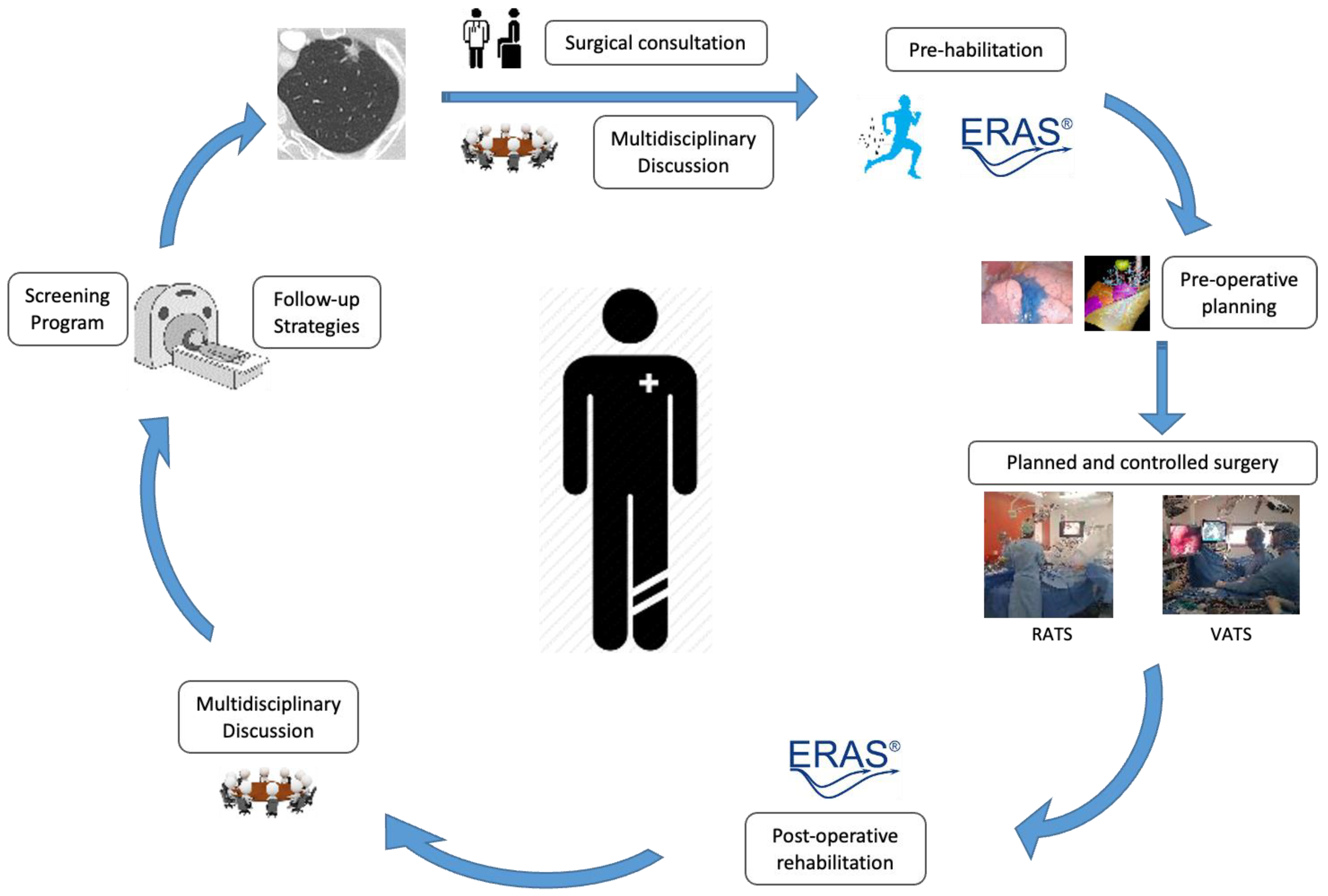
2.2. Innovation in Perioperative Management
2.2.1. Enhanced Recovery after Surgery (ERAS)
2.2.2. Prehabilitation
2.3. The Era of Precision in Thoracic Surgery: Customizing Treatment Approaches
2.3.1. The Role of Multimodal Approaches and Preoperative Planning
2.3.2. Sublobar Resection: Wedge Resection and Segmentectomy
3. Second Primary Lung Cancer and Recurrence: Approaching the Second Line
3.1. Second Primary Lung Cancer: Impact on Survival and Prognosis
3.2. Advances in Diagnostic Techniques and Surgical Approaches for Managing Second Lung Cancer
3.3. Management of Recurrence after Lung Cancer Treatment
4. Salvage Surgery in Advanced NSCLC—Third Line
4.1. Improved Outcomes in Metastatic Cancers Treated with Immunotherapy
4.2. Salvage Surgery: Safety and Feasibility
4.3. Patient Selection Criteria for Salvage or Rescue Surgery
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA. Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2022, 17, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Leleu, O.; Basille, D.; Auquier, M.; Clarot, C.; Hoguet, E.; Pétigny, V.; Addi, A.A.; Milleron, B.; Chauffert, B.; Berna, P.; et al. Lung Cancer Screening by Low-Dose CT Scan: Baseline Results of a French Prospective Study. Clin. Lung Cancer 2020, 21, 145–152. [Google Scholar] [CrossRef]
- National Lung Screening Trial Research Team. Lung Cancer Incidence and Mortality with Extended Follow-up in the National Lung Screening Trial. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 1732–1742. [Google Scholar] [CrossRef]
- Pastorino, U.; Silva, M.; Sestini, S.; Sabia, F.; Boeri, M.; Cantarutti, A.; Sverzellati, N.; Sozzi, G.; Corrao, G.; Marchianò, A. Prolonged Lung Cancer Screening Reduced 10-Year Mortality in the MILD Trial: New Confirmation of Lung Cancer Screening Efficacy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1162–1169. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and Survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Garraway, L.A. Genomics-Driven Oncology: Framework for an Emerging Paradigm. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Senft, D.; Leiserson, M.D.M.; Ruppin, E.; Ronai, Z.A. Precision Oncology: The Road Ahead. Trends Mol. Med. 2017, 23, 874–898. [Google Scholar] [CrossRef] [PubMed]
- Barlesi, F.; Mazieres, J.; Merlio, J.-P.; Debieuvre, D.; Mosser, J.; Lena, H.; Ouafik, L.; Besse, B.; Rouquette, I.; Westeel, V.; et al. Routine Molecular Profiling of Patients with Advanced Non-Small-Cell Lung Cancer: Results of a 1-Year Nationwide Programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet Lond. Engl. 2016, 387, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Oncogene-Addicted Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The Biology and Management of Non-Small Cell Lung Cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Mack, P.; Scagliotti, G.V.; Aggarwal, C.; Arcila, M.E.; Barlesi, F.; Bivona, T.; Diehn, M.; Dive, C.; Dziadziuszko, R.; et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021, 16, 1647–1662. [Google Scholar] [CrossRef]
- Wang, S.; Yang, D.M.; Rong, R.; Zhan, X.; Fujimoto, J.; Liu, H.; Minna, J.; Wistuba, I.I.; Xie, Y.; Xiao, G. Artificial Intelligence in Lung Cancer Pathology Image Analysis. Cancers 2019, 11, 1673. [Google Scholar] [CrossRef] [Green Version]
- Coudray, N.; Ocampo, P.S.; Sakellaropoulos, T.; Narula, N.; Snuderl, M.; Fenyö, D.; Moreira, A.L.; Razavian, N.; Tsirigos, A. Classification and Mutation Prediction from Non-Small Cell Lung Cancer Histopathology Images Using Deep Learning. Nat. Med. 2018, 24, 1559–1567. [Google Scholar] [CrossRef]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR-TKI Therapy in 155 Patients with EGFR-Mutant Lung Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef] [Green Version]
- Kunimasa, K.; Oka, T.; Hara, S.; Yamada, N.; Oizumi, S.; Miyashita, Y.; Kamada, R.; Funamoto, T.; Kawachi, H.; Kawamura, T.; et al. Osimertinib Is Associated with Reversible and Dose-Independent Cancer Therapy-Related Cardiac Dysfunction. Lung Cancer Amst. Neth. 2021, 153, 186–192. [Google Scholar] [CrossRef]
- Jänne, P.A.; Yang, J.C.-H.; Kim, D.-W.; Planchard, D.; Ohe, Y.; Ramalingam, S.S.; Ahn, M.-J.; Kim, S.-W.; Su, W.-C.; Horn, L.; et al. AZD9291 in EGFR Inhibitor-Resistant Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 1689–1699. [Google Scholar] [CrossRef]
- Scheffler, M.; Wiesweg, M.; Michels, S.; Nogová, L.; Kron, A.; Herold, T.; Scheel, A.H.; Metzenmacher, M.; Eberhardt, W.E.; Reis, H.; et al. Rebiopsy in Advanced Non-Small Cell Lung Cancer, Clinical Relevance and Prognostic Implications. Lung Cancer 2022, 168, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.H.; Kim, H.R.; Ahn, B.C.; Heo, S.J.; Kim, J.H.; Cho, B.C. Real-World Analysis of the Efficacy of Rebiopsy and EGFR Mutation Test of Tissue and Plasma Samples in Drug-Resistant Non-Small Cell Lung Cancer. Yonsei Med. J. 2019, 60, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef] [Green Version]
- Horn, L.; Spigel, D.R.; Vokes, E.E.; Holgado, E.; Ready, N.; Steins, M.; Poddubskaya, E.; Borghaei, H.; Felip, E.; Paz-Ares, L.; et al. Nivolumab Versus Docetaxel in Previously Treated Patients with Advanced Non–Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J. Clin. Oncol. 2017, 35, 3924–3933. [Google Scholar] [CrossRef]
- Xi, J.; Du, Y.; Hu, Z.; Liang, J.; Bian, Y.; Chen, Z.; Sui, Q.; Zhan, C.; Li, M.; Guo, W. Long-Term Outcomes Following Neoadjuvant or Adjuvant Chemoradiotherapy for Stage I-IIIA Non-Small Cell Lung Cancer: A Propensity-Matched Analysis. J. Thorac. Dis. 2020, 12, 3043–3056. [Google Scholar] [CrossRef]
- Shu, C.A.; Gainor, J.F.; Awad, M.M.; Chiuzan, C.; Grigg, C.M.; Pabani, A.; Garofano, R.F.; Stoopler, M.B.; Cheng, S.K.; White, A.; et al. Neoadjuvant Atezolizumab and Chemotherapy in Patients with Resectable Non-Small-Cell Lung Cancer: An Open-Label, Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol. 2020, 21, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant Atezolizumab after Adjuvant Chemotherapy in Resected Stage IB-IIIA Non-Small-Cell Lung Cancer (IMpower010): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet Lond. Engl. 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Saw, S.P.L.; Ong, B.-H.; Chua, K.L.M.; Takano, A.; Tan, D.S.W. Revisiting Neoadjuvant Therapy in Non-Small-Cell Lung Cancer. Lancet Oncol. 2021, 22, e501–e516. [Google Scholar] [CrossRef]
- Gaudreau, P.-O.; Negrao, M.V.; Mitchell, K.G.; Reuben, A.; Corsini, E.M.; Li, J.; Karpinets, T.V.; Wang, Q.; Diao, L.; Wang, J.; et al. Neoadjuvant Chemotherapy Increases Cytotoxic T Cell, Tissue Resident Memory T Cell, and B Cell Infiltration in Resectable NSCLC. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021, 16, 127–139. [Google Scholar] [CrossRef]
- Travis, W.D.; Dacic, S.; Wistuba, I.; Sholl, L.; Adusumilli, P.; Bubendorf, L.; Bunn, P.; Cascone, T.; Chaft, J.; Chen, G.; et al. Iaslc multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens following neoadjuvant therapy. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 709–740. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- El Husseini, K.; Piton, N.; De Marchi, M.; Grégoire, A.; Vion, R.; Blavier, P.; Thiberville, L.; Baste, J.-M.; Guisier, F. Lung Cancer Surgery after Treatment with Anti-PD1/PD-L1 Immunotherapy for Non-Small-Cell Lung Cancer: A Case—Cohort Study. Cancers 2021, 13, 4915. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.S.; Carnochan, F.M.; Tin, M. Thoracoscopy Assisted Pulmonary Lobectomy. Thorax 1993, 48, 921–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giudicelli, R.; Thomas, P.; Lonjon, T.; Ragni, J.; Bulgare, J.C.; Ottomani, R.; Fuentes, P. Major Pulmonary Resection by Video Assisted Mini-Thoracotomy. Initial Experience in 35 Patients. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 1994, 8, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Vansteenkiste, J.; Crinò, L.; Dooms, C.; Douillard, J.Y.; Faivre-Finn, C.; Lim, E.; Rocco, G.; Senan, S.; Van Schil, P.; Veronesi, G.; et al. 2nd ESMO Consensus Conference on Lung Cancer: Early-Stage Non-Small-Cell Lung Cancer Consensus on Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1462–1474. [Google Scholar] [CrossRef]
- Remon, J.; Soria, J.-C.; Peters, S. Early and Locally Advanced Non-Small-Cell Lung Cancer: An Update of the ESMO Clinical Practice Guidelines Focusing on Diagnosis, Staging, Systemic and Local Therapy. Ann. Oncol. 2021, 32, 1637–1642. [Google Scholar] [CrossRef]
- Pagès, P.-B.; Delpy, J.-P.; Orsini, B.; Gossot, D.; Baste, J.-M.; Thomas, P.; Dahan, M.; Bernard, A. Epithor Project French Society of Thoracic and Cardiovascular Surgery Propensity Score Analysis Comparing Videothoracoscopic Lobectomy with Thoracotomy: A French Nationwide Study. Ann. Thorac. Surg. 2016, 101, 1370–1378. [Google Scholar] [CrossRef]
- Bendixen, M.; Jørgensen, O.D.; Kronborg, C.; Andersen, C.; Licht, P.B. Postoperative Pain and Quality of Life after Lobectomy via Video-Assisted Thoracoscopic Surgery or Anterolateral Thoracotomy for Early Stage Lung Cancer: A Randomised Controlled Trial. Lancet Oncol. 2016, 17, 836–844. [Google Scholar] [CrossRef]
- Lim, E.; Batchelor, T.; Shackcloth, M.; Dunning, J.; McGonigle, N.; Brush, T.; Dabner, L.; Harris, R.; Mckeon, H.E.; Paramasivan, S.; et al. Study Protocol for VIdeo Assisted Thoracoscopic Lobectomy versus Conventional Open LobEcTomy for Lung Cancer, a UK Multicentre Randomised Controlled Trial with an Internal Pilot (the VIOLET Study). BMJ Open 2019, 9, e029507. [Google Scholar] [CrossRef] [Green Version]
- Lim, E.; Harris, R.A.; McKeon, H.E.; Batchelor, T.J.; Dunning, J.; Shackcloth, M.; Anikin, V.; Naidu, B.; Belcher, E.; Loubani, M.; et al. Impact of Video-Assisted Thoracoscopic Lobectomy versus Open Lobectomy for Lung Cancer on Recovery Assessed Using Self-Reported Physical Function: VIOLET RCT. Health Technol. Assess. Winch. Engl. 2022, 26, 1–162. [Google Scholar] [CrossRef] [PubMed]
- Falcoz, P.-E.; Puyraveau, M.; Thomas, P.-A.; Decaluwe, H.; Hürtgen, M.; Petersen, R.H.; Hansen, H.; Brunelli, A. ESTS Database Committee and ESTS Minimally Invasive Interest Group Video-Assisted Thoracoscopic Surgery versus Open Lobectomy for Primary Non-Small-Cell Lung Cancer: A Propensity-Matched Analysis of Outcome from the European Society of Thoracic Surgeon Database. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2016, 49, 602–609. [Google Scholar] [CrossRef]
- O’Sullivan, K.E.; Kreaden, U.S.; Hebert, A.E.; Eaton, D.; Redmond, K.C. A Systematic Review and Meta-Analysis of Robotic versus Open and Video-Assisted Thoracoscopic Surgery Approaches for Lobectomy. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 526–534. [Google Scholar] [CrossRef]
- Huang, L.; Shen, Y.; Onaitis, M. Comparative Study of Anatomic Lung Resection by Robotic vs. Video-Assisted Thoracoscopic Surgery. J. Thorac. Dis. 2019, 11, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S.H.; MacDonald, J.K.; Gilbert, S.; Khan, A.Z.; Kim, Y.T.; Louie, B.E.; Blair Marshall, M.; Santos, R.S.; Scarci, M.; Shargal, Y.; et al. Optimal Approach to Lobectomy for Non-Small Cell Lung Cancer: Systemic Review and Meta-Analysis. Innovations 2019, 14, 90–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Li, X.; Zhao, S.; Wang, J.; Zhang, W.; Sun, G. Robot-Assisted Thoracic Surgery versus Video-Assisted Thoracic Surgery for Lung Lobectomy or Segmentectomy in Patients with Non-Small Cell Lung Cancer: A Meta-Analysis. BMC Cancer 2021, 21, 498. [Google Scholar] [CrossRef]
- Montagne, F.; Chaari, Z.; Bottet, B.; Sarsam, M.; Mbadinga, F.; Selim, J.; Guisier, F.; Gillibert, A.; Baste, J.-M. Long-Term Survival Following Minimally Invasive Lung Cancer Surgery: Comparing Robotic-Assisted and Video-Assisted Surgery. Cancers 2022, 14, 2611. [Google Scholar] [CrossRef]
- Kneuertz, P.J.; D’Souza, D.M.; Richardson, M.; Abdel-Rasoul, M.; Moffatt-Bruce, S.D.; Merritt, R.E. Long-Term Oncologic Outcomes After Robotic Lobectomy for Early-Stage Non–Small-Cell Lung Cancer Versus Video-Assisted Thoracoscopic and Open Thoracotomy Approach. Clin. Lung Cancer 2020, 21, 214–224.e2. [Google Scholar] [CrossRef]
- Kent, M.S.; Hartwig, M.G.; Vallières, E.; Abbas, A.E.; Cerfolio, R.J.; Dylewski, M.R.; Fabian, T.; Herrera, L.J.; Jett, K.G.; Lazzaro, R.S.; et al. Pulmonary Open, Robotic, and Thoracoscopic Lobectomy (PORTaL) Study: An Analysis of 5721 Cases. Ann. Surg. 2023, 277, 528–533. [Google Scholar] [CrossRef]
- Tang, A.; Raja, S.; Bribriesco, A.C.; Raymond, D.P.; Sudarshan, M.; Murthy, S.C.; Ahmad, U. Robotic Approach Offers Similar Nodal Upstaging to Open Lobectomy for Clinical Stage I Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2020, 110, 424–433. [Google Scholar] [CrossRef]
- Hennon, M.W.; DeGraaff, L.H.; Groman, A.; Demmy, T.L.; Yendamuri, S. The Association of Nodal Upstaging with Surgical Approach and Its Impact on Long-Term Survival after Resection of Non-Small-Cell Lung Cancer. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2020, 57, 888–895. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, Y.; Jiang, H.; Xu, J.; Yu, D. Thoracotomy Is Better than Thoracoscopic Lobectomy in the Lymph Node Dissection of Lung Cancer: A Systematic Review and Meta-Analysis. World J. Surg. Oncol. 2016, 14, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.-F.J.; Kumar, A.; Deng, J.Z.; Raman, V.; Lui, N.S.; D’Amico, T.A.; Berry, M.F. A National Analysis of Short-Term Outcomes and Long-Term Survival Following Thoracoscopic Versus Open Lobectomy for Clinical Stage II Non-Small-Cell Lung Cancer. Ann. Surg. 2021, 273, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Zirafa, C.; Aprile, V.; Ricciardi, S.; Romano, G.; Davini, F.; Cavaliere, I.; Alì, G.; Fontanini, G.; Melfi, F. Nodal Upstaging Evaluation in NSCLC Patients Treated by Robotic Lobectomy. Surg. Endosc. 2019, 33, 153–158. [Google Scholar] [CrossRef]
- Medbery, R.L.; Gillespie, T.W.; Liu, Y.; Nickleach, D.C.; Lipscomb, J.; Sancheti, M.S.; Pickens, A.; Force, S.D.; Fernandez, F.G. Nodal Upstaging Is More Common with Thoracotomy than with VATS During Lobectomy for Early-Stage Lung Cancer: An Analysis from the National Cancer Data Base. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Kneuertz, P.J.; Cheufou, D.H.; D’Souza, D.M.; Mardanzai, K.; Abdel-Rasoul, M.; Theegarten, D.; Moffatt-Bruce, S.D.; Aigner, C.; Merritt, R.E. Propensity-Score Adjusted Comparison of Pathologic Nodal Upstaging by Robotic, Video-Assisted Thoracoscopic, and Open Lobectomy for Non-Small Cell Lung Cancer. J. Thorac. Cardiovasc. Surg. 2019, 158, 1457–1466.e2. [Google Scholar] [CrossRef] [PubMed]
- Durey, B.; Djerada, Z.; Boujibar, F.; Besnier, E.; Montagne, F.; Baste, J.-M.; Dusseaux, M.-M.; Compere, V.; Clavier, T.; Selim, J. Erector Spinae Plane Block versus Paravertebral Block after Thoracic Surgery for Lung Cancer: A Propensity Score Study. Cancers 2023, 15, 2306. [Google Scholar] [CrossRef]
- Weiss, W. Operative Mortality and Five-Year Survival Rates in Men with Bronchogenic Carcinoma. Chest 1974, 66, 483–487. [Google Scholar] [CrossRef]
- Pagès, P.-B.; Cottenet, J.; Mariet, A.-S.; Bernard, A.; Quantin, C. In-Hospital Mortality Following Lung Cancer Resection: Nationwide Administrative Database. Eur. Respir. J. 2016, 47, 1809–1817. [Google Scholar] [CrossRef]
- Berg, E.; Madelaine, L.; Baste, J.-M.; Dahan, M.; Thomas, P.; Falcoz, P.-E.; Martinod, E.; Bernard, A.; Pagès, P.-B. Interest of Anatomical Segmentectomy over Lobectomy for Lung Cancer: A Nationwide Study. J. Thorac. Dis. 2021, 13, 3587–3596. [Google Scholar] [CrossRef]
- Dumitra, T.-C.; Molina, J.-C.; Mouhanna, J.; Nicolau, I.; Renaud, S.; Aubin, L.; Siblini, A.; Mulder, D.; Ferri, L.; Spicer, J. Feasibility Analysis for the Development of a Video-Assisted Thoracoscopic (VATS) Lobectomy 23-Hour Recovery Pathway. Can. J. Surg. J. Can. Chir. 2020, 63, E349–E358. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H. Multimodal Approach to Control Postoperative Pathophysiology and Rehabilitation. Br. J. Anaesth. 1997, 78, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Eckardt, R.; Scholtz, K.; Neuner, B.; von Dossow-Hanfstingl, V.; Sehouli, J.; Stief, C.G.; Wernecke, K.-D.; Spies, C.D. PERATECS Group Patient Empowerment Improved Perioperative Quality of Care in Cancer Patients Aged ≥ 65 Years—A Randomized Controlled Trial. PLoS ONE 2015, 10, e0137824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crabtree, T.D.; Puri, V.; Bell, J.M.; Bontumasi, N.; Patterson, G.A.; Kreisel, D.; Krupnick, A.S.; Meyers, B.F. Outcomes and Perception of Lung Surgery with Implementation of a Patient Video Education Module: A Prospective Cohort Study. J. Am. Coll. Surg. 2012, 214, 816–821.e2. [Google Scholar] [CrossRef]
- Salati, M.; Brunelli, A.; Xiumè, F.; Refai, M.; Pompili, C.; Sabbatini, A. Does Fast-Tracking Increase the Readmission Rate after Pulmonary Resection? A Case-Matched Study. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2012, 41, 1083–1087; discussion 1087. [Google Scholar] [CrossRef] [Green Version]
- Muehling, B.M.; Halter, G.L.; Schelzig, H.; Meierhenrich, R.; Steffen, P.; Sunder-Plassmann, L.; Orend, K.-H. Reduction of Postoperative Pulmonary Complications after Lung Surgery Using a Fast Track Clinical Pathway. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2008, 34, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Gravier, F.-E.; Smondack, P.; Prieur, G.; Medrinal, C.; Combret, Y.; Muir, J.-F.; Baste, J.-M.; Cuvelier, A.; Boujibar, F.; Bonnevie, T. Effects of Exercise Training in People with Non-Small Cell Lung Cancer before Lung Resection: A Systematic Review and Meta-Analysis. Thorax 2022, 77, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Boujibar, F.; Gillibert, A.; Gravier, F.E.; Gillot, T.; Bonnevie, T.; Cuvelier, A.; Baste, J.-M. Performance at Stair-Climbing Test Is Associated with Postoperative Complications after Lung Resection: A Systematic Review and Meta-Analysis. Thorax 2020, 75, 791–797. [Google Scholar] [CrossRef]
- Sebio García, R.; Yáñez-Brage, M.I.; Giménez Moolhuyzen, E.; Salorio Riobo, M.; Lista Paz, A.; Borro Mate, J.M. Preoperative Exercise Training Prevents Functional Decline after Lung Resection Surgery: A Randomized, Single-Blind Controlled Trial. Clin. Rehabil. 2017, 31, 1057–1067. [Google Scholar] [CrossRef]
- Rochester, C.L.; Vogiatzis, I.; Holland, A.E.; Lareau, S.C.; Marciniuk, D.D.; Puhan, M.A.; Spruit, M.A.; Masefield, S.; Casaburi, R.; Clini, E.M.; et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: Enhancing Implementation, Use, and Delivery of Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2015, 192, 1373–1386. [Google Scholar] [CrossRef] [Green Version]
- Gravier, F.-E.; Smondack, P.; Boujibar, F.; Prieur, G.; Medrinal, C.; Combret, Y.; Muir, J.-F.; Baste, J.-M.; Cuvelier, A.; Debeaumont, D.; et al. Prehabilitation Sessions Can Be Provided More Frequently in a Shortened Regimen with Similar or Better Efficacy in People with Non-Small Cell Lung Cancer: A Randomised Trial. J. Physiother. 2022, 68, 43–50. [Google Scholar] [CrossRef]
- Brunelli, A.; Charloux, A.; Bolliger, C.T.; Rocco, G.; Sculier, J.-P.; Varela, G.; Licker, M.; Ferguson, M.K.; Faivre-Finn, C.; Huber, R.M.; et al. ERS/ESTS Clinical Guidelines on Fitness for Radical Therapy in Lung Cancer Patients (Surgery and Chemo-Radiotherapy). Eur. Respir. J. 2009, 34, 17–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boujibar, F.; Gravier, F.-E.; Selim, J.; Baste, J.-M. Preoperative Assessment for Minimally Invasive Lung Surgery: Need an Update? Thorac. Cancer 2021, 12, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Fennelly, J.; Potter, L.; Pompili, C.; Brunelli, A. Performance in the Shuttle Walk Test Is Associated with Cardiopulmonary Complications after Lung Resections. J. Thorac. Dis. 2017, 9, 789–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mainguene, J.; Basse, C.; Girard, P.; Beaucaire-Danel, S.; Cao, K.; Brian, E.; Grigoroiu, M.; Gossot, D.; Luporsi, M.; Perrot, L.; et al. Surgical or Medical Strategy for Locally-Advanced, Stage IIIA/B-N2 Non-Small Cell Lung Cancer: Reproducibility of Decision-Making at a Multidisciplinary Tumor Board. Lung Cancer Amst. Neth. 2022, 163, 51–58. [Google Scholar] [CrossRef]
- Rusch, V.W. Initiating the Era of “Precision” Lung Cancer Surgery. N. Engl. J. Med. 2023, 388, 557–558. [Google Scholar] [CrossRef]
- Montagne, F.; Guisier, F.; Venissac, N.; Baste, J.-M. The Role of Surgery in Lung Cancer Treatment: Present Indications and Future Perspectives—State of the Art. Cancers 2021, 13, 3711. [Google Scholar] [CrossRef]
- Sarsam, M.; Baste, J.-M.; Thiberville, L.; Salaun, M.; Lachkar, S. How Bronchoscopic Dye Marking Can Help Minimally Invasive Lung Surgery. J. Clin. Med. 2022, 11, 3246. [Google Scholar] [CrossRef]
- Baste, J.M.; Soldea, V.; Lachkar, S.; Rinieri, P.; Sarsam, M.; Bottet, B.; Peillon, C. Development of a Precision Multimodal Surgical Navigation System for Lung Robotic Segmentectomy. J. Thorac. Dis. 2018, 10, S1195–S1204. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, T.; Sato, T.; Shimizu, K. Technical Advances in Segmentectomy for Lung Cancer: A Minimally Invasive Strategy for Deep, Small, and Impalpable Tumors. Cancers 2021, 13, 3137. [Google Scholar] [CrossRef]
- Lachkar, S.; Baste, J.-M.; Thiberville, L.; Peillon, C.; Rinieri, P.; Piton, N.; Guisier, F.; Salaun, M. Pleural Dye Marking Using Radial Endobronchial Ultrasound and Virtual Bronchoscopy before Sublobar Pulmonary Resection for Small Peripheral Nodules. Respir. Int. Rev. Thorac. Dis. 2018, 95, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Aoun, H.D.; Littrup, P.J.; Heath, K.E.; Adam, B.; Prus, M.; Beydoun, R.; Baciewcz, F. Methylene Blue/Collagen Mixture for CT-Guided Presurgical Lung Nodule Marking: High Efficacy and Safety. J. Vasc. Interv. Radiol. JVIR 2020, 31, 1682.e1–1682.e7. [Google Scholar] [CrossRef]
- Nardini, M.; Bilancia, R.; Paul, I.; Jayakumar, S.; Papoulidis, P.; ElSaegh, M.; Hartley, R.; Richardson, M.; Misra, P.; Migliore, M.; et al. 99 mTechnetium and Methylene Blue Guided Pulmonary Nodules Resections: Preliminary British Experience. J. Thorac. Dis. 2018, 10, 1015–1021. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Lin, Y.; Zheng, L.; Liang, Y.; Zhao, L.; Wen, Y.; Zhang, R.; Huang, Z.; Yang, L.; Zhao, D.; et al. A New Method for Accurately Localizing and Resecting Pulmonary Nodules. J. Thorac. Dis. 2020, 12, 4973–4984. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, C.; Yue, W.; Lu, M.; Tian, H. Comparison of Computed Tomographic Imaging-Guided Hook Wire Localization and Electromagnetic Navigation Bronchoscope Localization in the Resection of Pulmonary Nodules: A Retrospective Cohort Study. Sci. Rep. 2020, 10, 21459. [Google Scholar] [CrossRef]
- Mariolo, A.V.; Vieira, T.; Stern, J.-B.; Perrot, L.; Caliandro, R.; Escande, R.; Brian, E.; Grigoroiu, M.; Boddaert, G.; Gossot, D.; et al. Electromagnetic Navigation Bronchoscopy Localization of Lung Nodules for Thoracoscopic Resection. J. Thorac. Dis. 2021, 13, 4371–4377. [Google Scholar] [CrossRef]
- Piolanti, M.; Coppola, F.; Papa, S.; Pilotti, V.; Mattioli, S.; Gavelli, G. Ultrasonographic Localization of Occult Pulmonary Nodules during Video-Assisted Thoracic Surgery. Eur. Radiol. 2003, 13, 2358–2364. [Google Scholar] [CrossRef] [PubMed]
- Gossot, D.; Lafouasse, C.; Kovacs, E.; Seguin-Givelet, A. Sublobar Resection for Early-Stage Lung Cancer: The Issue of Safety Margins. Eur. J. Cardiothorac. Surg. 2023, 63, ezad055. [Google Scholar] [CrossRef]
- Brunelli, A.; Decaluwe, H.; Gonzalez, M.; Gossot, D.; Petersen, R.H.; Augustin, F.; Assouad, J.; Baste, J.M.; Batirel, H.; Falcoz, P.E.; et al. European Society of Thoracic Surgeons Expert Consensus Recommendations on Technical Standards of Segmentectomy for Primary Lung Cancer. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2023, 63, ezad224. [Google Scholar] [CrossRef]
- Decaluwe, H.; Petersen, R.H.; Hansen, H.; Piwkowski, C.; Augustin, F.; Brunelli, A.; Schmid, T.; Papagiannopoulos, K.; Moons, J.; Gossot, D.; et al. Major Intraoperative Complications during Video-Assisted Thoracoscopic Anatomical Lung Resections: An Intention-to-Treat Analysis. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2015, 48, 588–598; discussion 599. [Google Scholar] [CrossRef] [Green Version]
- Bottet, B.; Rivera, C.; Dahan, M.; Falcoz, P.-E.; Jaillard, S.; Baste, J.-M.; Seguin-Givelet, A.; de la Tour, R.B.; Bellenot, F.; Rind, A.; et al. Reporting of Patient Safety Incidents in Minimally Invasive Thoracic Surgery: A National Registered Thoracic Surgeons Experience for Improvement of Patient Safety. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac129. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Chandrakumar, D.; Gupta, S.; Yan, T.D.; Tian, D.H. Could Less Be More?—A Systematic Review and Meta-Analysis of Sublobar Resections versus Lobectomy for Non-Small Cell Lung Cancer According to Patient Selection. Lung Cancer Amst. Neth. 2015, 89, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Winckelmans, T.; Decaluwé, H.; De Leyn, P.; Van Raemdonck, D. Segmentectomy or Lobectomy for Early-Stage Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2020, 57, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Saji, H.; Aokage, K.; Watanabe, S.-I.; Okada, M.; Mizusawa, J.; Nakajima, R.; Tsuboi, M.; Nakamura, S.; Nakamura, K.; et al. Comparison of Pulmonary Segmentectomy and Lobectomy: Safety Results of a Randomized Trial. J. Thorac. Cardiovasc. Surg. 2019, 158, 895–907. [Google Scholar] [CrossRef]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus Lobectomy in Small-Sized Peripheral Non-Small-Cell Lung Cancer (JCOG0802/WJOG4607L): A Multicentre, Open-Label, Phase 3, Randomised, Controlled, Non-Inferiority Trial. Lancet Lond. Engl. 2022, 399, 1607–1617. [Google Scholar] [CrossRef]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jones, D.R.; Conti, M.; Ashrafi, A.S.; et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef]
- Choi, E.; Luo, S.J.; Aredo, J.V.; Backhus, L.M.; Wilkens, L.R.; Su, C.C.; Neal, J.W.; Le Marchand, L.; Cheng, I.; Wakelee, H.A.; et al. The Survival Impact of Second Primary Lung Cancer in Patients with Lung Cancer. JNCI J. Natl. Cancer Inst. 2021, 114, 618–625. [Google Scholar] [CrossRef]
- Johnson, B.E. Second Lung Cancers in Patients after Treatment for an Initial Lung Cancer. J. Natl. Cancer Inst. 1998, 90, 1335–1345. [Google Scholar] [CrossRef]
- Zakowski, M.F.; Ladanyi, M.; Kris, M.G. EGFR Mutations in Small-Cell Lung Cancers in Patients Who Have Never Smoked. N. Engl. J. Med. 2006, 355, 213–215. [Google Scholar] [CrossRef]
- Yin, X.; Li, Y.; Wang, H.; Jia, T.; Wang, E.; Luo, Y.; Wei, Y.; Qin, Z.; Ma, X. Small Cell Lung Cancer Transformation: From Pathogenesis to Treatment. Semin. Cancer Biol. 2022, 86, 595–606. [Google Scholar] [CrossRef]
- Nicholson, S.A.; Beasley, M.B.; Brambilla, E.; Hasleton, P.S.; Colby, T.V.; Sheppard, M.N.; Falk, R.; Travis, W.D. Small Cell Lung Carcinoma (SCLC): A Clinicopathologic Study of 100 Cases with Surgical Specimens. Am. J. Surg. Pathol. 2002, 26, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Oser, M.G.; Niederst, M.J.; Sequist, L.V.; Engelman, J.A. Transformation from Non-Small-Cell Lung Cancer to Small-Cell Lung Cancer: Molecular Drivers and Cells of Origin. Lancet Oncol. 2015, 16, e165–e172. [Google Scholar] [CrossRef] [Green Version]
- Clamon, G.; Zeitler, W.; An, J.; Hejleh, T.A. Transformational Changes between Non-Small Cell and Small Cell Lung Cancer-Biological and Clinical Relevance-A Review. Am. J. Clin. Oncol. 2020, 43, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Marcoux, N.; Gettinger, S.N.; O’Kane, G.; Arbour, K.C.; Neal, J.W.; Husain, H.; Evans, T.L.; Brahmer, J.R.; Muzikansky, A.; Bonomi, P.D.; et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 278–285. [Google Scholar] [CrossRef]
- Roca, E.; Gurizzan, C.; Amoroso, V.; Vermi, W.; Ferrari, V.; Berruti, A. Outcome of Patients with Lung Adenocarcinoma with Transformation to Small-Cell Lung Cancer Following Tyrosine Kinase Inhibitors Treatment: A Systematic Review and Pooled Analysis. Cancer Treat. Rev. 2017, 59, 117–122. [Google Scholar] [CrossRef]
- De Leyn, P.; Dooms, C.; Kuzdzal, J.; Lardinois, D.; Passlick, B.; Rami-Porta, R.; Turna, A.; Van Schil, P.; Venuta, F.; Waller, D.; et al. Revised ESTS Guidelines for Preoperative Mediastinal Lymph Node Staging for Non-Small-Cell Lung Cancer. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2014, 45, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Lachkar, S.; Perrot, L.; Gervereau, D.; De Marchi, M.; Morisse Pradier, H.; Dantoing, E.; Piton, N.; Thiberville, L.; Guisier, F.; Salaün, M. Radial-EBUS and Virtual Bronchoscopy Planner for Peripheral Lung Cancer Diagnosis: How It Became the First-Line Endoscopic Procedure. Thorac. Cancer 2022, 13, 2854–2860. [Google Scholar] [CrossRef]
- Fournier, C.; Hermant, C.; Gounant, V.; Escarguel, B.; Thibout, Y.; Lachkar, S.; Raspaud, C.; Vergnon, J.-M. Diagnostic of Mediastinal Lymphadenopathy in Extrathoracic Cancer: A Place for EBUS-TBNA in Real Life Practice? Respir. Med. Res. 2019, 75, 1–4. [Google Scholar] [CrossRef]
- Navani, N.; Spiro, S.G.; Janes, S.M. Mediastinal Staging of NSCLC with Endoscopic and Endobronchial Ultrasound. Nat. Rev. Clin. Oncol. 2009, 6, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Yarmus, L.; Akulian, J.; Wahidi, M.; Chen, A.; Steltz, J.P.; Solomon, S.L.; Yu, D.; Maldonado, F.; Cardenas-Garcia, J.; Molena, D.; et al. A Prospective Randomized Comparative Study of Three Guided Bronchoscopic Approaches for Investigating Pulmonary Nodules: The PRECISION-1 Study. Chest 2020, 157, 694–701. [Google Scholar] [CrossRef]
- Simoff, M.J.; Pritchett, M.A.; Reisenauer, J.S.; Ost, D.E.; Majid, A.; Keyes, C.; Casal, R.F.; Parikh, M.S.; Diaz-Mendoza, J.; Fernandez-Bussy, S.; et al. Shape-Sensing Robotic-Assisted Bronchoscopy for Pulmonary Nodules: Initial Multicenter Experience Using the IonTM Endoluminal System. BMC Pulm. Med. 2021, 21, 322. [Google Scholar] [CrossRef] [PubMed]
- Manhire, A.; Charig, M.; Clelland, C.; Gleeson, F.; Miller, R.; Moss, H.; Pointon, K.; Richardson, C.; Sawicka, E. BTS Guidelines for Radiologically Guided Lung Biopsy. Thorax 2003, 58, 920–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Liang, Z.; Li, P.; Wang, Q. CT-Guided Core Needle Biopsy of Mediastinal Nodes through a Transpulmonary Approach: Retrospective Analysis of the Procedures Conducted over Six Years. Eur. Radiol. 2017, 27, 3401–3407. [Google Scholar] [CrossRef]
- Zakkar, M.; Tan, C.; Hunt, I. Is Video Mediastinoscopy a Safer and More Effective Procedure than Conventional Mediastinoscopy? Interact. Cardiovasc. Thorac. Surg. 2012, 14, 81–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardet, J.; Zaimi, R.; Dakhil, B.; Couffinhal, J.C.; Raynaud, C.; Bagan, P. Outpatient thoracoscopic resection of lung nodules within a fast-track recovery program. Rev. Mal. Respir. 2016, 33, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Bagan, P.; Berna, P.; De Dominicis, F.; Lafitte, S.; Zaimi, R.; Dakhil, B.; Das Neves Pereira, J.-C. Outpatient thoracic surgery: Evolution of the indications, current applications and limits. Rev. Mal. Respir. 2016, 33, 899–904. [Google Scholar] [CrossRef]
- Abid, W.; Seguin-Givelet, A.; Brian, E.; Grigoroiu, M.; Girard, P.; Girard, N.; Gossot, D. Second Pulmonary Resection for a Second Primary Lung Cancer: Analysis of Morbidity and Survival. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2021, 59, 1287–1294. [Google Scholar] [CrossRef]
- Endo, C.; Sakurada, A.; Notsuda, H.; Noda, M.; Hoshikawa, Y.; Okada, Y.; Kondo, T. Results of Long-Term Follow-Up of Patients with Completely Resected Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2012, 93, 1061–1068. [Google Scholar] [CrossRef]
- Gourcerol, D.; Scherpereel, A.; Debeugny, S.; Porte, H.; Cortot, A.B.; Lafitte, J.-J. Relevance of an Extensive Follow-up after Surgery for Nonsmall Cell Lung Cancer. Eur. Respir. J. 2013, 42, 1357–1364. [Google Scholar] [CrossRef]
- Jeong, W.G.; Choi, H.; Chae, K.J.; Kim, J. Prognosis and Recurrence Patterns in Patients with Early Stage Lung Cancer: A Multi-State Model Approach. Transl. Lung Cancer Res. 2022, 11, 1279–1291. [Google Scholar] [CrossRef]
- Sonoda, D.; Matsuura, Y.; Kondo, Y.; Ichinose, J.; Nakao, M.; Ninomiya, H.; Nishio, M.; Okumura, S.; Satoh, Y.; Mun, M. A Reasonable Definition of Oligo-Recurrence in Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2022, 23, 82–90. [Google Scholar] [CrossRef]
- Sihoe, A.D.L.; Van Schil, P. Non-Small Cell Lung Cancer: When to Offer Sublobar Resection. Lung Cancer 2014, 86, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, L.; Menichelli, C.; Casamassima, F.; Aristei, C.; Borghesi, S.; Ingrosso, G.; Draghini, L.; Tagliagambe, A.; Badellino, S.; di Monale, E.; et al. Stereotactic Radiotherapy for Lung Oligometastases. Rep. Pract. Oncol. Radiother. J. Gt. Cancer Cent. Poznan Pol. Soc. Radiat. Oncol. 2022, 27, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Yamakado, K.; Takaki, H.; Kashima, M.; Uraki, J.; Nakatsuka, A.; Takao, M.; Taguchi, O.; Yamada, T.; Takeda, K. Lung Radiofrequency Ablation for the Treatment of Unresectable Recurrent Non-Small-Cell Lung Cancer after Surgical Intervention. Cardiovasc. Intervent. Radiol. 2012, 35, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Riely, G.J. Systemic Therapy for Locally Advanced and Metastatic Non-Small Cell Lung Cancer: A Review. JAMA 2019, 322, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Romero-Vielva, L.; Viteri, S.; Moya-Horno, I.; Toscas, J.I.; Maestre-Alcácer, J.A.; Ramón y Cajal, S.; Rosell, R. Salvage Surgery after Definitive Chemo-Radiotherapy for Patients with Non-Small Cell Lung Cancer. Lung Cancer 2019, 133, 117–122. [Google Scholar] [CrossRef]
- Ueno, T.; Yamashita, M.; Yamashita, N.; Uomoto, M.; Kawamata, O.; Sano, Y.; Inokawa, H.; Hirayama, S.; Okazaki, M.; Toyooka, S. Safety of Salvage Lung Resection after Immunotherapy for Unresectable Non-Small Cell Lung Cancer. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 812–817. [Google Scholar] [CrossRef]
- Bott, M.J.; Cools-Lartigue, J.; Tan, K.S.; Dycoco, J.; Bains, M.S.; Downey, R.J.; Huang, J.; Isbell, J.M.; Molena, D.; Park, B.J.; et al. Safety and Feasibility of Lung Resection After Immunotherapy for Metastatic or Unresectable Tumors. Ann. Thorac. Surg. 2018, 106, 178–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.; Liu, J.; Cai, X.; Chen, J.; Rocco, G.; Petersen, R.H.; Brunelli, A.; Ng, C.S.H.; D’Amico, T.A.; Liang, W.; et al. Radical Minimally Invasive Surgery after Immuno-Chemotherapy in Initially-Unresectable Stage IIIB Non-Small Cell Lung Cancer. Ann. Surg. 2022, 275, e600–e602. [Google Scholar] [CrossRef] [PubMed]
- Bertolaccini, L.; Galetta, D.; Sedda, G.; de Marinis, F.; Spaggiari, L. Safety Analysis of Salvage Surgery for Advanced Stages or Metastatic Lung Cancers. Thorac. Cardiovasc. Surg. 2022, 70, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Etienne, H.; Fournel, L.; Mordant, P.; Delatour, B.R.; Pfeuty, K.; Frey, G.; Seguin-Givelet, A.; Fourdrain, A.; Lancelin, C.; Berna, P.; et al. Anatomic Lung Resection after Immune Checkpoint Inhibitors for Initially Unresectable Advanced-Staged Non-Small Cell Lung Cancer: A Retrospective Cohort Analysis. J. Thorac. Dis. 2023, 15, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E.; Hicks, R.J.; Dimitrakopoulou-Strauss, A.; Dercle, L.; Iravani, A.; Seban, R.D.; Sachpekidis, C.; Humbert, O.; Gheysens, O.; Glaudemans, A.W.J.M.; et al. Joint EANM/SNMMI/ANZSNM Practice Guidelines/Procedure Standards on Recommended Use of [18F]FDG PET/CT Imaging during Immunomodulatory Treatments in Patients with Solid Tumors Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2323–2341. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottet, B.; Piton, N.; Selim, J.; Sarsam, M.; Guisier, F.; Baste, J.-M. Beyond the Frontline: A Triple-Line Approach of Thoracic Surgeons in Lung Cancer Management—State of the Art. Cancers 2023, 15, 4039. https://doi.org/10.3390/cancers15164039
Bottet B, Piton N, Selim J, Sarsam M, Guisier F, Baste J-M. Beyond the Frontline: A Triple-Line Approach of Thoracic Surgeons in Lung Cancer Management—State of the Art. Cancers. 2023; 15(16):4039. https://doi.org/10.3390/cancers15164039
Chicago/Turabian StyleBottet, Benjamin, Nicolas Piton, Jean Selim, Matthieu Sarsam, Florian Guisier, and Jean-Marc Baste. 2023. "Beyond the Frontline: A Triple-Line Approach of Thoracic Surgeons in Lung Cancer Management—State of the Art" Cancers 15, no. 16: 4039. https://doi.org/10.3390/cancers15164039
APA StyleBottet, B., Piton, N., Selim, J., Sarsam, M., Guisier, F., & Baste, J.-M. (2023). Beyond the Frontline: A Triple-Line Approach of Thoracic Surgeons in Lung Cancer Management—State of the Art. Cancers, 15(16), 4039. https://doi.org/10.3390/cancers15164039







