Comparative Assessment of Different Ultrasound Technologies in the Detection of Prostate Cancer: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
- (1)
- Diagnostic accuracy of transrectal SWE ultrasound in the detection of prostate cancer.
- (2)
- Diagnostic accuracy of CEUS in the detection of prostate cancer.
- (3)
- Diagnostic accuracy of micro-ultrasound in the detection of prostate cancer.
- (4)
- Diagnostic accuracy of multiparametric ultrasound in the detection of prostate cancer.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction
2.3. Quality Assessment
2.4. Data Analyses
3. Results
3.1. Literature Search and Study Selection
3.2. Study Characteristics
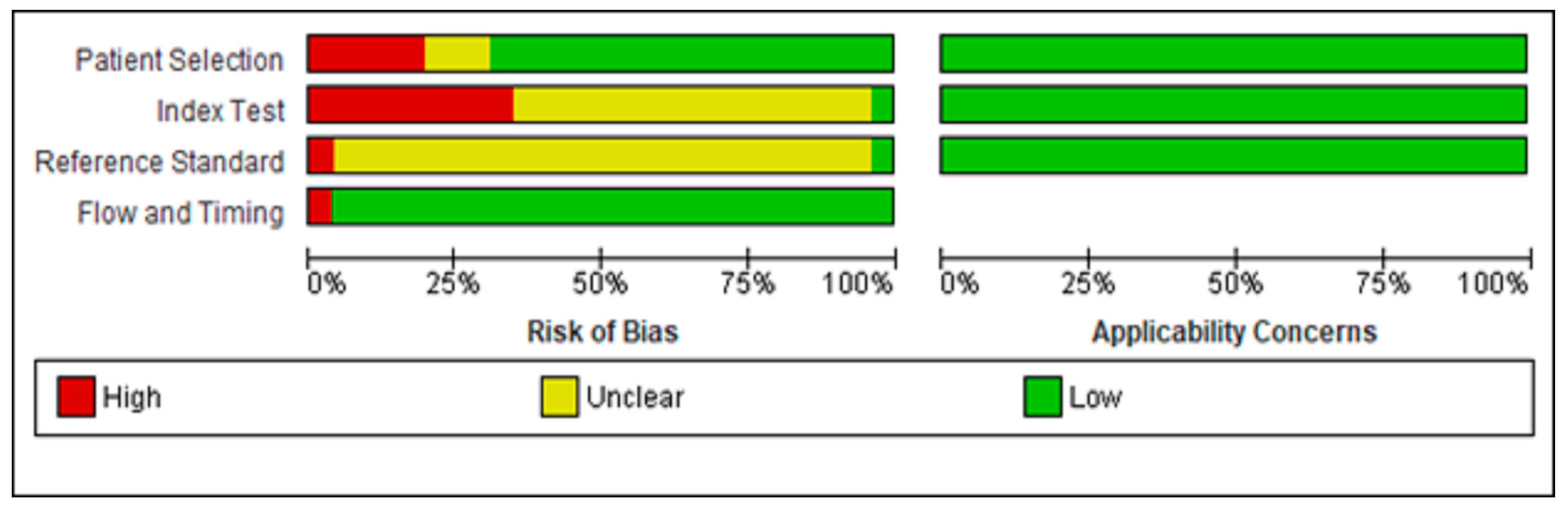
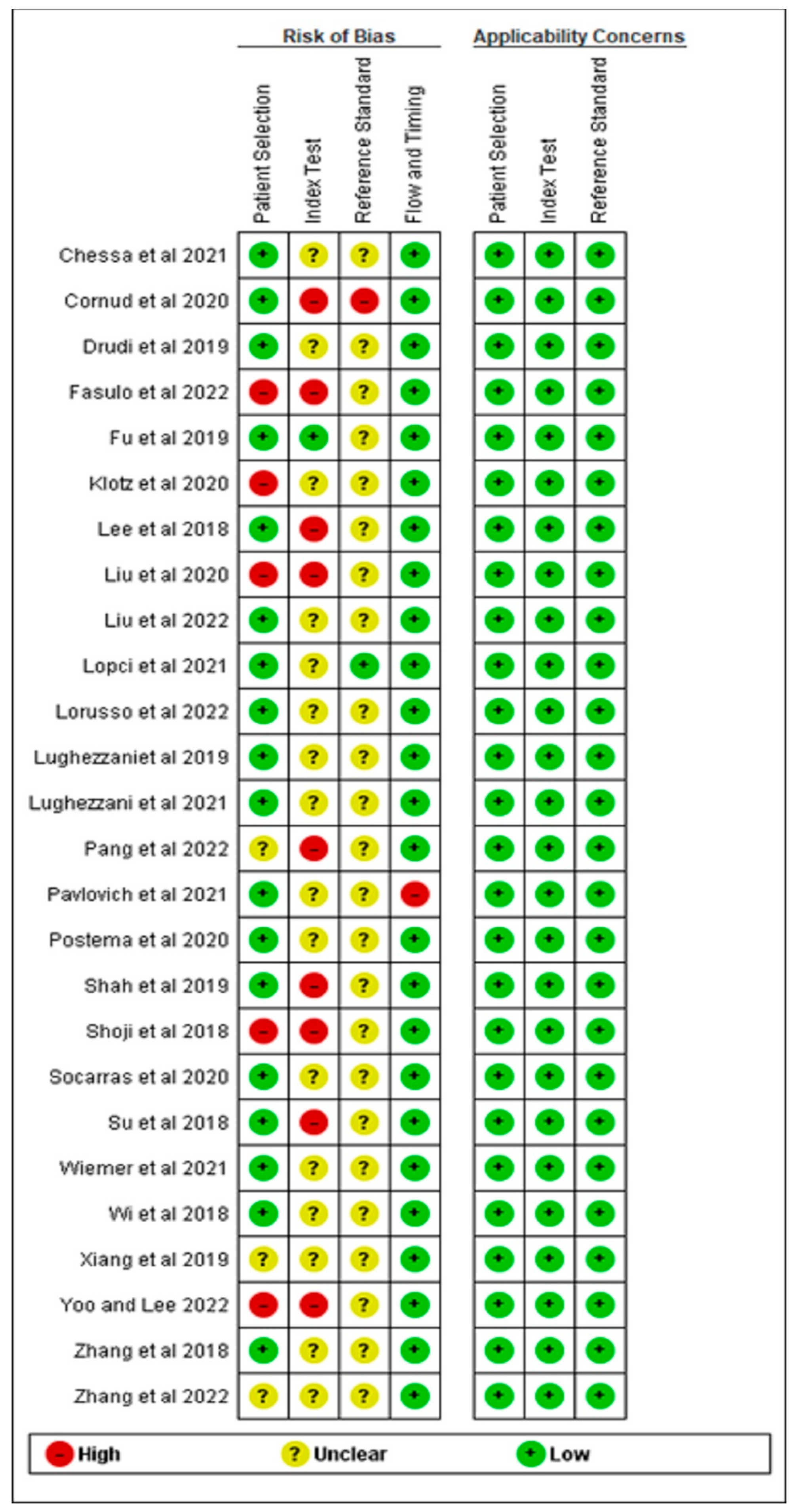
3.3. Quality Assessment
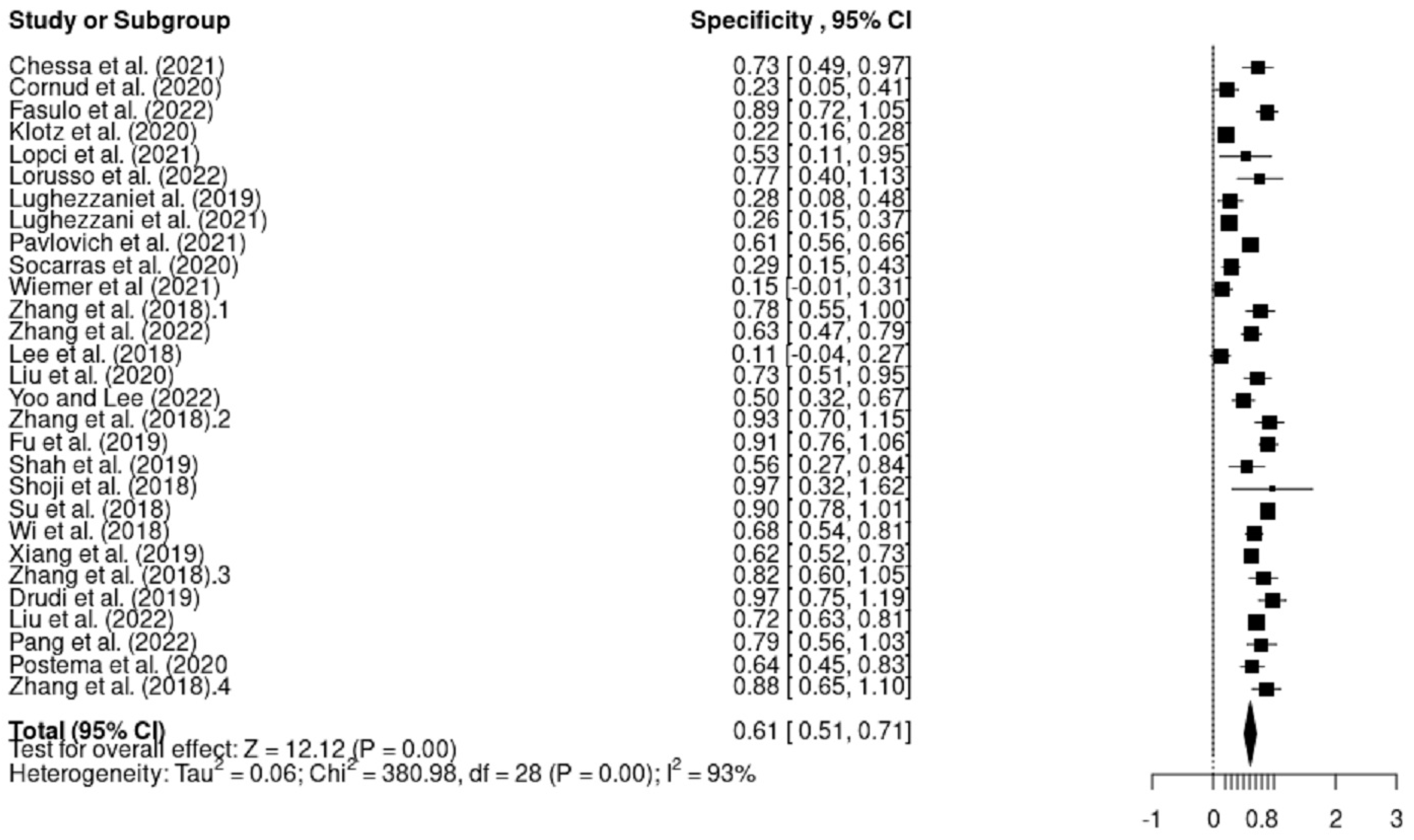
3.4. Sensitivity and Specificity Analysis
3.4.1. Heterogeneity
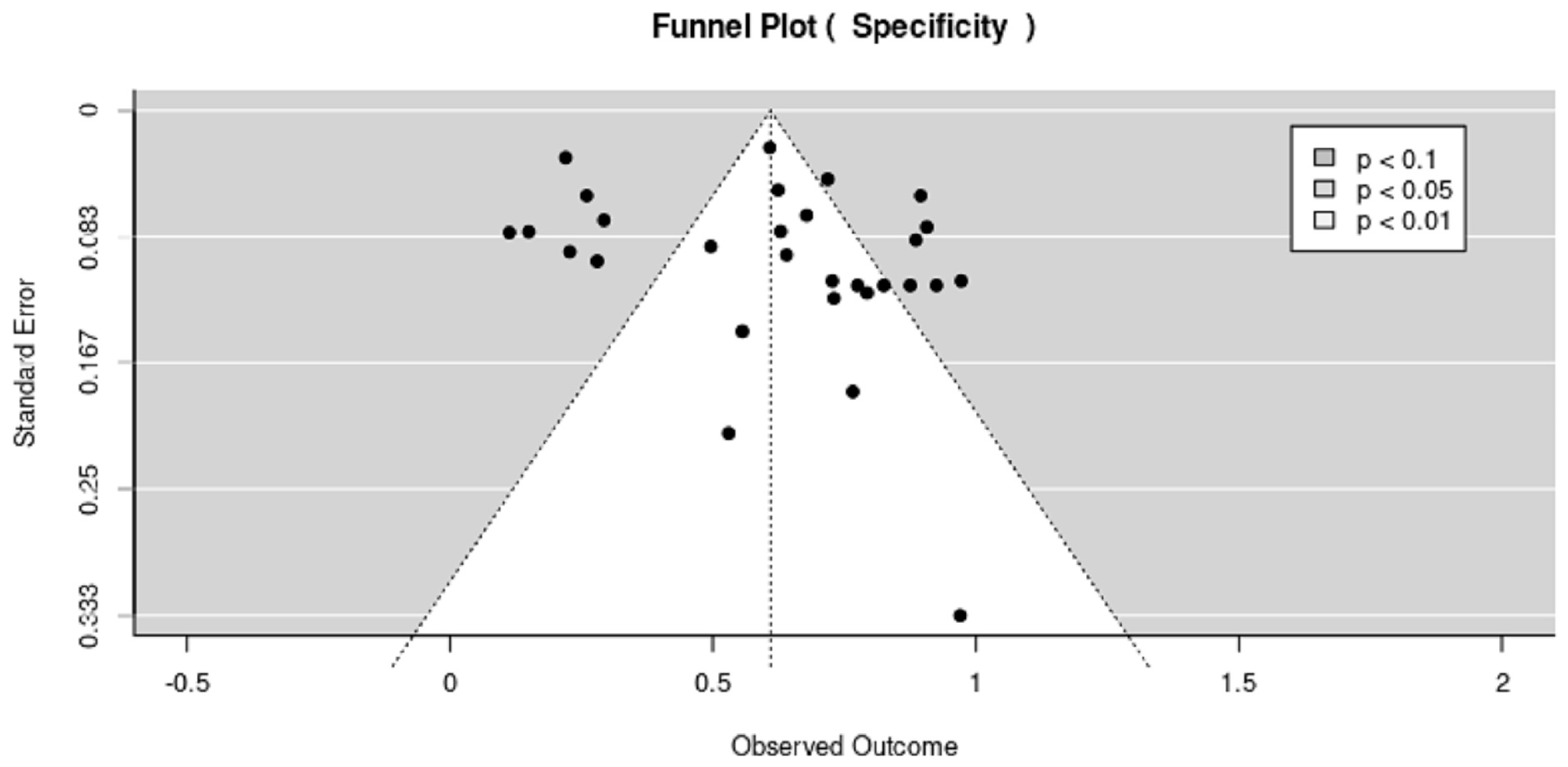
3.4.2. Publication Bias
4. Discussion
4.1. Main Findings of the Study in the Context of the Reported Literature
4.2. Limitations of the Review
4.3. Clinical Implications of the Review
- -
- Transrectal ultrasonography (TRUS) is a frequently employed method for prostate imaging and biopsy guiding. It is known to allow the prostate gland to be more visible and assists in the detection of any abnormal areas. Thus, we recommend that clinicians combine magnetic resonance imaging and TRUS to accurately identify prostate cancers. Real-time ultrasound and previously acquired MRI images can be combined in this way to improve the visibility of any questionable lesions and direct biopsy needles to the right places.
- -
- Micro ultrasound, a more recent imaging technique, provides better prostate visibility and resolution than traditional ultrasound. Thus, we recommend that clinicians use micro ultrasound to accurately detect prostate cancer as it increases the accuracy of biopsies and decreases unnecessary procedures. In addition, it is thought to improve the detection and localization of any questionable lesions within the prostate.
- -
- Multi-parametric ultrasound can be applied longitudinally to track disease development and evaluate treatment effectiveness. Clinicians can assess modifications to tumor size, vascularity and tissue features over time by comparing serial mpUS scans. These data can aid in assessing the efficacy of therapy, spotting recurrent illness and directing future management choices.
- -
- Compared to other imaging modalities such as MRI, mpUS can be carried out by utilizing either transrectal or transperineal techniques, both of which are minimally invasive. This makes mpUS a practical and well-tolerated choice for routine testing and monitoring in prostate cancer patients.
- -
- Targeted biopsies of questionable spots found on imaging can be guided using mpUS. mpUS can precisely identify and locate concerning lesions by combining imaging modalities such as B-mode, contrast-enhanced ultrasound and elastography. This increases the chance of finding prostate cancer and decreases the number of unnecessary biopsies by enabling more-accurate and focused sampling during biopsies.
- -
- mpUS can also assist in categorizing prostate cancer risk. Using a variety of measurements, including tumor size, vascularity and tissue stiffness, mpUS can determine the cancer’s aggressiveness and stage. Clinicians can use this risk stratification to guide their planning and decision-making for patient care, assisting them in selecting the best course of action.
4.4. Research Implications of the Review
- -
- There is still a lack of studies on the performance of mpUS in the detection of prostate cancer. Thus, future research should ideally focus on the diagnostic accuracy of mpUS. In addition, further studies are required on the diagnostic accuracy of Micro-US, mpUS, grayscale, elastography and CEUS modalities in asymptomatic men for the early detection of prostate cancer, although this would require large populations and may be very expensive.
- -
- We recommend comparative evaluation of various ultrasound modalities. For instance, there is still a lack of studies that compare the performance and diagnostic efficacy of different ultrasound technologies used to find prostate cancer. Transrectal ultrasound (TRUS) and transperineal ultrasound (TPUS) can be compared, and the efficacy of fusion imaging—which combines traditional ultrasound with an assessment of the prospective advantages of new technologies such as micro ultrasound—can also be evaluated.
- -
- We recommend future research on the viability and efficacy of more recent ultrasound methods for the detection of prostate cancer, such as micro ultrasonography, contrast-enhanced ultrasound (CEUS) and multiparametric ultrasound, and to investigate how such methods assist in improving the sensitivity, specificity and location of suspected lesions inside the prostate gland.
- -
- We recommend future validation studies to evaluate the effectiveness of ultrasound technologies in a range of patient populations, including those with various risk profiles or clinical traits. This can assist in determining the generalizability and usability of ultrasound techniques for the detection of prostate cancer in different contexts.
- -
- We recommend future longitudinal research on the long-term results and influence on patient care, for instance, the long-term effects and impact on patient management of the application of various ultrasound technologies for the identification of prostate cancer. To establish the therapeutic relevance and consequences of these technologies, future research should consider elements including biopsy accuracy, treatment decision-making, surveillance techniques and patient outcomes.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Ilic, D.; Neuberger, M.M.; Djulbegovic, M.; Dahm, P. Screening for prostate cancer. Cochrane Database Syst. Rev. 2013, 2013, CD004720. [Google Scholar] [CrossRef] [PubMed]
- Ageeli, W.; Wei, C.; Zhang, X.; Szewcyk-Bieda, M.; Wilson, J.; Li, C.; Nabi, G. Quantitative ultrasound shear wave elastography (USWE)-measured tissue stiffness correlates with PIRADS scoring of MRI and Gleason score on whole-mount histopathology of prostate cancer: Implications for ultrasound image-guided targeting approach. Insights into Imaging 2021, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lumbreras, B.; Parker, L.A.; Caballero-Romeu, J.P.; Gómez-Pérez, L.; Puig-García, M.; López-Garrigós, M.; García, N.; Hernández-Aguado, I. Variables Associated with False-Positive PSA Results: A Cohort Study with Real-World Data. Cancers 2023, 15, 261. [Google Scholar] [CrossRef] [PubMed]
- Pron, G. Prostate-Specific Antigen (PSA)-Based Population Screening for Prostate Cancer: An Evidence-Based Analysis. Ont. Heal. Technol. Assess. Ser. 2015, 15, 1–64. [Google Scholar]
- Stroumbakis, N.; Cookson, M.S.; Reuter, V.E.; Fair, W.R. Clinical significance of repeat sextant biopsies in prostate cancer patients. Urology 1997, 49, 113–118. [Google Scholar] [CrossRef]
- Turkbey, B.; Choyke, P.L. Future Perspectives and Challenges of Prostate MR Imaging. Radiol. Clin. N. Am. 2018, 56, 327–337. [Google Scholar] [CrossRef]
- Schröder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Määttänen, L.; Lilja, H.; et al. Screening and prostate cancer mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014, 384, 2027–2035. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Zhang, J.; Chen, L.; Yuan, Y.; Xu, D. Study on the Diagnostic Value of Contrast-Enhanced Ultrasound and Magnetic Resonance Imaging in Prostate Cancer. Evidence-Based Complement. Altern. Med. 2022, 2022, 1–6. [Google Scholar] [CrossRef]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Boehm, K.; Salomon, G.; Beyer, B.; Schiffmann, J.; Simonis, K.; Graefen, M.; Budaeus, L. Shear wave elastography for localization of prostate cancer lesions and assessment of elasticity thresholds: Implications for targeted biopsies and active surveillance protocols. J. Urol. 2015, 193, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Cosgrove, D.; Brock, M.; Cantisani, V.; Correas, J.M.; Postema, A.W.; Salomon, G.; Tsutsumi, M.; Xu, H.-X.; Dietrich, C.F. WFUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography: Part 5. Prostate. Ultrasound Med. Biol. 2017, 43, 27–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junker, D.; De Zordo, T.; Quentin, M.; Ladurner, M.; Bektic, J.; Horniger, W.; Jaschke, W.; Aigner, F. Real-time elastography of the prostate. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postema, A.; Mischi, M.; De La Rosette, J.; Wijkstra, H. Multiparametric ultrasound in the detection of prostate cancer: A systematic review. World J. Urol. 2015, 33, 1651–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannaerts, C.K.; Wildeboer, R.R.; Postema, A.W.; Hagemann, J.; Budäus, L.; Tilki, D.; Mischi, M.; Wijkstra, H.; Salomon, G. Multiparametric ultrasound: Evaluation of greyscale, shear wave elastography and contrast-enhanced ultrasound for prostate cancer detection and localization in correlation to radical prostatectomy specimens. BMC Urol. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Postema, A.W.; Frinking, P.J.; Smeenge, M.; De Reijke, T.M.; De la Rosette, J.J.; Tranquart, F.; Wijkstra, H. Dynamic contrast-enhanced ultrasound parametric imaging for the detection of prostate cancer. BJU Int. 2016, 117, 598–603. [Google Scholar] [CrossRef]
- Good, D.W.; Stewart, G.D.; Hammer, S.; Scanlan, P.; Shu, W.; Phipps, S.; Reuben, R.; McNeill, A.S. Elasticity as a biomarker for prostate cancer: A systematic review. BJU Int. 2014, 113, 523–534. [Google Scholar] [CrossRef]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Shear-Wave Elastography for Detection of Prostate Cancer: A Systematic Review and Diagnostic Meta-Analysis. AJR Am. J. Roentgenol. 2017, 209, 806–814. [Google Scholar] [CrossRef]
- Correas, J.-M.; Tissier, A.-M.; Khairoune, A.; Khoury, G.; Eiss, D.; Hélénon, O. Ultrasound elastography of the prostate: State of the art. Diagn. Interv. Imaging 2013, 94, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.B.; O’brien, C.; Correas, J.-M.; Ghai, S. Multiparametric ultrasound and micro-ultrasound in prostate cancer: A comprehensive review. Br. J. Radiol. 2022, 95, 20210633. [Google Scholar] [CrossRef]
- Gao, Y.; Liao, X.; Lu, L.; Wang, L.; Ma, Y.; Qin, H.; Yan, X.; Guo, P. Contrast-enhanced transrectal ultrasonography for the detection of diffuse prostate cancer. Clin. Radiol. 2016, 71, 258–264. [Google Scholar] [CrossRef]
- Qi, T.; Chen, Y.; Zhu, Y.; Jiang, J.; Wang, L.; Qi, J. Contrast-enhanced transrectal ultrasonography for detection and localization of prostate index tumor: Correlation with radical prostatectomy findings. Urology 2014, 84, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.W.; Li, H.L.; Du, J.; Xia, J.G.; Guo, Y.F.; Xin, M.; Li, F.H. Contrast-enhanced ultrasonography with contrast-tuned imaging technology for the detection of prostate cancer: Comparison with conventional ultrasonography. BJU Int. 2012, 109, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Seitz, M.; Gratzke, C.; Schlenker, B.; Buchner, A.; Karl, A.; Roosen, A.; Singer, B.B.; Bastian, P.J.; Ergün, S.; Stief, C.G.; et al. Contrast-enhanced transrectal ultrasound (CE-TRUS) with cadence-contrast pulse sequence (CPS) technology for the identification of prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2011, 29, 295–301. [Google Scholar] [CrossRef]
- Kunz, P.; Kiesl, S.; Groß, S.; Kauczor, H.-U.; Schmidmaier, G.; Fischer, C. Intra-observer and Device-Dependent Inter-observer Reliability of Contrast-Enhanced Ultrasound for Muscle Perfusion Quantification. Ultrasound Med. Biol. 2020, 46, 275–285. [Google Scholar] [CrossRef]
- Gurwin, A.; Kowalczyk, K.; Knecht-Gurwin, K.; Stelmach, P.; Nowak, Ł.; Krajewski, W.; Szydełko, T.; Małkiewicz, B. Alternatives for MRI in Prostate Cancer Diagnostics—Review of Current Ultrasound-Based Techniques. Cancers 2022, 14, 1859. [Google Scholar] [CrossRef]
- Ghai, S.; Van der Kwast, T. Suspicious findings on micro-ultrasound imaging and early detection of prostate cancer. Urol. Case Rep. 2018, 16, 98–100. [Google Scholar] [CrossRef]
- Panic, N.; Leoncini, E.; de Belvis, G.; Ricciardi, W.; Boccia, S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE 2013, 8, e83138. [Google Scholar] [CrossRef] [Green Version]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Luo, Y.; Wang, Y.; Wu, M.; Memmott, B.; Gao, J. Diagnostic performance of multiparametric transrectal ultrasound in localized prostate cancer: A comparative study with magnetic resonance imaging. J. Ultrasound Med. 2019, 38, 1823–1830. [Google Scholar] [CrossRef]
- Pavlovich, C.P.; Hyndman, M.E.; Eure, G.; Ghai, S.; Caumartin, Y.; Herget, E.; Young, J.D.; Wiseman, D.; Caughlin, C.; Gray, R.; et al. A multi-institutional randomized controlled trial comparing first-generation transrectal high-resolution micro-ultrasound with conventional frequency transrectal ultrasound for prostate biopsy. BJUl Compass. 2021, 2, 126–133. [Google Scholar] [CrossRef]
- Chessa, F.; Schiavina, R.; Amelio, E.; Gaudiano, C.; Giusti, D.; Bianchi, L.; Pultrone, C.; Marcelli, E.; Distefano, C.; Lodigiani, L.; et al. Diagnostic accuracy of the Novel 29 MHz micro-ultrasound “ExactVuTM” for the detection of clinically significant prostate cancer: A prospective single institutional study. A step forward in the diagnosis of prostate cancer. Arch. Ital. Urol. Androl. 2021, 93, 132–138. [Google Scholar] [CrossRef]
- Cornud, F.; Lefevre, A.; Flam, T.; Dumonceau, O.; Galiano, M.; Soyer, P.; Camparo, P.; Barral, M. MRI-directed high-frequency (29MhZ) TRUS-guided biopsies: Initial results of a single-center study. Eur. Radiol. 2020, 30, 4838–4846. [Google Scholar] [CrossRef]
- Fasulo, V.; Buffi, N.M.; Regis, F.; Paciotti, M.; Persico, F.; Maffei, D.; Uleri, A.; Saita, A.; Casale, P.; Hurle, R.; et al. Use of high-resolution micro-ultrasound to predict extraprostatic extension of prostate cancer prior to surgery: A prospective single-institutional study. World J. Urol. 2022, 40, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Lughezzani, G.; Maffei, D.; Sanchez, A.; Pereira, J.G.; Staerman, F.; Cash, H.; Luger, F.; Lopez, L.; Sanchez-Salas, R.; et al. Comparison of micro-ultrasound and multiparametric magnetic resonance imaging for prostate cancer: A multicenter, prospective analysis. Can. Urol. Assoc. J. 2020, 15, E11–E16. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E.; Lughezzani, G.; Castello, A.; Colombo, P.; Casale, P.; Saita, A.; Buffi, N.M.; Guazzoni, G.; Chiti, A.; Lazzeri, M. PSMA-PET and micro-ultrasound potential in the diagnostic pathway of prostate cancer. Clin. Transl. Oncol. 2021, 23, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, V.; Kabre, B.; Pignot, G.; Branger, N.; Pacchetti, A.; Thomassin-Piana, J.; Brunelle, S.; Gregori, A.; Salem, N.; Musi, G.; et al. Comparison Between Micro-Ultrasound and Multiparametric MRI Regarding the Correct Identification of Prostate Cancer Lesions. Clin. Genitourin. Cancer 2022, 20, e339–e345. [Google Scholar] [CrossRef]
- Lughezzani, G.; Maffei, D.; Saita, A.; Paciotti, M.; Diana, P.; Buffi, N.M.; Colombo, P.; Elefante, G.M.; Hurle, R.; Lazzeri, M.; et al. Diagnostic Accuracy of Microultrasound in Patients with a Suspicion of Prostate Cancer at Magnetic Resonance Imaging: A Single-institutional Prospective Study. Eur. Urol. Focus 2021, 7, 1019–1026. [Google Scholar] [CrossRef]
- Lughezzani, G.; Saita, A.; Lazzeri, M.; Paciotti, M.; Maffei, D.; Lista, G.; Hurle, R.; Buffi, N.M.; Guazzoni, G.; Casale, P. Comparison of the Diagnostic Accuracy of Micro-ultrasound and Magnetic Resonance Imaging/Ultrasound Fusion Targeted Biopsies for the Diagnosis of Clinically Significant Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 329–332. [Google Scholar] [CrossRef]
- Wiemer, L.; Hollenbach, M.; Heckmann, R.; Kittner, B.; Plage, H.; Reimann, M.; Asbach, P.; Friedersdorff, F.; Schlomm, T.; Hofbauer, S.; et al. Evolution of Targeted Prostate Biopsy by Adding Micro-Ultrasound to the Magnetic Resonance Imaging Pathway. Eur. Urol. Focus 2021, 7, 1292–1299. [Google Scholar] [CrossRef]
- Socarrás, M.E.R.; Rivas, J.G.; Rivera, V.C.; Elbers, J.R.; González, L.L.; Mercado, I.M.; del Alamo, J.F.; del Dago, P.J.; Sancha, F.G. Prostate Mapping for Cancer Diagnosis: The Madrid Protocol. Transperineal Prostate Biopsies Using Multiparametric Magnetic Resonance Imaging Fusion and Micro-Ultrasound Guided Biopsies. J. Urol. 2020, 204, 726–733. [Google Scholar] [CrossRef]
- Zhang, X.; Hong, H.; Liang, D. The combined value of mpUS and mpMRI-TRUS fusion for the diagnosis of clinically significant prostate cancer. Cancer Imaging 2022, 22, 1–10. [Google Scholar] [CrossRef]
- Yoo, J.W.; Lee, K.S. Usefulness of grayscale values measuring hypoechoic lesions for predicting prostate cancer: An experimental pilot study. Prostate Int. 2022, 10, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wu, S.; Huang, L. Contrast-enhanced ultrasound evaluation of the prostate before transrectal ultrasound-guided biopsy can improve diagnostic sensitivity: A STARD-compliant article. Medicine 2020, 99, E19946. [Google Scholar] [CrossRef]
- Lee, K.S.; Koo, K.C.; Chung, B.H. Quantitation of hypoechoic lesions for the prediction and Gleason grading of prostate cancer: A prospective study. World J. Urol. 2018, 36, 1059–1065. [Google Scholar] [CrossRef]
- Wei, C.; Li, C.; Szewczyk-Bieda, M.; Upreti, D.; Lang, S.; Huang, Z.; Nabi, G. Performance Characteristics of Transrectal Shear Wave Elastography Imaging in the Evaluation of Clinically Localized Prostate Cancer: A Prospective Study. J. Urol. 2018, 200, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Xiang, L.-H.; Fang, Y.; Wan, J.; Xu, G.; Yao, M.-H.; Ding, S.-S.; Liu, H.; Wu, R. Shear-wave elastography: Role in clinically significant prostate cancer with false-negative magnetic resonance imaging. Eur. Radiol. 2019, 29, 6682–6689. [Google Scholar] [CrossRef]
- Shoji, S.; Hashimoto, A.; Nakamura, T.; Hiraiwa, S.; Sato, H.; Sato, Y.; Tajiri, T.; Miyajima, A. Novel application of three-dimensional shear wave elastography in the detection of clinically significant prostate cancer. Biomed. Rep. 2018, 8, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Su, R.; Xu, G.; Xiang, L.; Ding, S.; Wu, R. A Novel Scoring System for Prediction of Prostate Cancer Based on Shear Wave Elastography and Clinical Parameters. Urology 2018, 121, 112–117. [Google Scholar] [CrossRef]
- Shah, D.; Sai, V.; Ramasamy, N.; Thirunavukkarasu, C.; Kumaresan, N. Transrectal Ultrasound Elastography-Evaluating Clinical Implications to Differentiate between Benign and Malignant Lesion of Prostate: A Prospective Observational Study. Int. J. Sci. Study 2020, 8, 64–67. [Google Scholar]
- Fu, S.; Tang, Y.; Tan, S.; Zhao, Y.; Cui, L. Diagnostic Value of Transrectal Shear Wave Elastography for Prostate Cancer Detection in Peripheral Zone: Comparison with Magnetic Resonance Imaging. J. Endourol. 2020, 34, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Xiang, L.-H.; Xu, G.; Dong, L.; Sun, Y.; Ye, B.; Zhang, Y.; Xu, H. The potential of a nomogram combined PI-RADS v2.1 and contrast-enhanced ultrasound (CEUS) to reduce unnecessary biopsies in prostate cancer diagnostics. Br. J. Radiol. 2022, 95, 20220209. [Google Scholar] [CrossRef]
- Drudi, F.M.; Cantisani, V.; Angelini, F.; Ciccariello, M.; Messineo, D.; Ettorre, E.; Liberatore, M.; Scialpi, M. Multiparametric MRI versus multiparametric US in the detection of prostate cancer. Anticancer Res. 2019, 39, 3101–3110. [Google Scholar] [CrossRef]
- Postema, A.W.; Gayet, M.C.W.; van Sloun, R.J.G.; Wildeboer, R.R.; Mannaerts, C.K.; Savci-Heijink, C.D.; Schalk, S.G.; Kajtazovic, A.; van der Poel, H.; Mulders, P.F.A.; et al. Contrast-enhanced ultrasound with dispersion analysis for the localization of prostate cancer: Correlation with radical prostatectomy specimens. World J. Urol. 2020, 38, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Aigner, F.; Mitterberger, M.; Rehder, P.; Pallwein, L.; Junker, D.; Horninger, W.; Frauscher, F. Status of Transrectal Ultrasound Imaging of the Prostate. J. Endourol. 2010, 24, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent—Update 2013. Eur. Urol. 2014, 65, 124–137. [Google Scholar] [CrossRef]
- Pummer, K.; Rieken, M.; Augustin, H.; Gutschi, T.; Shariat, S.F. Innovations in diagnostic imaging of localized prostate cancer. World J. Urol. 2014, 32, 881–890. [Google Scholar] [CrossRef]
- Sano, F.; Uemura, H. The Utility and Limitations of Contrast-Enhanced Ultrasound for the Diagnosis and Treatment of Prostate Cancer. Sensors 2015, 15, 4947–4957. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, S. A Review of Imaging Methods for Prostate Cancer Detection. Biomed. Eng. Comput. Biol. 2016, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, S.A. Re: Shear Wave Ultrasound Elastography of the Prostate: Initial Results. J. Urol. 2013, 189, 229. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, Y.; Malik, H.; Zhang, X.; Alqahtani, S.; Upreti, D.; Szewczyk-Bieda, M.; Lang, S.; Nabi, G. Prediction of Postprostatectomy Biochemical Recurrence Using Quantitative Ultrasound Shear Wave Elastography Imaging. Front. Oncol. 2019, 9, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Ma, X.; Zhan, W.; Zhu, F.; Li, M.; Huang, J.; Li, Y.; Xue, L.; Liu, L.; Wei, Y. Real-time elastography in the diagnosis of patients suspected of having prostate cancer: A meta-analysis. Ultrasound Med. Biol. 2014, 40, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Chen, M.; Gao, Y.; Yao, Y.; Chen, L.; Xu, D. Transrectal sonoelastography in the detection of prostate cancers: A meta-analysis. BJU Int. 2012, 110, E614–E620. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, R.; Wu, Y.; Jing, J.; Chen, S.; Zhang, G.; Xu, B.; Liu, C.; Chen, M. Micro-Ultrasound Imaging for Accuracy of Diagnosis in Clinically Significant Prostate Cancer: A Meta-Analysis. Front. Oncol. 2019, 9, 1368. [Google Scholar] [CrossRef] [PubMed]



| Inclusion | Exclusion | |
|---|---|---|
| Settings | All Countries | None |
| Participants | Male patients (all ages) with a suspicion of prostate cancer, based on an elevated serum PSA concentration or abnormal digital rectal examination | Females |
| Modality | Studies that used the following devices: Multiparametric ultrasound Micro ultrasound Grayscale Elastography Contrast-enhanced ultrasound | Studies that did not use the following devices: Multiparametric ultrasound Micro ultrasound Grayscale Elastography Contrast-enhanced ultrasound |
| Outcomes | Studies that report sensitivity, specificity, positive predictive value and negative predictive value | Studies that do not report sensitivity, specificity, positive predictive value or negative predictive value |
| Study Type | In vivo studies Prospective and retrospective studies Randomized Clinical trial Non-randomized | In vitro studies Review articles Systematic review |
| Publication Type | Journal articles | Conference abstract, study protocol, report, dissertation, books and non-professional journal |
| Publication Year | Publication date 2018 and after | Publication date before 2018 |
| Language | English | All other languages |
| Modality | Authors (Year) | Number of Cases | Sensitivity (%) | Specificity (%) | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|---|
| Micro-US | Chessa et al. (2021) [32] | 68 | 68 | 73 | 93 | 31 | 69 |
| Cornud et al. (2020) [33] | 118 | 100 | 23 | 75 | 100 | 77 | |
| Fasulo et al. (2022) [34] | 140 | 72 | 89 | 83 | 81 | ||
| Klotz et al. (2020) [35] | 1040 | 94 | 22 | 44 | 85 | 50 | |
| Lopci et al. (2021) [36] | 25 | 80 | 53 | 35 | 89 | 61 | |
| Lorusso et al. (2022) [37] | 32 | 77 | 77 | 64 | 86 | 77 | |
| Lughezzaniet al. (2019) [39] | 104 | 94 | 28 | 40 | 90 | 49 | |
| Lughezzani et al. (2021) [38] | 320 | 90 | 26 | 41 | 82 | 49 | |
| Pavlovich et al. (2021) [31] | 1676 | 19 | 92 | 63 | 61 | ||
| Socarras et al. (2020) [41] | 194 | 99 | 29 | 62 | 96 | ||
| Wiemer et al. (2021) [40] | 159 | 95 | 15 | 52 | 75 | 54 | |
| mpUS | Zhang et al. (2018) [30] | 78 | 97 | 78 | 80 | 97 | 87 |
| Zhang et al. (2022) [42] | 160 | 84 | 64 | 72 | 79 | ||
| Grayscale | Lee et al. (2018) [45] | 157 | 81 | 11 | 47 | 38 | |
| Liu et al. (2020) [44] | 82 | 60 | 73 | 71 | 63 | 67 | |
| Yoo and Lee (2022) [43] | 127 | 58 | 50 | 33 | 73 | 52 | |
| Zhang et al. (2018) [30] | 78 | 61 | 93 | 89 | 71 | 77 | |
| Shear wave elastography | Fu et al. (2019) [51] | 172 | 79 | 91 | 71 | 94 | |
| Shah et al. (2019) [50] | 50 | 83 | 56 | 61 | 79 | 68 | |
| Shoji et al. (2018) [48] | 12 | 58 | 97 | 86 | 87 | ||
| Su et al. (2018) [49] | 320 | 77 | 90 | 83 | 85 | ||
| Wei et al. (2018) [46] | 212 | 97 | 68 | 96 | |||
| Xiang et al. (2019) [47] | 367 | 79 | 62 | 47 | 87 | 67 | |
| Zhang et al. (2018) [30] | 78 | 90 | 83 | 83 | 89 | 86 | |
| CEUS | Drudi et al. (2019) [53] | 82 | 40 | 97 | 94 | 55 | 63 |
| Liu et al. (2022) [52] | 490 | 81 | 72 | 97 | 92 | 74 | |
| Pang et al. (2022) [9] | 72 | 72 | 79 | 84 | 66 | 75 | |
| Postema et al. (2020) [54] | 113 | 81 | 64 | ||||
| Zhang et al. (2018) [30] | 78 | 84 | 88 | 87 | 85 | 86 |
| Author (Year) | Study Design | Population | Methodology | Device | Biopsies/Radical Prostatectomy | Outcome Measures | Conclusions |
|---|---|---|---|---|---|---|---|
| Chessa et al. (2021) [32] | Prospective database | 68 patients with biopsy-proven PCa. | Patients received mpMRI, which resulted in the discovery of an index lesion with a PIRADS-v2 score of at least 3. A fusion biopsy was performed. Micro-ultrasound images were taken of all males who had prostate cancer at the level of the index lesion as determined by biopsy. | ExactVuTM and mpMRI | Fusion biopsy was imaged by ExactVuTM | Sensitivity, specificity, PPV and NPV. | A high resolution of the prostatic peripheral zone is provided by ExactVuTM, which may advance the triage tool’s ability to identify csPCa. |
| Cornud et al. (2020) [33] | Retrospective single-center | 118 patients with a rising PSA level. | Micro-US-guided biopsy performed on patients with MRI guidance. All of the lesions that could be seen on the Micro-US were targeted without the use of image fusion, which was performed for the lesions that could be seen on the MRI and/or the micro ultrasound. | Micro-US and bp-MRI | MRI and TRUS-guided biopsies | Sensitivity and specificity. | The use of Micro-US as a complementary examination to bp-MRI may provide some potential in its ability to localize targets. |
| Fasulo et al. (2022) [34] | Prospective | 140 patients with biopsy-proven prostate cancer. | A side-free endorectal probe and a 29 MHz ExactVuTM Micro-US device were used for Micro-US imaging on all patients the day before RARP. | Micro-US | Radical prostatectomy | Sensitivity, specificity, negative predictive value and positive predictive value. AUC. | Micro-ultrasound could effectively predict EPE in patients scheduled for RARP based on the final pathology report. |
| Klotz et al. (2020) [35] | Multi-center prospective registry | 1040 patients were diagnosed with PCa based on abnormal digital rectal examination and/or increased PSA. | Biopsies were collected from micro-ultrasound and mpMRI targets. Systematic biopsy was taken up to 14 cores. | Micro ultrasound and mpMRI | Micro ultrasound and mpMRI | Sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV). | In comparison to mpMRI, micro ultrasound demonstrated a similar or greater sensitivity and similar specificity for csPCs. For targeted biopsy and prostate screening, micro ultrasound provides an inexpensive, one-session selection. |
| Lopci et al. (2021) [36] | Pilot prospective single-institutional clinical trial | 25 patients with suspicion of prostate cancer. | Patients were given 68 Ga-PSMA PET/TRUS fusion biopsy assignments, and their results were compared to PRI-MUS system grading. | 68 Ga-PSMA PET/CT, Micro-US, TRUS biopsy | Comparison of PRI-M with Ga-PSMA PET/TRUS fusion biopsy | Sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV). | The diagnostic performance of 68Ga-PSMA PET/CT is better than PRI-MUS protocol. |
| Lorusso et al. (2022) [37] | Retrospective | 32 patients with biopsy-proven PCs. | Patients diagnosed with prostate cancer using micro-ultrasound imaging and scheduled for radical prostatectomy. | Micro-US and mpMRI | Radical prostatectomy | Sensitivity, specificity, negative and positive predictive values, and accuracy. | In diagnosing prostate cancer index lesions, micro ultrasound showed high reliability, comparable to mpMRI in terms of performance. |
| Lughezzani et al. (2019) [39] | Prospective single-institutional clinical trial | 104 patients with a clinical suspicion of prostate cancer who were examined consecutively. | All patients had micro-ultrasound-targeted biopsies conducted by urologists who were unaware of the results of the mpMRI scans. Substantially, 12-core systematic and MRI/US-fusion-targeted biopsy were performed. | Micro-US and MRI/US | Micro-US-targeted biopsiesMRI/US fusion targeted | Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). | Micro ultrasound can provide more details about the absence or presence of csPCa in patients who have clinical suspicion of prostate cancer. |
| Lughezzani et al. (2021) [38] | Prospective cohort | 320 patients with a suspicion of PC based on high PSA test. | Patients had micro-ultrasound scan prior to biopsy utilizing ExactVu system. | MRI and Micro-US | MRI and Micro-US | Sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV). | For directed prostate biopsies, micro ultrasound is a capable imaging scanning technique. |
| Pavlovich et al. (2021) [31] | Prospective randomized clinical trial | 1676 candidates received prostate biopsy with unknown PCa. | One of two biopsy techniques—conventional ultrasonography or micro ultrasound—was assigned randomly to each patient. | Conventional ultrasound and micro ultrasound | Conventional ultrasound and micro ultrasound | Per-patient detection of csPCa. | Micro-US was not clearly superior to conventional ultrasound in detecting csPC during biopsy. |
| Socarras et al. (2020) [41] | Retrospective | 194 patients with suspicion of PCa. | Transperineal prostate biopsies technique utilizing ultrasound fusion targeted biopsy and real-time targeted Micro-US were performed on all patients. | Micro-ultrasound-guided biopsy and multiparametric MRI | Transperineal biopsies | Sensitivity, specificity, PPV and NPV. | High diagnostic accuracy for csPCa and PCa, preventing infectious complications associated with biopsy. |
| Wiemer et al. (2021) [40] | Prospective cohort | 159 patients with a clinical suspicion of PCa. | Patients with clinical suspicion of prostate cancer had TRUS biopsy by micro-ultrasound (ExactVu) system. Prior to prostate biopsy, all patients underwent mpMRI. | NTB, MRI-TB, Micro-US-TB, NTB + MRI-TB, NTB + Micro-US-TB, Micro-US-TB + MRI-TB | Systematic biopsy and targeted cores | Sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV). | Micro-US has advantages over mpMRI-targeted biopsies. It is feasible to replace conventional ultrasonography and eliminate routine systematic biopsies in a unique biopsy strategy that uses Micro-US and mpMRI only for targeted biopsies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, D.; Kernohan, N.; Li, C.; Nabi, G. Comparative Assessment of Different Ultrasound Technologies in the Detection of Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 4105. https://doi.org/10.3390/cancers15164105
Alghamdi D, Kernohan N, Li C, Nabi G. Comparative Assessment of Different Ultrasound Technologies in the Detection of Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(16):4105. https://doi.org/10.3390/cancers15164105
Chicago/Turabian StyleAlghamdi, Dareen, Neil Kernohan, Chunhui Li, and Ghulam Nabi. 2023. "Comparative Assessment of Different Ultrasound Technologies in the Detection of Prostate Cancer: A Systematic Review and Meta-Analysis" Cancers 15, no. 16: 4105. https://doi.org/10.3390/cancers15164105
APA StyleAlghamdi, D., Kernohan, N., Li, C., & Nabi, G. (2023). Comparative Assessment of Different Ultrasound Technologies in the Detection of Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers, 15(16), 4105. https://doi.org/10.3390/cancers15164105







