ASSET: Auto-Segmentation of the Seventeen SEgments for Ventricular Tachycardia Ablation in Radiation Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Treatment
2.2. Patient Imaging
2.3. Left Ventricular Segmentation
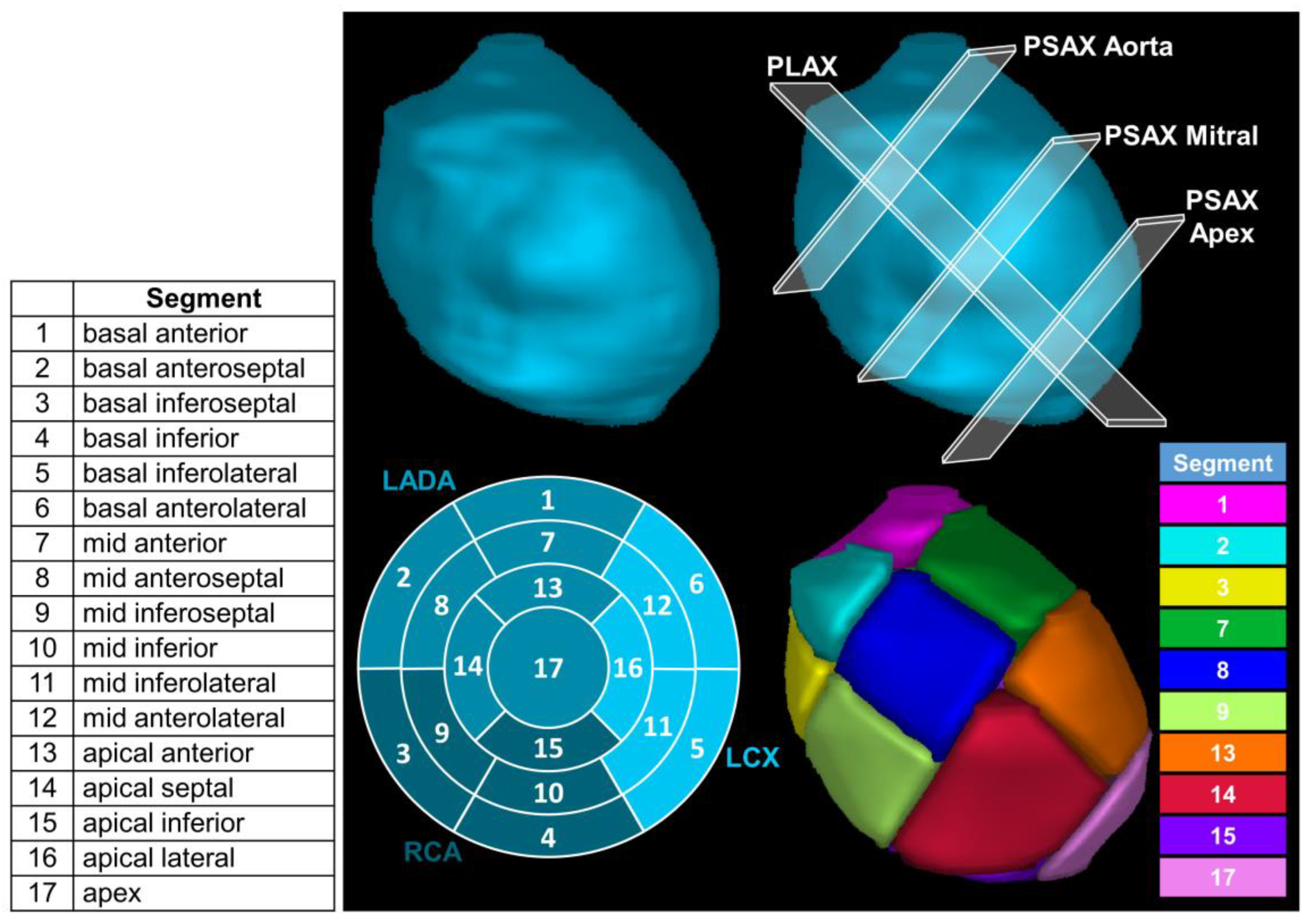
2.4. Definition of 17 Left Ventricular Segments
2.5. Applying the ASSET Model
2.6. Data Extraction
2.7. Dosimetric Assessment
2.8. Qualitative Analysis
- (1)
- Clinically unacceptable
- (2)
- Major modifications required
- (3)
- Moderate modifications required
- (4)
- Minor modifications required
- (5)
- Clinically acceptable
3. Results
3.1. Generation of the 17 Segments
3.2. Model Performance
3.3. Retrospective Dosimetric Assessment
3.4. Prospective Patient Treatment
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayinger, M.; Kovacs, B.; Tanadini-Lang, S.; Ehrbar, S.; Wilke, L.; Chamberlain, M.; Moreira, A.; Weitkamp, N.; Brunckhorst, C.; Duru, F.; et al. First magnetic resonance imaging-guided cardiac radioablation of sustained ventricular tachycardia. Radiother. Oncol. 2020, 152, 203–207. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Bailey, K.R.; Chaouki, A.S.; Deshmukh, A.J.; Gautam, S.; Kim, R.J.; Kramer, D.B.; Lambrakos, L.K.; Nasser, N.H.; Sorajja, D. Systematic review for the 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018, 138, e392–e414. [Google Scholar] [PubMed]
- Dinov, B.; Fiedler, L.; Schönbauer, R.; Bollmann, A.; Rolf, S.; Piorkowski, C.; Hindricks, G.; Arya, A. Outcomes in Catheter Ablation of Ventricular Tachycardia in Dilated Nonischemic Cardiomyopathy Compared with Ischemic Cardiomyopathy. Circulation 2014, 129, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, R.G. Radiofrequency ablation: A review of current knowledge, therapeutic perspectives, complications, and contraindications. Int. J. Biosens. Bioelectron. 2018, 4, 56–58. [Google Scholar]
- Cuculich, P.S.; Schill, M.R.; Kashani, R.; Mutic, S.; Lang, A.; Cooper, D.; Faddis, M.; Gleva, M.; Noheria, A.; Smith, T.W.; et al. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N. Engl. J. Med. 2017, 377, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Blanck, O.; Ipsen, S.; Chan, M.K.; Bauer, R.; Kerl, M.; Hunold, P.; Jacobi, V.; Bruder, R.; Schweikard, A.; Rades, D.; et al. Treatment Planning Considerations for Robotic Guided Cardiac Radiosurgery for Atrial Fibrillation. Cureus 2016, 8, e705. [Google Scholar] [PubMed] [Green Version]
- Gach, H.M.; Green, O.L.; Cuculich, P.S.; Wittland, E.J.; Marko, A.; Luchtefeld, M.E.; Entwistle, J.M.; Yang, D.; Wilber, D.J.; Mutic, S.; et al. Lessons Learned from the First Human Low-Field MRI Guided Radiation Therapy of the Heart in the Presence of an Implantable Cardiac Defibrillator. Pract. Radiat. Oncol. 2019, 9, 274–279. [Google Scholar]
- Chin, R.; Hayase, J.; Hu, P.; Cao, M.; Deng, J.; Ajijola, O.; Do, D.; Vaseghi, M.; Buch, E.; Khakpour, H.; et al. Non-invasive stereotactic body radiation therapy for refractory ventricular arrhythmias: An institutional experience. J. Interv. Card. Electrophysiol. 2020, 61, 535–543. [Google Scholar]
- Robinson, C.G.; Samson, P.P.; Moore, K.M.; Hugo, G.D.; Knutson, N.; Mutic, S.; Goddu, S.M.; Lang, A.; Cooper, D.H.; Faddis, M.; et al. Phase I/II trial of electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation 2019, 139, 313–321. [Google Scholar] [CrossRef]
- Neuwirth, R.; Cvek, J.; Knybel, L.; Jiravsky, O.; Molenda, L.; Kodaj, M.; Fiala, M.; Peichl, P.; Feltl, D.; Januška, J.; et al. Stereotactic radiosurgery for ablation of ventricular tachycardia. EP Eur. 2019, 21, 1088–1095. [Google Scholar] [CrossRef]
- American Heart Association Writing Group on Myocardial Segmentation, Imaging R for C; Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar] [PubMed] [Green Version]
- Picano, E.; Sicari, R.; Landi, P.; Raciti, M.; Pingitore, A.; Vassalle, C.; Mathias, W.; Lowenstein, J.; Petix, N.; Gigli, G.; et al. The multicentre trial philosophy in stress echocardiography: Lessons learned fom the EPIC study. Eur. Heart J. 1995, 16, 2–4. [Google Scholar] [PubMed]
- Rashid, S.; Rapacchi, S.; Vaseghi, M.; Tung, R.; Shivkumar, K.; Finn, J.P.; Hu, P.; Hong, K.; Collins, J.D.; Freed, B.H.; et al. Improved Late Gadolinium Enhancement MR Imaging for Patients with Implanted Cardiac Devices. Radiology 2014, 270, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Kawel, N.; Turkbey, E.B.; Carr, J.J.; Eng, J.; Gomes, A.S.; Hundley, W.G.; Johnson, C.; Masri, S.C.; Prince, M.R.; van der Geest, R.J.; et al. Normal Left Ventricular Myocardial Thickness for Middle-Aged and Older Subjects with Steady-State Free Precession Cardiac Magnetic Resonance. Circ. Cardiovasc. Imaging 2012, 5, 500–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvadurai, B.; Puntmann, V.; Bluemke, D.; Ferrari, V.A.; Friedrich, M.G.; Kramer, C.M.; Kwong, R.Y.; Lombardi, M.; Prasad, S.K.; Rademakers, F.E.; et al. Definition of Left Ventricular Segments for Cardiac Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2017, 11, 926–928. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, J.; Afzal, M.; Okabe, T.; Harfi, T.T.; Tong, M.S.; Thomas, E.; Hugo, G.; Cuculich, P.; Robinson, C.; Williams, T.M. Method and Atlas to Enable Targeting for Cardiac Radioablation Employing the American Heart Association Segmented Model. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Picano, E. Segmentation of the Left Ventricle. In Stress Echocardiography; Picano, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 57–65. [Google Scholar]
- Jolliffe, I.T. Principal Components in Regression Analysis. In Principal Component Analysis; Jolliffe, I.T., Ed.; Springer Series in Statistics; Springer: New York, NY, USA, 1986; pp. 129–155. [Google Scholar]
- Hedberg, H.; Kristensen, F.; Nilsson, P.; Owall, V. A low complexity architecture for binary image erosion and dilation using structuring element decomposition. In Proceedings of the 2005 IEEE International Symposium on Circuits and Systems, Kobe, Japan, 23–26 May 2005; Volume 4, pp. 3431–3434. [Google Scholar]
- Kumarasiri, A.; Siddiqui, F.; Liu, C.; Yechieli, R.; Shah, M.; Pradhan, D.; Zhong, H.; Chetty, I.J.; Kim, J. Deformable image registration based automatic CT-to-CT contour propagation for head and neck adaptive radiotherapy in the routine clinical setting. Med. Phys. 2014, 41, 121712. [Google Scholar] [CrossRef]
- Morris, E.D.; Ghanem, A.I.; Pantelic, M.V.; Walker, E.M.; Han, X.; Glide-Hurst, C.K. Cardiac Substructure Segmentation and Dosimetry Using a Novel Hybrid Magnetic Resonance and Computed Tomography Cardiac Atlas. Int. J. Radiat. Oncol. 2019, 103, 985–993. [Google Scholar] [CrossRef]
- Morris, E.D.; Ghanem, A.I.; Dong, M.; Pantelic, M.V.; Walker, E.M.; Glide-Hurst, C.K. Cardiac substructure segmentation with deep learning for improved cardiac sparing. Med. Phys. 2020, 47, 576–586. [Google Scholar] [CrossRef]
- Mortazi, A.; Burt, J.; Bagci, U. Multi-planar deep segmentation networks for cardiac substructures from MRI and CT. In International Workshop on Statistical Atlases and Computational Models of the Heart; Springer: Cham, Switzerland, 2017; pp. 199–206. [Google Scholar]
- Avendi, M.R.; Kheradvar, A.; Jafarkhani, H. A combined deep-learning and deformable-model approach to fully automatic segmentation of the left ventricle in cardiac MRI. Med. Image Anal. 2016, 30, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Liao, Z.; Pan, T.; Milgrom, S.A.; Pinnix, C.C.; Shi, A.; Tang, L.; Yang, J.; Liu, Y.; Gomez, D.; et al. Cardiac atlas development and validation for automatic segmentation of cardiac substructures. Radiother. Oncol. 2017, 122, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piers, S.R.; Zeppenfeld, K. Imaging-guided Ventricular Tachycardia Ablation. Arrhythm. Electrophysiol. Rev. 2013, 2, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharp, G.; Fritscher, K.D.; Pekar, V.; Peroni, M.; Shusharina, N.; Veeraraghavan, H.; Yang, J. Vision 20/20: Perspectives on automated image segmentation for radiotherapy. Med. Phys. 2014, 41, 050902. [Google Scholar] [CrossRef] [Green Version]
- Mayinger, M.; Boda-Heggemann, J.; Mehrhof, F.; Krug, D.; Hohmann, S.; Xie, J.; Ehrbar, S.; Kovacs, B.; Merten, R.; Grehn, M.; et al. Quality assurance process within the RAdiosurgery for VENtricular TAchycardia (RAVENTA) trial for the fusion of electroanatomical mapping and radiotherapy planning imaging data in cardiac radioablation. Phys. Imaging Radiat. Oncol. 2023, 25, 100406. [Google Scholar] [CrossRef] [PubMed]



| Comparison Between Observers | Observers Compared with ASSET | ||||
|---|---|---|---|---|---|
| Patient | Average DSC | Average MDA (mm) | Average DSC | Average MDA (mm) | Median and IQR Qualitative Score |
| 1 | 0.85 ± 0.07 | 0.83 ± 0.48 | 0.83 ± 0.07 | 0.95 ± 0.54 | 5.0 (5.0–5.0) |
| 2 | 0.81 ± 0.09 | 1.16 ± 0.59 | 0.73 ± 0.06 | 1.33 ± 0.66 | 5.0 (5.0–5.0) |
| 3 | 0.78 ± 0.09 | 1.45 ± 0.75 | 0.84 ± 0.04 | 0.87 ± 0.25 | 5.0 (4.3–5.0) |
| 4 | 0.87 ± 0.04 | 0.62 ± 0.24 | 0.82 ± 0.07 | 0.99 ± 0.52 | 5.0 (5.0–5.0) |
| 5 | 0.81 ± 0.09 | 1.08 ± 0.63 | 0.81 ± 0.07 | 1.04 ± 0.50 | 5.0 (5.0–5.0) |
| 6 | 0.72 ± 0.11 | 1.68 ± 1.08 | 0.74 ± 0.11 | 1.63 ± 0.98 | 5.0 (5.0–5.0) |
| 7 | 0.82 ± 0.06 | 0.76 ± 0.27 | 0.79 ± 0.08 | 1.03 ± 0.48 | 5.0 (5.0–5.0) |
| 8 | 0.87 ± 0.07 | 0.57 ± 0.29 | 0.85 ± 0.07 | 0.66 ± 0.28 | 5.0 (4.3–5.0) |
| 9 | 0.85 ± 0.07 | 0.62 ± 0.25 | 0.82 ± 0.07 | 0.83 ± 0.38 | 5.0 (4.3–5.0) |
| 10 | 0.91 ± 0.03 | 0.51 ± 0.13 | 0.89 ± 0.06 | 0.60 ± 0.39 | 5.0 (5.0–5.0) |
| Average ± SD | Median and IQR | ||||
| Average ± SD | 0.83 ± 0.07 | 0.93 ± 0.47 | 0.81 ± 0.06 | 0.99 ± 0.49 | 5.0 (5.0–5.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morris, E.; Chin, R.; Wu, T.; Smith, C.; Nejad-Davarani, S.; Cao, M. ASSET: Auto-Segmentation of the Seventeen SEgments for Ventricular Tachycardia Ablation in Radiation Therapy. Cancers 2023, 15, 4062. https://doi.org/10.3390/cancers15164062
Morris E, Chin R, Wu T, Smith C, Nejad-Davarani S, Cao M. ASSET: Auto-Segmentation of the Seventeen SEgments for Ventricular Tachycardia Ablation in Radiation Therapy. Cancers. 2023; 15(16):4062. https://doi.org/10.3390/cancers15164062
Chicago/Turabian StyleMorris, Eric, Robert Chin, Trudy Wu, Clayton Smith, Siamak Nejad-Davarani, and Minsong Cao. 2023. "ASSET: Auto-Segmentation of the Seventeen SEgments for Ventricular Tachycardia Ablation in Radiation Therapy" Cancers 15, no. 16: 4062. https://doi.org/10.3390/cancers15164062
APA StyleMorris, E., Chin, R., Wu, T., Smith, C., Nejad-Davarani, S., & Cao, M. (2023). ASSET: Auto-Segmentation of the Seventeen SEgments for Ventricular Tachycardia Ablation in Radiation Therapy. Cancers, 15(16), 4062. https://doi.org/10.3390/cancers15164062






