Peptidylarginine Deiminase Type 2 Predicts Tumor Progression and Poor Prognosis in Patients with Curatively Resected Biliary Tract Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
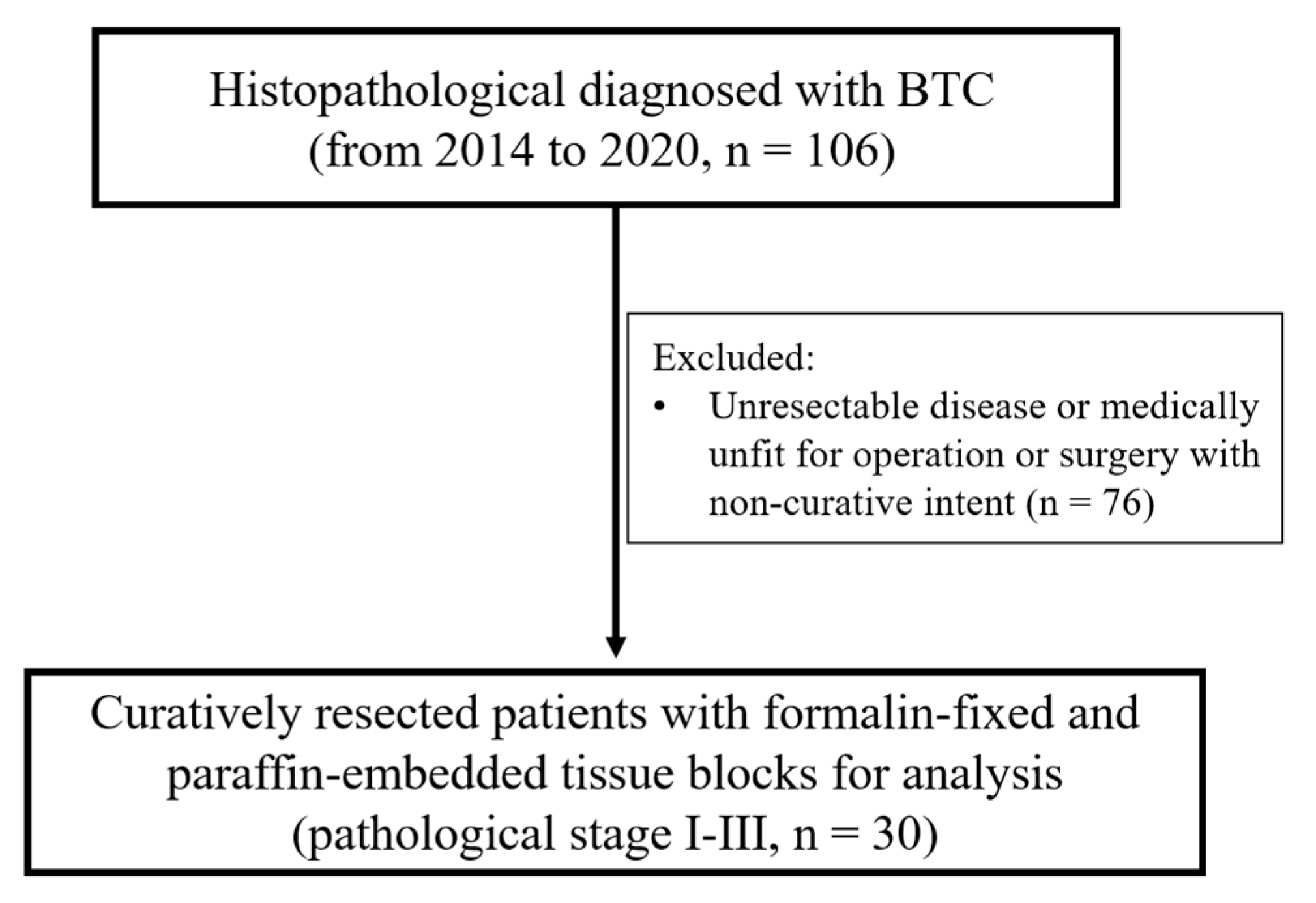
2.1. Patient Characteristics
2.2. Cancer Treatment
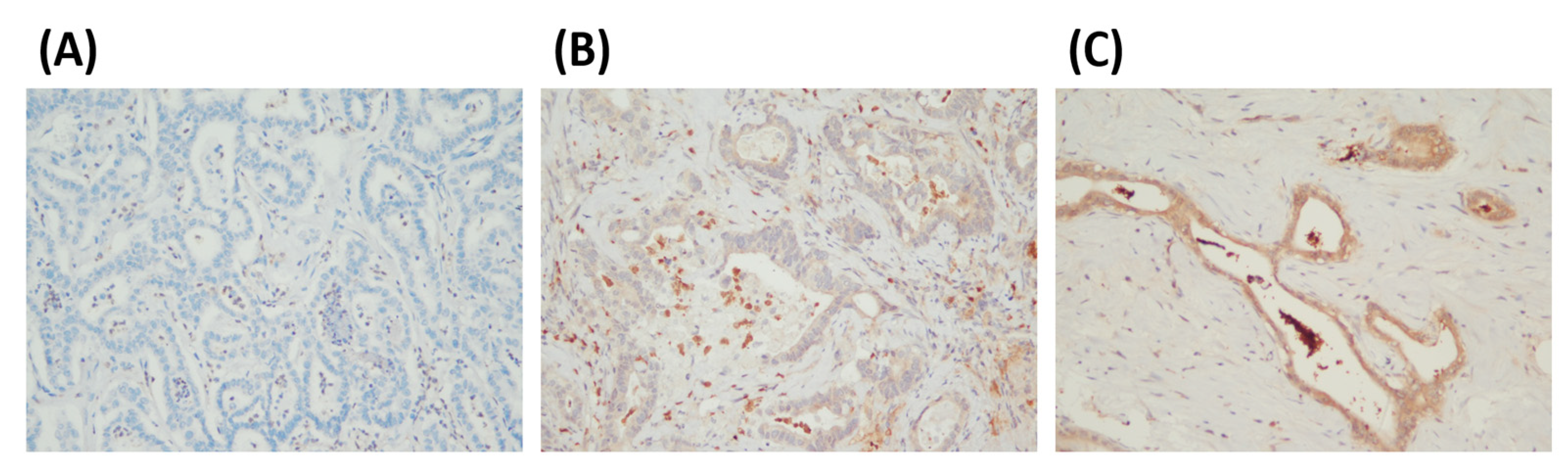
2.3. PADI2 Immunohistochemical Evaluation
2.4. Statistical Analysis
3. Results
3.1. Relationship between PADI2 Expression and Patients’ Clinicopathological Characteristics
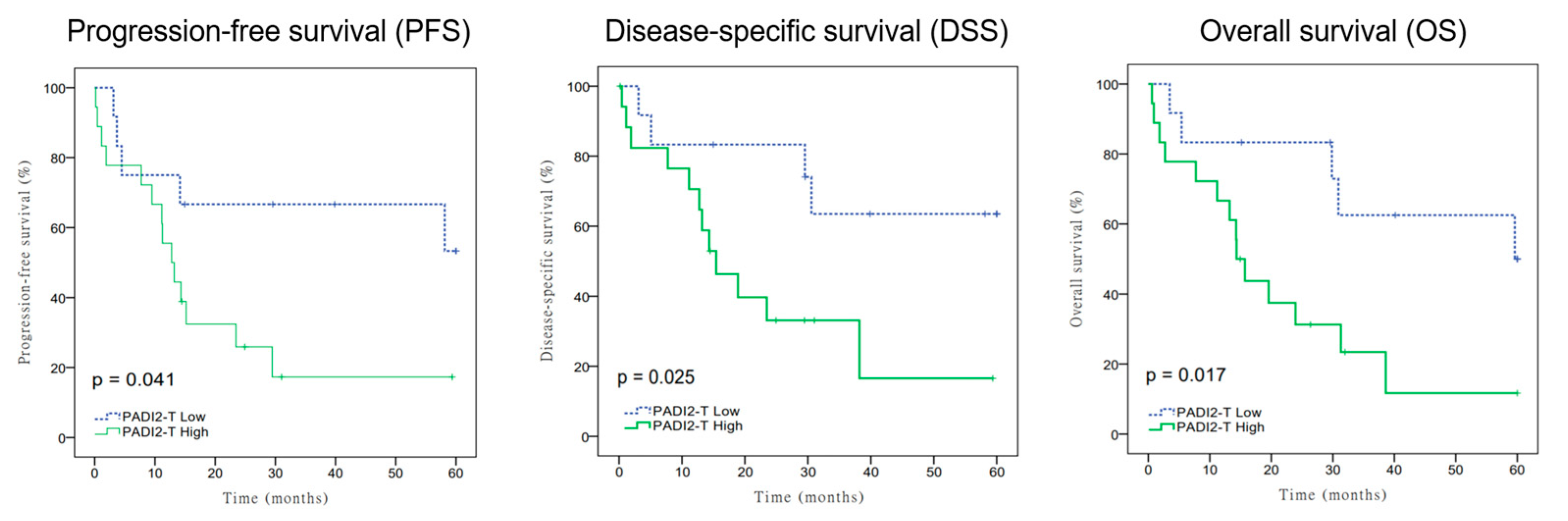
3.2. Relationship of PADI2 Expression on Progress-Free Survival (PFS), Disease-Specific Survival (DSS), and Overall Survival (OS)
3.3. Univariate and Multivariate Analysis of PADI2 Expression on Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valle, J.W.; Lamarca, A.; Goyal, L.; Barriuso, J.; Zhu, A.X. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017, 7, 943–962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Miao, K.; Sun, H.; Deng, C.X. Tumor heterogeneity reshapes the tumor microenvironment to influence drug resistance. Int. J. Biol. Sci. 2022, 18, 3019–3033. [Google Scholar] [CrossRef]
- Tariq, N.U.; McNamara, M.G.; Valle, J.W. Biliary tract cancers: Current knowledge, clinical candidates and future challenges. Cancer Manag. Res. 2019, 11, 2623–2642. [Google Scholar] [CrossRef]
- Tshering, G.; Dorji, P.W.; Chaijaroenkul, W.; Na-Bangchang, K. Biomarkers for the Diagnosis of Cholangiocarcinoma: A Systematic Review. Am. J. Trop. Med. Hyg. 2018, 98, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Hensen, S.M.; Pruijn, G.J. Methods for the detection of peptidylarginine deiminase (PAD) activity and protein citrullination. Mol. Cell Proteom. 2014, 13, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, P.; Tian, Y.; Ouyang, W.; Ho, J.W.; Alam, H.B.; Li, Y. Peptidylarginine Deiminase 2 in Host Immunity: Current Insights and Perspectives. Front. Immunol. 2021, 12, 761946. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, B.; Mittereder, N.; Chaerkady, R.; Strain, M.; An, L.L.; Rahman, S.; Ma, W.; Low, C.P.; Chan, D.; et al. Spontaneous Secretion of the Citrullination Enzyme PAD2 and Cell Surface Exposure of PAD4 by Neutrophils. Front. Immunol. 2017, 8, 1200. [Google Scholar] [CrossRef]
- Vossenaar, E.R.; Radstake, T.R.; van der Heijden, A.; van Mansum, M.A.; Dieteren, C.; de Rooij, D.J.; Barrera, P.; Zendman, A.J.; van Venrooij, W.J. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann. Rheum. Dis. 2004, 63, 373–381. [Google Scholar] [CrossRef]
- Beato, M.; Sharma, P. Peptidyl Arginine Deiminase 2 (PADI2)-Mediated Arginine Citrullination Modulates Transcription in Cancer. Int. J. Mol. Sci. 2020, 21, 1351. [Google Scholar] [CrossRef]
- Cherrington, B.D.; Zhang, X.; McElwee, J.L.; Morency, E.; Anguish, L.J.; Coonrod, S.A. Potential role for PAD2 in gene regulation in breast cancer cells. PLoS ONE 2012, 7, e41242. [Google Scholar] [CrossRef]
- Wang, L.; Song, G.; Zhang, X.; Feng, T.; Pan, J.; Chen, W.; Yang, M.; Bai, X.; Pang, Y.; Yu, J.; et al. PADI2-Mediated Citrullination Promotes Prostate Cancer Progression. Cancer Res. 2017, 77, 5755–5768. [Google Scholar] [CrossRef] [PubMed]
- Gijon, M.; Metheringham, R.L.; Toss, M.S.; Paston, S.J.; Durrant, L.G. The Clinical and Prognostic Significance of Protein Arginine Deiminases 2 and 4 in Colorectal Cancer. Pathobiology 2022, 89, 38–48. [Google Scholar] [CrossRef] [PubMed]
- McElwee, J.L.; Mohanan, S.; Griffith, O.L.; Breuer, H.C.; Anguish, L.J.; Cherrington, B.D.; Palmer, A.M.; Howe, L.R.; Subramanian, V.; Causey, C.P.; et al. Identification of PADI2 as a potential breast cancer biomarker and therapeutic target. BMC Cancer 2012, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Z.; Zhang, G.; Wang, T.; Ma, Y.; Guo, W. Down-regulation of PADI2 prevents proliferation and epithelial-mesenchymal transition in ovarian cancer through inhibiting JAK2/STAT3 pathway in vitro and in vivo, alone or in combination with Olaparib. J. Transl. Med. 2020, 18, 357. [Google Scholar] [CrossRef]
- Cantariño, N.; Musulén, E.; Valero, V.; Peinado, M.A.; Perucho, M.; Moreno, V.; Forcales, S.V.; Douet, J.; Buschbeck, M. Downregulation of the Deiminase PADI2 Is an Early Event in Colorectal Carcinogenesis and Indicates Poor Prognosis. Mol. Cancer Res. 2016, 14, 841–848. [Google Scholar] [CrossRef]
- Loos, T.; Mortier, A.; Gouwy, M.; Ronsse, I.; Put, W.; Lenaerts, J.P.; Van Damme, J.; Proost, P. Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: A naturally occurring posttranslational modification of chemokines and new dimension of immunoregulation. Blood 2008, 112, 2648–2656. [Google Scholar] [CrossRef]
- Proost, P.; Loos, T.; Mortier, A.; Schutyser, E.; Gouwy, M.; Noppen, S.; Dillen, C.; Ronsse, I.; Conings, R.; Struyf, S.; et al. Citrullination of CXCL8 by peptidylarginine deiminase alters receptor usage, prevents proteolysis, and dampens tissue inflammation. J. Exp. Med. 2008, 205, 2085–2097. [Google Scholar] [CrossRef]
- Lee, H.J.; Joo, M.; Abdolrasulnia, R.; Young, D.G.; Choi, I.; Ware, L.B.; Blackwell, T.S.; Christman, B.W. Peptidylarginine deiminase 2 suppresses inhibitory {kappa}B kinase activity in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Biol. Chem. 2010, 285, 39655–39662. [Google Scholar] [CrossRef]
- Mohanan, S.; Cherrington, B.D.; Horibata, S.; McElwee, J.L.; Thompson, P.R.; Coonrod, S.A. Potential role of peptidylarginine deiminase enzymes and protein citrullination in cancer pathogenesis. Biochem. Res. Int. 2012, 2012, 895343. [Google Scholar] [CrossRef]
- Amin, M.B.; American Joint Committee on Cancer. AJCC Cancer Staging Manual, 8th ed.; American Joint Committee on Cancer/Springer: Chicago, IL, USA, 2017; p. xvii. 1024p. [Google Scholar]
- Edge, S.B.; American Joint Committee on Cancer. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010; p. xix. 718p. [Google Scholar]
- Kim, B.J.; Newhook, T.E.; Tzeng, C.D.; Ikoma, N.; Chiang, Y.J.; Chun, Y.S.; Vauthey, J.N.; Tran Cao, H.S. Lymphadenectomy and margin-negative resection for biliary tract cancer surgery in the United States—Differential technical performance by approach. J. Surg. Oncol. 2022, 126, 658–666. [Google Scholar] [CrossRef]
- Rizzo, A.; Brandi, G. Adjuvant systemic treatment in resected biliary tract cancer: State of the art, controversies, and future directions. Cancer Treat. Res. Commun. 2021, 27, 100334. [Google Scholar] [CrossRef]
- Palloni, A.; Frega, G.; De Lorenzo, S.; Rizzo, A.; Abbati, F.; Deserti, M.; Tavolari, S.; Brandi, G. Adjuvant treatment in biliary tract cancer. Transl. Cancer Res. 2019, 8, S289–S296. [Google Scholar] [CrossRef] [PubMed]
- Brunner, T.B.; Eccles, C.L. Radiotherapy and chemotherapy as therapeutic strategies in extrahepatic biliary duct carcinoma. Strahlenther. Onkol. 2010, 186, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Lin, H.Y.; Hung, S.K.; Chiou, W.Y.; Lee, M.S. Role of modern radiotherapy in managing patients with hepatocellular carcinoma. World J. Gastroenterol. 2021, 27, 2434–2457. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Hung, S.K.; Chiou, W.Y.; Lee, M.S.; Shen, B.J.; Chen, L.C.; Liu, D.W.; Tsai, W.T.; Lin, P.H.; Shih, Y.T.; et al. Significant symptoms alleviation and tumor volume reduction after combined simultaneously integrated inner-escalated boost and volumetric-modulated arc radiotherapy in a patient with unresectable bulky hepatocellular carcinoma: A care-compliant case report. Medicine 2016, 95, e4717. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Chan, M.W.Y.; Lin, H.Y.; Chiou, W.Y.; Lin, R.I.; Chen, C.A.; Lee, M.S.; Chi, C.L.; Chen, L.C.; Huang, L.W.; et al. IRAK2, an IL1R/TLR Immune Mediator, Enhances Radiosensitivity via Modulating Caspase 8/3-Mediated Apoptosis in Oral Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 647175. [Google Scholar] [CrossRef]
- Yu, C.C.; Lin, H.Y.; Hsieh, C.H.; Chan, M.W.Y.; Chiou, W.Y.; Lee, M.S.; Chi, C.L.; Lin, R.I.; Hsu, F.C.; Chen, L.C.; et al. IRAK2, an Immune and Radiation-Response Gene, Correlates with Advanced Disease Features but Predicts Higher Post-Irradiation Local Control in Non-Metastatic and Resected Oral Cancer Patients. Int. J. Mol. Sci. 2023, 24, 6903. [Google Scholar] [CrossRef]
- Lin, H.Y.; Hung, S.K.; Lee, M.S.; Chiou, W.Y.; Huang, T.T.; Tseng, C.E.; Shih, L.Y.; Lin, R.I.; Lin, J.M.; Lai, Y.H.; et al. DNA methylome analysis identifies epigenetic silencing of FHIT as a determining factor for radiosensitivity in oral cancer: An outcome-predicting and treatment-implicating study. Oncotarget 2015, 6, 915–934. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Liu, C.; Li, J.; Li, J.; Wu, H.; Wang, G.; Li, D. Importance of Normalization of Carbohydrate Antigen 19-9 in Patients with Intrahepatic Cholangiocarcinoma. Front. Oncol. 2021, 11, 780455. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim. Biophys. Acta 2013, 1829, 1126–1135. [Google Scholar] [CrossRef]
- Liu, C.Y.; Lin, H.H.; Tang, M.J.; Wang, Y.K. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 2015, 6, 15966–15983. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- McElwee, J.L.; Mohanan, S.; Horibata, S.; Sams, K.L.; Anguish, L.J.; McLean, D.; Cvitaš, I.; Wakshlag, J.J.; Coonrod, S.A. PAD2 overexpression in transgenic mice promotes spontaneous skin neoplasia. Cancer Res. 2014, 74, 6306–6317. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Guedj, N.; Clapéron, A.; Nguyen Ho-Bouldoires, T.H.; Paradis, V.; Fouassier, L. Epithelial-mesenchymal transition in cholangiocarcinoma: From clinical evidence to regulatory networks. J. Hepatol. 2017, 66, 424–441. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Cao, Z.; Ding, Q.; Li, Z.; Zhang, C. Prognostic value of multiple epithelial mesenchymal transition-associated proteins in intrahepatic cholangiocarcinoma. Oncol. Lett. 2019, 18, 2059–2065. [Google Scholar] [CrossRef]
- Oh, C.R.; Kim, H.D.; Ryu, Y.M.; Lee, S.; Kim, D.; Lee, D.S.; Jeong, J.H.; Chang, H.M.; Ryoo, B.Y.; Kim, K.P.; et al. Epithelial-Mesenchymal Transition Phenotype and Peritumoral Immune Cell Infiltration in Advanced Biliary Tract Cancer. Anticancer Res. 2023, 43, 645–652. [Google Scholar] [CrossRef]
- Rubesa-Mihaljevic, R.; Babarovic, E.; Vrdoljak-Mozetic, D.; Stemberger-Papic, S.; Klaric, M.; Krasevic, M.; Jonjic, N. The Immunohistochemical Pattern of Epithelial-Mesenchymal Transition Markers In Endometrial Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 339–346. [Google Scholar] [CrossRef]
- Li, W.; Jia, H.; Wang, S.; Guo, X.; Zhang, X.; Zhang, L.; Wen, H.Y.; Fu, L. The presence of retraction clefts correlates with lymphovascular invasion and lymph node metastasis and predicts poor outcome: Analysis of 2497 breast cancer patients. Ann. Diagn. Pathol. 2022, 61, 152047. [Google Scholar] [CrossRef]
- Kariri, Y.A.; Aleskandarany, M.A.; Joseph, C.; Kurozumi, S.; Mohammed, O.J.; Toss, M.S.; Green, A.R.; Rakha, E.A. Molecular Complexity of Lymphovascular Invasion: The Role of Cell Migration in Breast Cancer as a Prototype. Pathobiology 2020, 87, 218–231. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, N.; Wang, X.; Huang, Y.; Zhou, X.; Zhang, D. Potential roles of lymphovascular space invasion based on tumor characteristics provide important prognostic information in T1 tumors with ER and HER2 positive breast cancer. Clin. Transl. Oncol. 2020, 22, 2275–2285. [Google Scholar] [CrossRef]
- Liang, C.; Jiang, H.; Sun, L.; Kang, S.; Cui, Z.; Wang, L.; Zhao, W.; Bin, X.; Lang, J.; Liu, P.; et al. Which factors predict parametrial involvement in stage IB cervical cancer? A Chinese multicentre study. Eur. J. Surg. Oncol. 2023. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, W.; Li, X.; Xu, D.; Liu, L. Prognostic value of lymphovascular space invasion in stage IA to IIB cervical cancer: A meta-analysis. Medicine 2023, 102, e33547. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, H.H.B.; Hardie, A.N.; Moncada-Torres, A.; Hogdall, C.K.; Bekkers, R.L.M.; Falconer, H.; Jensen, P.T.; Nijman, H.W.; van der Aa, M.A.; Martin, F.; et al. A federated approach to identify women with early-stage cervical cancer at low risk of lymph node metastases. Eur. J. Cancer 2023, 185, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dessi, M.; Marcus, D.; Russell, J.; Aboagye, E.O.; Ellis, L.B.; Sheeka, A.; Park, W.E.; Bharwani, N.; Ghaem-Maghami, S.; et al. Prediction of Deep Myometrial Infiltration, Clinical Risk Category, Histological Type, and Lymphovascular Space Invasion in Women with Endometrial Cancer Based on Clinical and T2-Weighted MRI Radiomic Features. Cancers 2023, 15, 2209. [Google Scholar] [CrossRef]
- Oliver-Perez, M.R.; Padilla-Iserte, P.; Arencibia-Sanchez, O.; Martin-Arriscado, C.; Muruzabal, J.C.; Diaz-Feijoo, B.; Cabrera, S.; Coronado, P.; Martin-Salamanca, M.B.; Pantoja-Garrido, M.; et al. Lymphovascular Space Invasion in Early-Stage Endometrial Cancer (LySEC): Patterns of Recurrence and Predictors. A Multicentre Retrospective Cohort Study of the Spain Gynecologic Oncology Group. Cancers 2023, 15, 2612. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, V.; Kontopantelis, E.; Cuccu, I.; Sgamba, L.; Golia D’Auge, T.; Pernazza, A.; Della Rocca, C.; Manganaro, L.; Catalano, C.; Perniola, G.; et al. Magnetic resonance imaging-radiomics in endometrial cancer: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2023. [Google Scholar] [CrossRef]
- Lin, H.Y.; Huang, T.T.; Lee, M.S.; Hung, S.K.; Lin, R.I.; Tseng, C.E.; Chang, S.M.; Chiou, W.Y.; Hsu, F.C.; Hsu, W.L.; et al. Unexpected close surgical margin in resected buccal cancer: Very close margin and DAPK promoter hypermethylation predict poor clinical outcomes. Oral Oncol. 2013, 49, 336–344. [Google Scholar] [CrossRef]
| PADI2 | |||||
|---|---|---|---|---|---|
| Variables | Low Expression (n = 12) | High Expression (n = 18) | p-Value | ||
| Median age (IQR), years | 71.50 | (60.5, 76.5) | 75.00 | (66.5, 77.0) | |
| Age: n (%) | 0.392 | ||||
| <65 | 4 | 57.1% | 3 | 42.9% | |
| ≥65 | 8 | 34.8% | 15 | 65.2% | |
| Gender: n (%) | 0.710 | ||||
| Male | 7 | 46.7% | 8 | 53.3% | |
| Female | 5 | 33.3% | 10 | 66.7% | |
| pN: n (%) | 0.465 | ||||
| N0 | 8 | 47.1% | 9 | 52.9% | |
| N1–2 | 4 | 30.8% | 9 | 69.2% | |
| p-Stage: n (%) | 0.704 | ||||
| Stage I–II | 9 | 42.9% | 12 | 57.1% | |
| Stage III | 3 | 33.3% | 6 | 66.7% | |
| Surgical margins | 0.456 | ||||
| <3 mm | 5 | 33.3% | 10 | 66.7% | |
| ≥3 mm | 7 | 46.7% | 8 | 53.3% | |
| Perineural invasion: n (%) | 0.135 | ||||
| Present | 4 | 25.0% | 12 | 75.0% | |
| Absent | 8 | 57.1% | 6 | 42.9% | |
| Lymphovascular invasion: n (%) | 0.060 | ||||
| Present | 3 | 20.0% | 12 | 80.0% | |
| Absent | 9 | 60.0% | 6 | 40.0% | |
| Chemotherapy: n (%) | 0.066 | ||||
| No | 12 | 48.0% | 13 | 52.0% | |
| Yes | 0 | 0.0% | 5 | 100.0% | |
| Radiotherapy: n (%) | 0.130 | ||||
| No | 12 | 46.2% | 14 | 53.8% | |
| Yes | 0 | 0.0% | 4 | 100.0% | |
| Variables | Dichotomized Units | OS | DSS | PFS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | ||
| Age | ≥65 vs. <65 | 0.765 | (0.274–2.133) | 0.609 | 0.842 | (0.270–2.626) | 0.767 | 0.701 | (0.252–1.953) | 0.497 |
| Gender | Male vs. Female | 1.143 | (0.463–2.824) | 0.772 | 1.021 | (0.379–2.751) | 0.968 | 1.018 | (0.413–2.508) | 0.969 |
| Surgical margins | ≥3 mm vs. <3 mm | 0.517 | (0.206–1.296) | 0.159 | 0.407 | (0.146–1.137) | 0.086 | 0.623 | (0.250–1.556) | 0.311 |
| p-Stage | III vs. I–II | 1.762 | (0.690–4.501) | 0.236 | 1.925 | (0.695–5.330) | 0.208 | 1.659 | (0.650–4.230) | 0.290 |
| Lymphovascular invasion | Present vs. Absent | 1.826 | (0.700–4.763) | 0.219 | 1.824 | (0.647–5.142) | 0.255 | 1.515 | (0.586–3.920) | 0.392 |
| PADI2 | High vs. Low | 3.399 | (1.183–9.766) | 0.023 | 3.519 | (1.101–11.250) | 0.034 | 2.869 | (1.002–8.222) | 0.050 |
| Variables | Dichotomized Units | OS | DSS | PFS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | ||
| Age | ≥65 vs. <65 | 0.476 | (0.111–2.033) | 0.316 | 0.470 | (0.096–2.300) | 0.351 | 0.490 | (0.119–2.013) | 0.322 |
| Gender | Male vs. Female | 0.932 | (0.256–3.392) | 0.915 | 0.827 | (0.204–3.355) | 0.790 | 0.850 | (0.248–2.917) | 0.797 |
| Surgical margins | ≥3 mm vs. <3 mm | 1.115 | (0.232–5.365) | 0.892 | 0.655 | (0.129–3.330) | 0.610 | 1.511 | (0.306–7.456) | 0.613 |
| p-Stage | III vs. I–II | 2.841 | (0.564–14.321) | 0.206 | 2.201 | (0.428–11.334) | 0.345 | 3.066 | (0.604–15.556) | 0.176 |
| Lymphovascular invasion | Present vs. Absent | 0.280 | (0.063–1.247) | 0.095 | 0.419 | (0.091–1.925) | 0.264 | 0.365 | (0.085–1.571) | 0.176 |
| PADI2 | High vs. Low | 8.449 | (1.843–38.740) | 0.006 | 6.166 | (1.274–29.840) | 0.024 | 5.676 | (1.226–26.280) | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-Y.; Yu, C.-C.; Chi, C.-L.; Wei, C.-K.; Yin, W.-Y.; Tseng, C.-E.; Li, S.-C. Peptidylarginine Deiminase Type 2 Predicts Tumor Progression and Poor Prognosis in Patients with Curatively Resected Biliary Tract Cancer. Cancers 2023, 15, 4131. https://doi.org/10.3390/cancers15164131
Lin H-Y, Yu C-C, Chi C-L, Wei C-K, Yin W-Y, Tseng C-E, Li S-C. Peptidylarginine Deiminase Type 2 Predicts Tumor Progression and Poor Prognosis in Patients with Curatively Resected Biliary Tract Cancer. Cancers. 2023; 15(16):4131. https://doi.org/10.3390/cancers15164131
Chicago/Turabian StyleLin, Hon-Yi, Chih-Chia Yu, Chen-Lin Chi, Chang-Kuo Wei, Wen-Yao Yin, Chih-En Tseng, and Szu-Chin Li. 2023. "Peptidylarginine Deiminase Type 2 Predicts Tumor Progression and Poor Prognosis in Patients with Curatively Resected Biliary Tract Cancer" Cancers 15, no. 16: 4131. https://doi.org/10.3390/cancers15164131
APA StyleLin, H. -Y., Yu, C. -C., Chi, C. -L., Wei, C. -K., Yin, W. -Y., Tseng, C. -E., & Li, S. -C. (2023). Peptidylarginine Deiminase Type 2 Predicts Tumor Progression and Poor Prognosis in Patients with Curatively Resected Biliary Tract Cancer. Cancers, 15(16), 4131. https://doi.org/10.3390/cancers15164131






