Immunotherapy in Acute Leukemias: Past Success Paves the Way for Future Progress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Early Immunotherapy and Allogenic Hematopoietic Stem Cell Transplantation
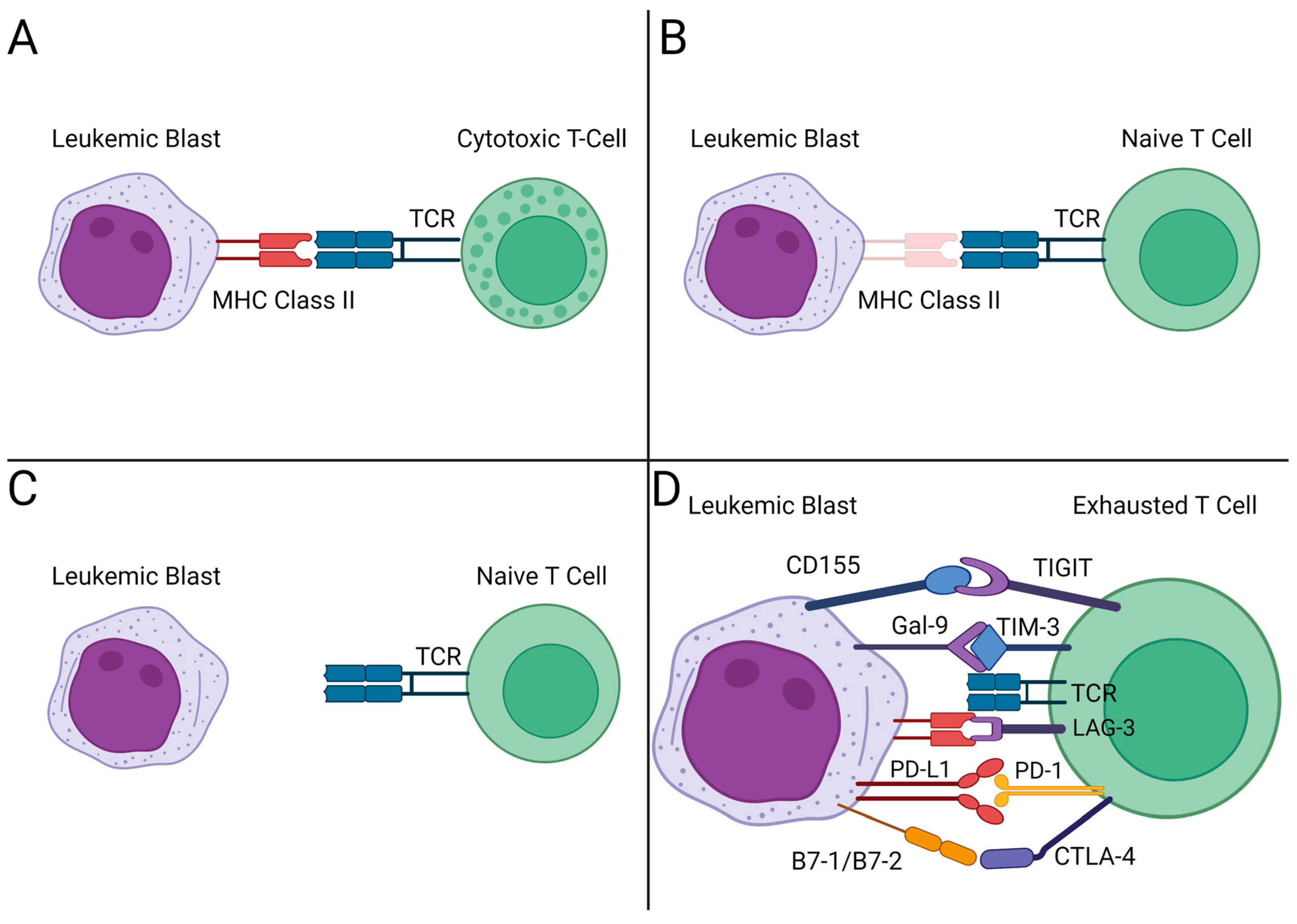
3. Immune Checkpoint Blockade
| Agent | Target | Malignancy | Outcomes | Trial/Development Phase | Reference |
|---|---|---|---|---|---|
| Monoclonal Antibodies | |||||
| Magrolimab | CD47/SIPRα | AML, first-line | Trial ongoing | Phase III clinical trial | [42] |
| Rituximab | CD20 | ALL | Ritux + chemo vs. chemo alone: 2-year event-free survival 65% vs. 52% (p = 0.04) | FDA approved | [48,49] |
| Immune Cell Engagers | |||||
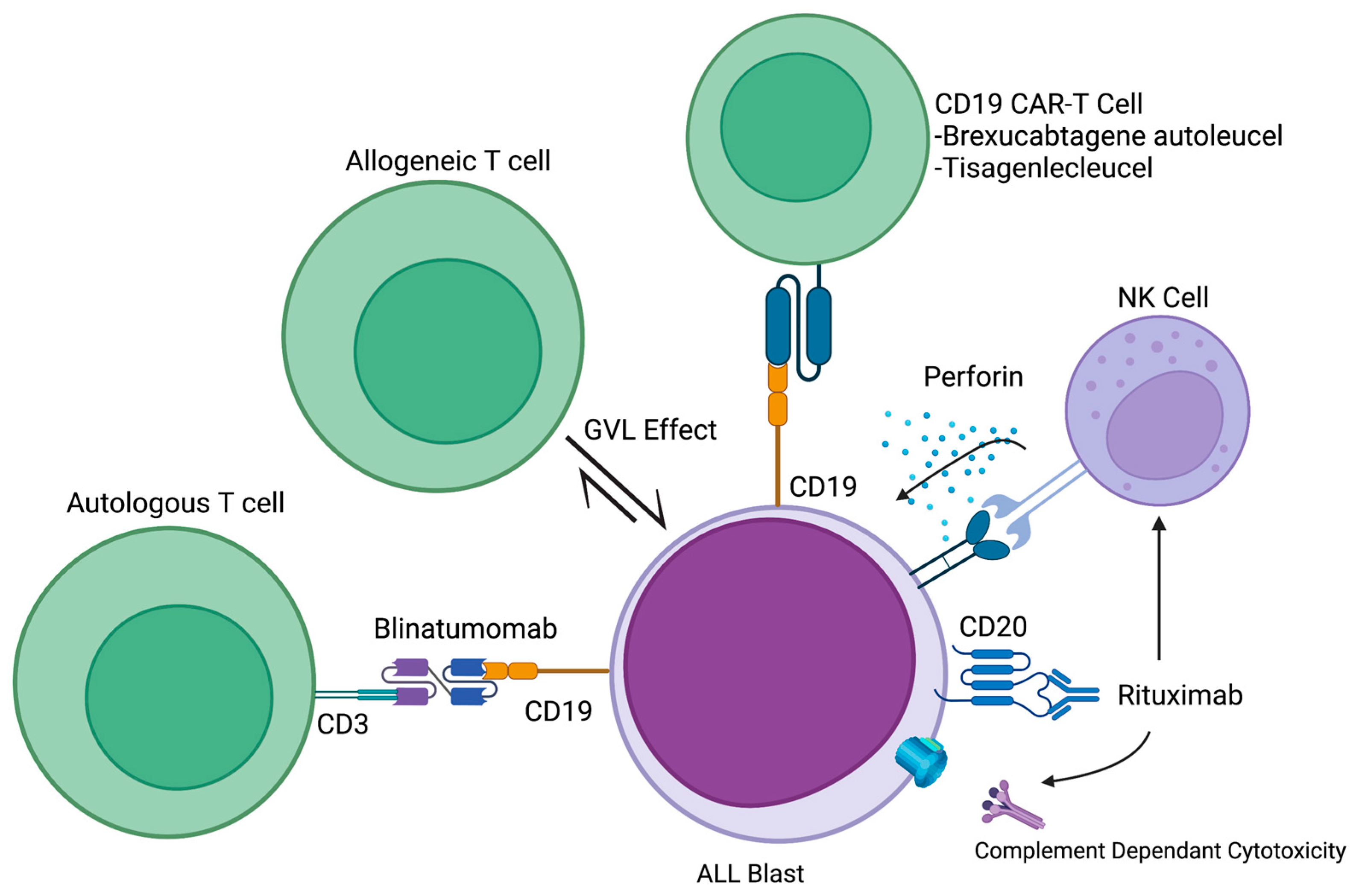
| Blinatumomab | CD19/CD3 | Relapsed and refractory ALL | Blina vs. chemo: median overall survival 7.7 months vs. 4 months (p = 0.01) | FDA approved | [50] |
| MRD + ALL | MRD after 1 cycle of blina: 80%, 95% CI [71–87] | FDA approved | [51] | ||
| Newly diagnosed Ph + ALL | Dasatinib induction followed by blina + dasatinib consolidation: 18-month survival 95%, MRD response rate 52% | Phase II clinical trial | [52] | ||
| Chimeric Antigen T-Cell Therapy (CAR-T) | |||||
| Tisagenlecleucel | CD19 | ALL, relapsed/refractory | Three-month complete remission rate: 81%, 95% CI [69–91] | FDA approved | [53] |
| Brexucabtagene autoleucel | CD19 | B-ALL, relapsed/refractory | Complete remission rate: 71%, 95% CI [57–82] | FDA approved | [54] |
4. Monoclonal Abs
4.1. Anti-CD20
4.2. Anti-CD38
5. Immune Cell Engagers (ICE)
6. Cellular-Based Therapies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coley, W.B., II. Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891, 14, 199–220. [Google Scholar] [CrossRef]
- McCarthy, E.F. The Toxins of William B. Coley and the Treatment of Bone and Soft-Tissue Sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar] [PubMed]
- Thomas, E.D.; Lochte, H.L.; Lu, W.C.; Ferrebee, J.W. Intravenous Infusion of Bone Marrow in Patients Receiving Radiation and Chemotherapy. N. Engl. J. Med. 1957, 257, 491–496. [Google Scholar] [CrossRef]
- Fefer, A.; Einstein, A.B.; Thomas, E.D.; Buckner, C.D.; Clift, R.A.; Glucksberg, H.; Neiman, P.E.; Storb, R. Bone-Marrow Transplantation for Hematologic Neoplasia in 16 Patients with Identical Twins. N. Engl. J. Med. 1974, 290, 1389–1393. [Google Scholar] [CrossRef]
- Thomas, E.D.; Buckner, C.D.; Clift, R.A.; Fefer, A.; Johnson, F.L.; Neiman, P.E.; Sale, G.E.; Sanders, J.E.; Singer, J.W.; Shulman, H.; et al. Marrow Transplantation for Acute Nonlymphoblastic Leukemia in First Remission. N. Engl. J. Med. 1979, 301, 597–599. [Google Scholar] [CrossRef]
- Mathe, G.; Amiel, J.L.; Schwarzenberg, L.; Cattan, A.; Schneider, M. Adoptive Immunotherapy of Acute Leukemia: Experimental and Clinical Results. Cancer Res. 1965, 25, 1525–1531. [Google Scholar]
- Weiden, P.L.; Flournoy, N.; Thomas, E.D.; Prentice, R.; Fefer, A.; Buckner, C.D.; Storb, R. Antileukemic Effect of Graft-versus-Host Disease in Human Recipients of Allogeneic-Marrow Grafts. N. Engl. J. Med. 1979, 300, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Weiden, P.L.; Sullivan, K.M.; Flournoy, N.; Storb, R.; Thomas, E.D. Antileukemic Effect of Chronic Graft-versus-Host Disease: Contribution to Improved Survival after Allogeneic Marrow Transplantation. N. Engl. J. Med. 1981, 304, 1529–1533. [Google Scholar] [CrossRef] [PubMed]
- Granot, N.; Storb, R. History of Hematopoietic Cell Transplantation: Challenges and Progress. Haematologica 2020, 105, 2716–2729. [Google Scholar] [CrossRef]
- Horowitz, M.M.; Gale, R.P.; Sondel, P.M.; Goldman, J.M.; Kersey, J.; Kolb, H.-J.; Rimm, A.A.; Ringdén, O.; Rozman, C.; Speck, B.; et al. Graft-Versus-Leukemia Reactions After Bone Marrow Transplantation. Blood 1990, 75, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.; de Wreede, L.C.; Brand, R.; van Biezen, A.; Dreger, P.; Mohty, M.; de Witte, T.M.; Kröger, N.; Ruutu, T. Sensitivity of Hematological Malignancies to Graft-versus-Host Effects: An EBMT Megafile Analysis. Leukemia 2014, 28, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Montoro, J.; Ceberio, I.; Hilden, P.; Maloy, M.A.; Barker, J.; Castro-Malaspina, H.; Dahi, P.; Koehne, G.; Perales, M.-A.; Ponce, D.; et al. Ex Vivo T Cell-Depleted Hematopoietic Stem Cell Transplantation for Adult Patients with Acute Myeloid Leukemia in First and Second Remission: Long-Term Disease-Free Survival with a Significantly Reduced Risk of Graft-versus-Host Disease. Biol. Blood Marrow Transpl. 2020, 26, 323–332. [Google Scholar] [CrossRef]
- Yeshurun, M.; Weisdorf, D.; Rowe, J.M.; Tallman, M.S.; Zhang, M.J.; Wang, H.L.; Saber, W.; de Lima, M.; Sandmaier, B.M.; Uy, G.; et al. The Impact of the Graft-versus-Leukemia Effect on Survival in Acute Lymphoblastic Leukemia. Blood Adv. 2019, 3, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Gillissen, M.A.; Kedde, M.; Jong, G.d.; Moiset, G.; Yasuda, E.; Levie, S.E.; Bakker, A.Q.; Claassen, Y.B.; Wagner, K.; Böhne, M.; et al. AML-Specific Cytotoxic Antibodies in Patients with Durable Graft-versus-Leukemia Responses. Blood 2018, 131, 131–143. [Google Scholar] [CrossRef]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic Cell Transplantation (HCT)-Specific Comorbidity Index: A New Tool for Risk Assessment before Allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef]
- Backhaus, D.; Brauer, D.; Pointner, R.; Bischof, L.; Vucinic, V.; Franke, G.-N.; Niederwieser, D.; Platzbecker, U.; Jentzsch, M.; Schwind, S. A High Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) Does Not Impair Outcomes after Non-Myeloablative Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia Patients 60 Years or Older. Bone Marrow Transpl. 2023, 58, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Vago, L.; Perna, S.K.; Zanussi, M.; Mazzi, B.; Barlassina, C.; Stanghellini, M.T.L.; Perrelli, N.F.; Cosentino, C.; Torri, F.; Angius, A.; et al. Loss of Mismatched HLA in Leukemia after Stem-Cell Transplantation. N. Engl. J. Med. 2009, 361, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Christopher, M.J.; Petti, A.A.; Rettig, M.P.; Miller, C.A.; Chendamarai, E.; Duncavage, E.J.; Klco, J.M.; Helton, N.M.; O’Laughlin, M.; Fronick, C.C.; et al. Immune Escape of Relapsed AML Cells after Allogeneic Transplantation. N. Engl. J. Med. 2018, 379, 2330–2341. [Google Scholar] [CrossRef]
- Gournay, V.; Vallet, N.; Peux, V.; Vera, K.; Bordenave, J.; Lambert, M.; Corneau, A.; Michonneau, D.; Peffault de Latour, R.; Caillat-Zucman, S.; et al. Immune Landscape after Allo-HSCT: TIGIT- and CD161-Expressing CD4 T Cells Are Associated with Subsequent Leukemia Relapse. Blood 2022, 140, 1305–1321. [Google Scholar] [CrossRef]
- Williams, P.; Basu, S.; Garcia-Manero, G.; Hourigan, C.S.; Oetjen, K.A.; Cortes, J.E.; Ravandi, F.; Jabbour, E.J.; Al-Hamal, Z.; Konopleva, M.; et al. The Distribution of T-Cell Subsets and the Expression of Immune Checkpoint Receptors and Ligands in Patients with Newly Diagnosed and Relapsed Acute Myeloid Leukemia. Cancer 2019, 125, 1470–1481. [Google Scholar] [CrossRef]
- Toffalori, C.; Zito, L.; Gambacorta, V.; Riba, M.; Oliveira, G.; Bucci, G.; Barcella, M.; Spinelli, O.; Greco, R.; Crucitti, L.; et al. Immune Signature Drives Leukemia Escape and Relapse after Hematopoietic Cell Transplantation. Nat. Med. 2019, 25, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Thanarajasingam, G.; Kim, H.T.; Cutler, C.; Ho, V.T.; Koreth, J.; Alyea, E.P.; Antin, J.H.; Soiffer, R.J.; Armand, P. Outcome and Prognostic Factors for Patients Who Relapse After Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transpl. 2013, 19, 1713–1718. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Kim, H.T.; Bachireddy, P.; Costello, C.; Liguori, R.; Savell, A.; Lukez, A.P.; Avigan, D.; Chen, Y.-B.; McSweeney, P.; et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N. Engl. J. Med. 2016, 375, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Holderried, T.A.W.; Fraccaroli, A.; Schumacher, M.; Heine, A.; Brossart, P.; Stelljes, M.; Klobuch, S.; Kröger, N.; Apostolova, P.; Finke, J.; et al. The Role of Checkpoint Blockade after Allogeneic Stem Cell Transplantation in Diseases Other than Hodgkin’s Lymphoma. Bone Marrow Transpl. 2019, 54, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Kim, H.T.; Costello, C.; Herrera, A.F.; Locke, F.L.; Maegawa, R.O.; Savell, A.; Mazzeo, M.; Anderson, A.; Boardman, A.P.; et al. A Multicenter Phase 1 Study of Nivolumab for Relapsed Hematologic Malignancies after Allogeneic Transplantation. Blood 2020, 135, 2182–2191. [Google Scholar] [CrossRef]
- Godfrey, J.; Liu, H.; Yu, J.; Tallarico, M.; Curran, E.; Artz, A.; Riedell, P.A.; Stock, W.; Karrison, T.; Fitzpatrick, C.; et al. Pembrolizumab for the Treatment of Disease Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Blood Adv. 2023, 7, 963–970. [Google Scholar] [CrossRef]
- Wang, A.Y.; Kline, J.; Stock, W.; Kosuri, S.; Artz, A.; Larson, R.A.; Riedell, P.A.; Bishop, M.; Liu, H. Unexpected Toxicities When Nivolumab Was Given as Maintenance Therapy Following Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transpl. 2020, 26, 1025–1027. [Google Scholar] [CrossRef]
- Yang, H.; Bueso-Ramos, C.; DiNardo, C.; Estecio, M.R.; Davanlou, M.; Geng, Q.-R.; Fang, Z.; Nguyen, M.; Pierce, S.; Wei, Y.; et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in Myelodysplastic Syndromes Is Enhanced by Treatment with Hypomethylating Agents. Leukemia 2014, 28, 1280–1288. [Google Scholar] [CrossRef]
- Daver, N.; Basu, S.; Garcia-Manero, G.; A Abbas, H.; Konopleva, M.; Kadia, T.M.; DiNardo, C.D.; Borthakur, G.; Alotaibi, A.S.; Pemmaraju, N.; et al. Azacitidine (AZA) with Nivolumab (Nivo), and AZA with Nivo + Ipilimumab (Ipi) in Relapsed/Refractory (R/R) Acute Myeloid Leukemia: Clinical and Immune Biomarkers of Response. Blood 2020, 136, 43–45. [Google Scholar] [CrossRef]
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef]
- Goswami, M.; Gui, G.; Dillon, L.W.; Lindblad, K.E.; Thompson, J.; Valdez, J.; Kim, D.-Y.; Ghannam, J.Y.; Oetjen, K.A.; Destefano, C.B.; et al. Pembrolizumab and Decitabine for Refractory or Relapsed Acute Myeloid Leukemia. J. Immunother. Cancer 2022, 10, e003392. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Boss, I.; Beach, C.L.; Copeland, W.B.; Thompson, E.; Fox, B.A.; Hasle, V.E.; Hellmann, A.; Taussig, D.C.; Tormo, M.; et al. A Randomized Phase 2 Trial of Azacitidine with or without Durvalumab as First-Line Therapy for Older Patients with AML. Blood Adv. 2022, 6, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Esteve, J.; Porkka, K. Sabatolimab plus Hypomethylating Agents (Hmas) in Patients (Pts) with High-/Very High-Risk Myelodysplastic Syndrome (Hr/Vhr-Mds) and Acute Myeloid Leukemia (Aml): Subgroup Analysis of a Phase 1 Study. Eur. Hematol. Assoc. EHA 2021, 324576, S168. [Google Scholar]
- Ferraro, F.; Miller, C.A.; Christensen, K.A.; Helton, N.M.; O’Laughlin, M.; Fronick, C.C.; Fulton, R.S.; Kohlschmidt, J.; Eisfeld, A.-K.; Bloomfield, C.D.; et al. Immunosuppression and Outcomes in Adult Patients with de Novo Acute Myeloid Leukemia with Normal Karyotypes. Proc. Natl. Acad. Sci. USA 2021, 118, e2116427118. [Google Scholar] [CrossRef] [PubMed]
- Vereecque, R.; Saudemont, A.; Quesnel, B. Cytosine Arabinoside Induces Costimulatory Molecule Expression in Acute Myeloid Leukemia Cells. Leukemia 2004, 18, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; Assi, R.; Daver, N.; Benton, C.B.; Kadia, T.; A Thompson, P.; Borthakur, G.; Alvarado, Y.; Jabbour, E.J.; Konopleva, M.; et al. Idarubicin, Cytarabine, and Nivolumab in Patients with Newly Diagnosed Acute Myeloid Leukaemia or High-Risk Myelodysplastic Syndrome: A Single-Arm, Phase 2 Study. Lancet Haematol. 2019, 6, e480–e488. [Google Scholar] [CrossRef]
- Zeidner, J.F.; Vincent, B.G.; Ivanova, A.; Moore, D.; McKinnon, K.P.; Wilkinson, A.D.; Mukhopadhyay, R.; Mazziotta, F.; Knaus, H.A.; Foster, M.C.; et al. Phase II Trial of Pembrolizumab after High-Dose Cytarabine in Relapsed/Refractory Acute Myeloid Leukemia. Blood Cancer Discov. 2021, 2, 616–629. [Google Scholar] [CrossRef]
- Chao, M.P.; Weissman, I.L.; Majeti, R. The CD47–SIRPα Pathway in Cancer Immune Evasion and Potential Therapeutic Implications. Curr. Opin. Immunol. 2012, 24, 225–232. [Google Scholar] [CrossRef]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D.; van Rooijen, N.; Weissman, I.L. CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Zhao, F.; Tseng, S.; Narayanan, C.; Shura, L.; Willingham, S.; Howard, M.; Prohaska, S.; Volkmer, J.; et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS ONE 2015, 10, e0137345. [Google Scholar] [CrossRef]
- Daver, N.; Senapati, J.; Maiti, A.; Loghavi, S.; Kadia, T.M.; DiNardo, C.D.; Pemmaraju, N.; Jabbour, E.; Montalban-Bravo, G.; Tang, G.; et al. Phase I/II Study of Azacitidine (AZA) with Venetoclax (VEN) and Magrolimab (Magro) in Patients (Pts) with Newly Diagnosed (ND) Older/Unfit or High-Risk Acute Myeloid Leukemia (AML) and Relapsed/Refractory (R/R) AML. Blood 2022, 140, 141–144. [Google Scholar] [CrossRef]
- Gilead Sciences. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety and Efficacy of Magrolimab Versus Placebo in Combination with Venetoclax and Azacitidine in Newly Diagnosed, Previously Untreated Patients with Acute Myeloid Leukemia Who Are Ineligible for Intensive Chemotherapy. 2023. Available online: https://www.clinicaltrials.gov (accessed on 25 May 2023).
- Boissel, N. ALL in Escape Room. Blood 2021, 137, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Blaeschke, F.; Willier, S.; Stenger, D.; Lepenies, M.; Horstmann, M.A.; Escherich, G.; Zimmermann, M.; Rojas Ringeling, F.; Canzar, S.; Kaeuferle, T.; et al. Leukemia-Induced Dysfunctional TIM-3+CD4+ Bone Marrow T Cells Increase Risk of Relapse in Pediatric B-Precursor ALL Patients. Leukemia 2020, 34, 2607–2620. [Google Scholar] [CrossRef] [PubMed]
- Hohtari, H.; Brück, O.; Blom, S.; Turkki, R.; Sinisalo, M.; Kovanen, P.E.; Kallioniemi, O.; Pellinen, T.; Porkka, K.; Mustjoki, S. Immune Cell Constitution in Bone Marrow Microenvironment Predicts Outcome in Adult ALL. Leukemia 2019, 33, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Tracy, S.I.; Venkatesh, H.; Hekim, C.; Heltemes-Harris, L.M.; Knutson, T.P.; Bachanova, V.; Farrar, M.A. Combining Nilotinib and PD-L1 Blockade Reverses CD4+ T-Cell Dysfunction and Prevents Relapse in Acute B-Cell Leukemia. Blood 2022, 140, 335–348. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). A Phase 1 Study of Blinatumomab in Combination with Checkpoint Inhibitor(s) of PD-1 (Nivolumab) or Both PD-1 (Nivolumab) and CTLA-4 (Ipilimumab) in Patients with Poor-Risk, Relapsed or Refractory CD19+ Precursor B-Lymphoblastic Leukemia. 2023. Available online: https://www.clinicaltrials.gov (accessed on 25 May 2023).
- Thomas, D.A.; O’Brien, S.; Faderl, S.; Garcia-Manero, G.; Ferrajoli, A.; Wierda, W.; Ravandi, F.; Verstovsek, S.; Jorgensen, J.L.; Bueso-Ramos, C.; et al. Chemoimmunotherapy with a Modified Hyper-CVAD and Rituximab Regimen Improves Outcome in de Novo Philadelphia Chromosome-Negative Precursor B-Lineage Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2010, 28, 3880–3889. [Google Scholar] [CrossRef]
- Maury, S.; Chevret, S.; Thomas, X.; Heim, D.; Leguay, T.; Huguet, F.; Chevallier, P.; Hunault, M.; Boissel, N.; Escoffre-Barbe, M.; et al. Rituximab in B-Lineage Adult Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2016, 375, 1044–1053. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.-M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Gökbuget, N.; Dombret, H.; Bonifacio, M.; Reichle, A.; Graux, C.; Faul, C.; Diedrich, H.; Topp, M.S.; Brüggemann, M.; Horst, H.-A.; et al. Blinatumomab for Minimal Residual Disease in Adults with B-Cell Precursor Acute Lymphoblastic Leukemia. Blood 2018, 131, 1522–1531. [Google Scholar] [CrossRef]
- Foà, R.; Bassan, R.; Vitale, A.; Elia, L.; Piciocchi, A.; Puzzolo, M.-C.; Canichella, M.; Viero, P.; Ferrara, F.; Lunghi, M.; et al. Dasatinib-Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N. Engl. J. Med. 2020, 383, 1613–1623. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.D.; Ghobadi, A.; Oluwole, O.O.; Logan, A.C.; Boissel, N.; Cassaday, R.D.; Leguay, T.; Bishop, M.R.; Topp, M.S.; Tzachanis, D.; et al. KTE-X19 for Relapsed or Refractory Adult B-Cell Acute Lymphoblastic Leukaemia: Phase 2 Results of the Single-Arm, Open-Label, Multicentre ZUMA-3 Study. Lancet 2021, 398, 491–502. [Google Scholar] [CrossRef]
- Maloney, D.G.; Grillo-López, A.J.; White, C.A.; Bodkin, D.; Schilder, R.J.; Neidhart, J.A.; Janakiraman, N.; Foon, K.A.; Liles, T.-M.; Dallaire, B.K.; et al. IDEC-C2B8 (Rituximab) Anti-CD20 Monoclonal Antibody Therapy in Patients with Relapsed Low-Grade Non-Hodgkin’s Lymphoma. Blood 1997, 90, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef]
- Maury, S.; Huguet, F.; Leguay, T.; Lacombe, F.; Maynadié, M.; Girard, S.; de Labarthe, A.; Kuhlein, E.; Raffoux, E.; Thomas, X.; et al. Adverse Prognostic Significance of CD20 Expression in Adults with Philadelphia Chromosome-Negative B-Cell Precursor Acute Lymphoblastic Leukemia. Haematologica 2010, 95, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.A.; O’Brien, S.; Jorgensen, J.L.; Cortes, J.; Faderl, S.; Garcia-Manero, G.; Verstovsek, S.; Koller, C.; Pierce, S.; Huh, Y.; et al. Prognostic Significance of CD20 Expression in Adults with de Novo Precursor B-Lineage Acute Lymphoblastic Leukemia. Blood 2009, 113, 6330–6337. [Google Scholar] [CrossRef]
- Bride, K.L.; Vincent, T.L.; Im, S.-Y.; Aplenc, R.; Barrett, D.M.; Carroll, W.L.; Carson, R.; Dai, Y.; Devidas, M.; Dunsmore, K.P.; et al. Preclinical Efficacy of Daratumumab in T-Cell Acute Lymphoblastic Leukemia. Blood 2018, 131, 995–999. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef]
- Naik, J.; Themeli, M.; de Jong-Korlaar, R.; Ruiter, R.W.J.; Poddighe, P.J.; Yuan, H.; de Bruijn, J.D.; Ossenkoppele, G.J.; Zweegman, S.; Smit, L.; et al. CD38 as a Therapeutic Target for Adult Acute Myeloid Leukemia and T-Cell Acute Lymphoblastic Leukemia. Haematologica 2019, 104, e100–e103. [Google Scholar] [CrossRef]
- Ofran, Y.; Ringelstein-Harlev, S.; Slouzkey, I.; Zuckerman, T.; Yehudai-Ofir, D.; Henig, I.; Beyar-Katz, O.; Hayun, M.; Frisch, A. Daratumumab for Eradication of Minimal Residual Disease in High-Risk Advanced Relapse of T-Cell/CD19/CD22-Negative Acute Lymphoblastic Leukemia. Leukemia 2020, 34, 293–295. [Google Scholar] [CrossRef]
- Cerrano, M.; Bonifacio, M.; Olivi, M.; Curti, A.; Malagola, M.; Dargenio, M.; Scattolin, A.M.; Papayannidis, C.; Forghieri, F.; Gurrieri, C.; et al. Daratumumab with or without Chemotherapy in Relapsed and Refractory Acute Lymphoblastic Leukemia. A Retrospective Observational Campus ALL Study. Haematologica 2022, 107, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Hogan, L.E.; Bhatla, T.; Teachey, D.T.; Sirvent, F.J.B.; Moppett, J.; Puyó, P.V.; Micalizzi, C.; R?Ssig, C.; Shukla, N.; Gilad, G.; et al. Efficacy and Safety of Daratumumab (DARA) in Pediatric and Young Adult Patients (Pts) with Relapsed/Refractory T-Cell Acute Lymphoblastic Leukemia (ALL) or Lymphoblastic Lymphoma (LL): Results from the Phase 2 DELPHINUS Study. J. Clin. Oncol. 2022, 40, 10001. [Google Scholar] [CrossRef]
- Diorio, C.; Teachey, D.T. Please Eat Me! Targeting CD47 and CD38 in T-ALL. Blood 2022, 140, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.A.; Ji, L.; Xu, X.; Devidas, M.; Hogan, L.E.; Borowitz, M.J.; Raetz, E.A.; Zugmaier, G.; Sharon, E.; Bernhardt, M.B.; et al. Effect of Postreinduction Therapy Consolidation with Blinatumomab vs. Chemotherapy on Disease-Free Survival in Children, Adolescents, and Young Adults With First Relapse of B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA 2021, 325, 833–842. [Google Scholar] [CrossRef]
- Locatelli, F.; Zugmaier, G.; Rizzari, C.; Morris, J.D.; Gruhn, B.; Klingebiel, T.; Parasole, R.; Linderkamp, C.; Flotho, C.; Petit, A.; et al. Effect of Blinatumomab vs Chemotherapy on Event-Free Survival Among Children with High-Risk First-Relapse B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA 2021, 325, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, S.; Bassan, R.; Vitale, A. Updated Results of the GIMEMA LAL2116 D-ALBA Trial for Newly Diagnosed Adults with Ph+ ALL. HemaSphere 2021, 5, e566. [Google Scholar]
- Jabbour, E.; Haddad, F.G.; Short, N.J.; Kantarjian, H. Treatment of Adults with Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia—From Intensive Chemotherapy Combinations to Chemotherapy-Free Regimens: A Review. JAMA Oncol. 2022, 8, 1340–1348. [Google Scholar] [CrossRef]
- Ravandi, F.; Walter, R.B.; Subklewe, M.; Buecklein, V.; Jongen-Lavrencic, M.; Paschka, P.; Ossenkoppele, G.J.; Kantarjian, H.M.; Hindoyan, A.; Agarwal, S.K.; et al. Updated Results from Phase I Dose-Escalation Study of AMG 330, a Bispecific T-Cell Engager Molecule, in Patients with Relapsed/Refractory Acute Myeloid Leukemia (R/R AML). J. Clin. Oncol. 2020, 38, 7508. [Google Scholar] [CrossRef]
- Subklewe, M.; Stein, A.; Walter, R.B.; Bhatia, R.; Wei, A.H.; Ritchie, D.; Bücklein, V.; Vachhani, P.; Dai, T.; Hindoyan, A. Preliminary Results from a Phase 1 First-in-Human Study of AMG 673, a Novel Half-Life Extended (HLE) Anti-CD33/CD3 BiTE® (Bispecific T-Cell Engager) in Patients with Relapsed/Refractory (R/R) Acute Myeloid Leukemia (AML). Blood 2019, 134, 833. [Google Scholar] [CrossRef]
- Ravandi, F.; Bashey, A.; Stock, W.; Foran, J.M.; Mawad, R.; Egan, D.; Blum, W.; Yang, A.; Pastore, A.; Johnson, C.; et al. Complete Responses in Relapsed/Refractory Acute Myeloid Leukemia (AML) Patients on a Weekly Dosing Schedule of Vibecotamab (XmAb14045), a CD123 x CD3 T Cell-Engaging Bispecific Antibody; Initial Results of a Phase 1 Study. Blood 2020, 136, 4–5. [Google Scholar] [CrossRef]
- Uy, G.L.; Aldoss, I.; Foster, M.C.; Sayre, P.H.; Wieduwilt, M.J.; Advani, A.S.; Godwin, J.E.; Arellano, M.L.; Sweet, K.L.; Emadi, A.; et al. Flotetuzumab as Salvage Immunotherapy for Refractory Acute Myeloid Leukemia. Blood 2021, 137, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Warlick, E.D.; Weisdorf, D.J.; Vallera, D.A.; Wangen, R.; Lewis, D.; Knox, J.; Schroeder, M.; Felices, M.; Miller, J.S. GTB-3550 TriKETM for the Treatment of High-Risk Myelodysplastic Syndromes (MDS) and Refractory/Relapsed Acute Myeloid Leukemia (AML) Safely Drives Natural Killer (NK) Cell Proliferation at Initial Dose Cohorts. Blood 2020, 136, 7–8. [Google Scholar] [CrossRef]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, M.C.; Hu, Z.-H.; Curran, K.; Laetsch, T.; Locke, F.; Rouce, R.; Pulsipher, M.A.; Phillips, C.L.; Keating, A.; Frigault, M.J.; et al. Real-World Evidence of Tisagenlecleucel for Pediatric Acute Lymphoblastic Leukemia and Non-Hodgkin Lymphoma. Blood Adv. 2020, 4, 5414–5424. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.D.; Ghobadi, A.; Oluwole, O.O.; Logan, A.C.; Boissel, N.; Cassaday, R.D.; Leguay, T.; Bishop, M.R.; Topp, M.S.; Tzachanis, D.; et al. Two-Year Follow-up of KTE-X19 in Patients with Relapsed or Refractory Adult B-Cell Acute Lymphoblastic Leukemia in ZUMA-3 and Its Contextualization with SCHOLAR-3, an External Historical Control Study. J. Hematol. Oncol. 2022, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Elsallab, M.; Ellithi, M.; Hempel, S.; Abdel-Azim, H.; Abou-el-Enein, M. Long-Term Response to Autologous Anti-CD19 Chimeric Antigen Receptor T Cells in Relapsed or Refractory B Cell Acute Lymphoblastic Leukemia: A Systematic Review and Meta-Analysis. Cancer Gene Ther. 2023, 30, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, R.; Jain, N.; Maus, M.V.; Boissel, N.; Graham, C.; Jozwik, A.; Yallop, D.; Konopleva, M.; Frigault, M.J.; Teshima, T.; et al. UCART19, a First-in-Class Allogeneic Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy for Adults with Relapsed or Refractory B-Cell Acute Lymphoblastic Leukaemia (CALM): A Phase 1, Dose-Escalation Trial. Lancet Haematol. 2022, 9, e833–e843. [Google Scholar] [CrossRef]
- Benjamin, R.; Graham, C.; Yallop, D.; Jozwik, A.; Mirci-Danicar, O.C.; Lucchini, G.; Pinner, D.; Jain, N.; Kantarjian, H.; Boissel, N.; et al. Genome-Edited, Donor-Derived Allogeneic Anti-CD19 Chimeric Antigen Receptor T Cells in Paediatric and Adult B-Cell Acute Lymphoblastic Leukaemia: Results of Two Phase 1 Studies. Lancet 2020, 396, 1885–1894. [Google Scholar] [CrossRef]
- Sheth, V.S.; Gauthier, J. Taming the Beast: CRS and ICANS after CAR T-Cell Therapy for ALL. Bone Marrow Transpl. 2021, 56, 552–566. [Google Scholar] [CrossRef]
- Roddie, C.; Dias, J.; O’Reilly, M.A.; Abbasian, M.; Cadinanos-Garai, A.; Vispute, K.; Bosshard-Carter, L.; Mitsikakou, M.; Mehra, V.; Roddy, H.; et al. Durable Responses and Low Toxicity After Fast Off-Rate CD19 Chimeric Antigen Receptor-T Therapy in Adults with Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2021, 39, 3352–3363. [Google Scholar] [CrossRef]
- Roddie, C.; Sandhu, K.S.; Tholouli, E.; Shaughnessy, P.; Barba, P.; Guerreiro, M.N.; Yallop, D.; Abedi, M.; Chaganti, S.; Ghobadi, A.; et al. Safety and Efficacy of Obecabtagene Autoleucel (Obe-Cel, AUTO1), a Fast-off Rate CD19 CAR, in Relapsed/Refractory Adult B-Cell Acute Lymphoblastic Leukemia (r/r B-ALL): Top Line Results of the Pivotal FELIX Study. J. Clin. Oncol. 2023, 41, 7000. [Google Scholar] [CrossRef]
- Hernani, R.; Benzaquén, A.; Solano, C. Toxicities Following CAR-T Therapy for Hematological Malignancies. Cancer Treat. Rev. 2022, 111, 102479. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Alotaibi, A.S.; Bücklein, V.; Subklewe, M. T-Cell-Based Immunotherapy of Acute Myeloid Leukemia: Current Concepts and Future Developments. Leukemia 2021, 35, 1843–1863. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Capanni, M.; Casucci, M.; Volpi, I.; Tosti, A.; Perruccio, K.; Urbani, E.; Negrin, R.S.; Martelli, M.F.; Velardi, A. Role of Natural Killer Cell Alloreactivity in HLA-Mismatched Hematopoietic Stem Cell Transplantation. Blood 1999, 94, 333–339. [Google Scholar] [CrossRef]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful Adoptive Transfer and in Vivo Expansion of Human Haploidentical NK Cells in Patients with Cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.A.; Miller, J.S. Exploring the NK Cell Platform for Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chergui, A.; Reagan, J.L. Immunotherapy in Acute Leukemias: Past Success Paves the Way for Future Progress. Cancers 2023, 15, 4137. https://doi.org/10.3390/cancers15164137
Chergui A, Reagan JL. Immunotherapy in Acute Leukemias: Past Success Paves the Way for Future Progress. Cancers. 2023; 15(16):4137. https://doi.org/10.3390/cancers15164137
Chicago/Turabian StyleChergui, Adel, and John L. Reagan. 2023. "Immunotherapy in Acute Leukemias: Past Success Paves the Way for Future Progress" Cancers 15, no. 16: 4137. https://doi.org/10.3390/cancers15164137
APA StyleChergui, A., & Reagan, J. L. (2023). Immunotherapy in Acute Leukemias: Past Success Paves the Way for Future Progress. Cancers, 15(16), 4137. https://doi.org/10.3390/cancers15164137





