Immunotherapy in Melanoma: Recent Advancements and Future Directions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Treatment Advancements and Innovation
2.1. Lymphocyte Activated Gene 3 (LAG-3)
2.2. Toll-Like Receptors (TLRs)
2.3. Vascular Endothelial Growth Factor (VEGF)
2.4. Immune-Mobilizing Monoclonal T Cell Receptors against Cancer (ImmTACs)
2.5. Strategies to Mitigate Immune-Related Adverse Events (irAEs)
2.6. Oncolytic Viruses
2.7. Adoptive Cellular Therapies
2.8. Vaccination
2.9. Combination Immunotherapy and BRAF/MEK Inhibition
2.10. T Cell Immunoreceptor with Immunoglobulin and Immunoreceptor Tyrosine-Based Inhibition Motif Domain (TIGIT)
2.11. Early-Stage Disease
2.11.1. Adjuvant Strategies
2.11.2. Neoadjuvant Strategies
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hugo, W.; Zaretsky, J.M.; Sun, L.; Song, C.; Moreno, B.H.; Hu-Lieskovan, S.; Berent-Maoz, B.; Pang, J.; Chmielowski, B.; Cherry, G.; et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016, 165, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Spaapen, R.M.; Zha, Y.; Williams, J.; Meng, Y.; Ha, T.T.; Gajewski, T.F. Up-Regulation of PD-L1, IDO, and T(Regs) in the Melanoma Tumor Microenvironment Is Driven by CD8(+) T Cells. Sci. Transl. Med. 2013, 5, 200ra116. [Google Scholar] [CrossRef]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive Resistance to Therapeutic PD-1 Blockade Is Associated with Upregulation of Alternative Immune Checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Sheehan, K.C.F.; Old, L.J.; Schreiber, R.D. IFN Unresponsiveness in LNCaP Cells Due to the Lack of JAK1 Gene Expression. Cancer Res. 2005, 65, 3447–3453. [Google Scholar] [CrossRef]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Restifo, N.P.; Smyth, M.J.; Snyder, A. Acquired Resistance to Immunotherapy and Future Challenges. Nat. Rev. Cancer 2016, 16, 121–126. [Google Scholar] [CrossRef]
- Durham, N.M.; Nirschl, C.J.; Jackson, C.M.; Elias, J.; Kochel, C.M.; Anders, R.A.; Drake, C.G. Lymphocyte Activation Gene 3 (LAG-3) Modulates the Ability of CD4 T-Cells to Be Suppressed In Vivo. PLoS ONE 2014, 9, e109080. [Google Scholar] [CrossRef]
- Woo, S.-R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-Cell Function to Promote Tumoral Immune Escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef]
- Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma|NEJM. Available online: https://www.nejm.org/doi/full/10.1056/nejmoa2109970 (accessed on 21 April 2023).
- Hamid, O.; Weise, A.; Kim, T.M.; Mckean, M.A.; Lakhani, N.J.; Kaczmar, J.; Papadopoulos, K.P.; Chen, S.; Mani, J.; Jankovic, V.; et al. Phase I Study of Fianlimab, a Human Lymphocyte Activation Gene-3 (LAG-3) Monoclonal Antibody, in Combination with Cemiplimab in Advanced Melanoma (Mel). Ann. Oncol. 2022, 33 (Suppl. S7), S356–S409. [Google Scholar]
- Hamid, O.; Lewis, K.; Weise, A.; McKean, M.; Papadopoulos, K.; Crown, J.; Thomas, S.; Girda, E.; Kaczmar, J.; Kim, K.; et al. Significant Durable Response with Fianlimab (Anti-LAG-3) and Cemiplimab (Anti-PD-1) in Advanced Melanoma: Post Adjuvant PD-1 Analysis. J. Clin. Oncol. 2023, 41, 9501. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Karapetyan, L.; Luke, J.J.; Davar, D. Toll-Like Receptor 9 Agonists in Cancer. OncoTargets Ther. 2020, 13, 10039–10060. [Google Scholar] [CrossRef] [PubMed]
- Haymaker, C.; Johnson, D.H.; Murthy, R.; Bentebibel, S.-E.; Uemura, M.I.; Hudgens, C.W.; Safa, H.; James, M.; Andtbacka, R.H.I.; Johnson, D.B.; et al. Tilsotolimod with Ipilimumab Drives Tumor Responses in Anti–PD-1 Refractory Melanoma. Cancer Discov. 2021, 11, 1996–2013. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Medina, T.; Kummar, S.; Amin, A.; Kalbasi, A.; Drabick, J.J.; Barve, M.; Daniels, G.A.; Wong, D.J.; Schmidt, E.V.; et al. SD-101 in Combination with Pembrolizumab in Advanced Melanoma: Results of a Phase Ib, Multicenter Study. Cancer Discov. 2018, 8, 1250–1257. [Google Scholar] [CrossRef]
- Milhem, M.; Zakharia, Y.; Davar, D.; Buchbinder, E.; Medina, T.; Daud, A.; Ribas, A.; Niu, J.; Gibney, G.; Margolin, K.; et al. 304 Intratumoral Injection of CMP-001, a Toll-like Receptor 9 (TLR9) Agonist, in Combination with Pembrolizumab Reversed Programmed Death Receptor 1 (PD-1) Blockade Resistance in Advanced Melanoma. J. Immunother. Cancer 2020, 8 (Suppl. S3), A186–A187. [Google Scholar] [CrossRef]
- Idera Pharmaceuticals Announces Results from ILLUMINATE-301 Trial of Tilsotolimod + Ipilimumab in Anti-PD-1 Refractory Advanced Melanoma|Aceragen, Inc. Available online: https://ir.iderapharma.com/news-releases/news-release-details/idera-pharmaceuticals-announces-results-illuminate-301-trial (accessed on 23 May 2023).
- Ribas, A.; Medina, T.; Kirkwood, J.M.; Zakharia, Y.; Gonzalez, R.; Davar, D.; Chmielowski, B.; Campbell, K.M.; Bao, R.; Kelley, H.; et al. Overcoming PD-1 Blockade Resistance with CpG-A Toll-Like Receptor 9 Agonist Vidutolimod in Patients with Metastatic Melanoma. Cancer Discov. 2021, 11, 2998–3007. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Karunamurthy, A.; Hartman, D.; DeBlasio, R.; Chauvin, J.-M.; Ding, Q.; Pagliano, O.; Rose, A.; Kirkwood, J.; Zarour, H. 303 Phase II Trial of Neoadjuvant Nivolumab (Nivo) and Intra-Tumoral (IT) CMP-001 in High-Risk Resectable Melanoma (Neo-C-Nivo): Final Results. J. Immunother. Cancer 2020, 8 (Suppl. 3), A185–A186. [Google Scholar] [CrossRef]
- Ohm, J.E.; Carbone, D.P. VEGF as a Mediator of Tumor-Associated Immunodeficiency. Immunol. Res. 2001, 23, 263–272. [Google Scholar] [CrossRef]
- Hodi, F.S.; Lawrence, D.; Lezcano, C.; Wu, X.; Zhou, J.; Sasada, T.; Zeng, W.; Giobbie-Hurder, A.; Atkins, M.B.; Ibrahim, N.; et al. Bevacizumab plus Ipilimumab in Patients with Metastatic Melanoma. Cancer Immunol. Res. 2014, 2, 632–642. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Larkin, J.; Sosman, J.A.; Lebbé, C.; Brady, B.; Neyns, B.; Schmidt, H.; Hassel, J.C.; Hodi, F.S.; Lorigan, P.; et al. Efficacy and Safety of Nivolumab Alone or in Combination With Ipilimumab in Patients With Mucosal Melanoma: A Pooled Analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 226–235. [Google Scholar] [CrossRef]
- Li, S.; Wu, X.; Yan, X.; Zhou, L.; Chi, Z.; Si, L.; Cui, C.; Tang, B.; Mao, L.; Lian, B.; et al. Toripalimab plus Axitinib in Patients with Metastatic Mucosal Melanoma: 3-Year Survival Update and Biomarker Analysis. J. Immunother. Cancer 2022, 10, e004036. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Fang, M.; Chen, Y.; Mao, L.; Zhang, P.; Lin, J.; Bai, X.; Cao, X.; Chen, Y.; Guo, J. Atezolizumab in Combination with Bevacizumab in Patients with Unresectable Locally Advanced or Metastatic Mucosal Melanoma: Interim Analysis of an Open-Label Phase II Trial. J. Clin. Oncol. 2021, 39 (Suppl. 15), 9511. [Google Scholar] [CrossRef]
- Arance, A.; de la Cruz-Merino, L.; Petrella, T.M.; Jamal, R.; Ny, L.; Carneiro, A.; Berrocal, A.; Márquez-Rodas, I.; Spreafico, A.; Atkinson, V.; et al. Phase II LEAP-004 Study of Lenvatinib Plus Pembrolizumab for Melanoma With Confirmed Progression on a Programmed Cell Death Protein-1 or Programmed Death Ligand 1 Inhibitor Given as Monotherapy or in Combination. J. Clin. Oncol. 2023, 41, 75–85. [Google Scholar] [CrossRef]
- Bossi, G.; Buisson, S.; Oates, J.; Jakobsen, B.K.; Hassan, N.J. ImmTAC-Redirected Tumour Cell Killing Induces and Potentiates Antigen Cross-Presentation by Dendritic Cells. Cancer Immunol. Immunother. CII 2014, 63, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.M.; Henson, V.; Slack, R.; Ng, J.; Hartzman, R.J.; Katovich Hurley, C. Frequencies of HLA-A2 Alleles in Five U.S. Population Groups. Predominance Of A*02011 and Identification of HLA-A*0231. Hum. Immunol. 2000, 61, 334–340. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.-F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Faje, A.T.; Lawrence, D.; Flaherty, K.; Freedman, C.; Fadden, R.; Rubin, K.; Cohen, J.; Sullivan, R.J. High-Dose Glucocorticoids for the Treatment of Ipilimumab-Induced Hypophysitis Is Associated with Reduced Survival in Patients with Melanoma. Cancer 2018, 124, 3706–3714. [Google Scholar] [CrossRef]
- Bai, X.; Hu, J.; Betof Warner, A.; Quach, H.T.; Cann, C.G.; Zhang, M.Z.; Si, L.; Tang, B.; Cui, C.; Yang, X.; et al. Early Use of High-Dose Glucocorticoid for the Management of IrAE Is Associated with Poorer Survival in Patients with Advanced Melanoma Treated with Anti-PD-1 Monotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 5993–6000. [Google Scholar] [CrossRef]
- Lechner, M.G.; Patel, A.Y.; Hugo, W.; Angell, T.E.; Cheng, M.I.; Pioso, M.S.; Hoang, A.T.; Yakobian, N.; McCarthy, E.C.; Chen, H.-C.; et al. Inhibition of the IL-17A Axis Protects against Immune-Related Adverse Events While Supporting Checkpoint Inhibitor Anti-Tumor Efficacy. bioRxiv 2022. bioRxiv: 2022.01.19.476844. [Google Scholar] [CrossRef]
- Bergqvist, V.; Hertervig, E.; Gedeon, P.; Kopljar, M.; Griph, H.; Kinhult, S.; Carneiro, A.; Marsal, J. Vedolizumab Treatment for Immune Checkpoint Inhibitor-Induced Enterocolitis. Cancer Immunol. Immunother. CII 2017, 66, 581–592. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.H.; Zobniw, C.M.; Trinh, V.A.; Ma, J.; Bassett, R.L.; Abdel-Wahab, N.; Anderson, J.; Davis, J.E.; Joseph, J.; Uemura, M.; et al. Infliximab Associated with Faster Symptom Resolution Compared with Corticosteroids Alone for the Management of Immune-Related Enterocolitis. J. Immunother. Cancer 2018, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, F.; Montfort, A.; Marcheteau, E.; Imbert, C.; Gilhodes, J.; Filleron, T.; Rochaix, P.; Andrieu-Abadie, N.; Levade, T.; Meyer, N.; et al. TNFα Blockade Overcomes Resistance to Anti-PD-1 in Experimental Melanoma. Nat. Commun. 2017, 8, 2256. [Google Scholar] [CrossRef]
- Bertrand, F.; Rochotte, J.; Colacios, C.; Montfort, A.; Tilkin-Mariamé, A.-F.; Touriol, C.; Rochaix, P.; Lajoie-Mazenc, I.; Andrieu-Abadie, N.; Levade, T.; et al. Blocking Tumor Necrosis Factor α Enhances CD8 T-Cell-Dependent Immunity in Experimental Melanoma. Cancer Res. 2015, 75, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, E.; Minute, L.; Otano, I.; Alvarez, M.; Ochoa, M.C.; Belsue, V.; de Andrea, C.; Rodriguez-Ruiz, M.E.; Perez-Gracia, J.L.; Marquez-Rodas, I.; et al. Prophylactic TNF Blockade Uncouples Efficacy and Toxicity in Dual CTLA-4 and PD-1 Immunotherapy. Nature 2019, 569, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Montfort, A.; Dufau, C.; Colacios, C.; Andrieu-Abadie, N.; Levade, T.; Filleron, T.; Delord, J.-P.; Ayyoub, M.; Meyer, N.; Ségui, B. Anti-TNF, a Magic Bullet in Cancer Immunotherapy? J. Immunother. Cancer 2019, 7, 303. [Google Scholar] [CrossRef] [PubMed]
- Montfort, A.; Filleron, T.; Virazels, M.; Dufau, C.; Milhès, J.; Pagès, C.; Olivier, P.; Ayyoub, M.; Mounier, M.; Lusque, A.; et al. Combining Nivolumab and Ipilimumab with Infliximab or Certolizumab in Patients with Advanced Melanoma: First Results of a Phase Ib Clinical Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 1037–1047. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Collichio, F.; Harrington, K.J.; Middleton, M.R.; Downey, G.; Öhrling, K.; Kaufman, H.L. Final Analyses of OPTiM: A Randomized Phase III Trial of Talimogene Laherparepvec versus Granulocyte-Macrophage Colony-Stimulating Factor in Unresectable Stage III-IV Melanoma. J. Immunother. Cancer 2019, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Dummer, R.; Johnson, D.; Michielin, O.; Martin-Algarra, S.; Treichel, S.; Chan, E.; Diede, S.; Ribas, A. 429 Long-Term Analysis of MASTERKEY-265 Phase 1b Trial of Talimogene Laherparepvec (T-VEC) plus Pembrolizumab in Patients with Unresectable Stage IIIB-IVM1c Melanoma. J. Immunother. Cancer 2020, 8 (Suppl. 3), A261. [Google Scholar] [CrossRef]
- Chesney, J.A.; Ribas, A.; Long, G.V.; Kirkwood, J.M.; Dummer, R.; Puzanov, I.; Hoeller, C.; Gajewski, T.F.; Gutzmer, R.; Rutkowski, P.; et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined with Pembrolizumab for Advanced Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 528–540. [Google Scholar] [CrossRef]
- Dummer, R.; Gyorki, D.E.; Hyngstrom, J.; Berger, A.C.; Conry, R.; Demidov, L.; Sharma, A.; Treichel, S.A.; Radcliffe, H.; Gorski, K.S.; et al. Neoadjuvant Talimogene Laherparepvec plus Surgery versus Surgery Alone for Resectable Stage IIIB-IVM1a Melanoma: A Randomized, Open-Label, Phase 2 Trial. Nat. Med. 2021, 27, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Rohaan, M.W.; Stahlie, E.H.A.; Franke, V.; Zijlker, L.P.; Wilgenhof, S.; van der Noort, V.; van Akkooi, A.C.J.; Haanen, J.B.A.G. Neoadjuvant Nivolumab + T-VEC Combination Therapy for Resectable Early Stage or Metastatic (IIIB-IVM1a) Melanoma with Injectable Disease: Study Protocol of the NIVEC Trial. BMC Cancer 2022, 22, 851. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Packard, B.S.; Aebersold, P.M.; Solomon, D.; Topalian, S.L.; Toy, S.T.; Simon, P.; Lotze, M.T.; Yang, J.C.; Seipp, C.A.; et al. Use of Tumor-Infiltrating Lymphocytes and Interleukin-2 in the Immunotherapy of Patients with Metastatic Melanoma. N. Engl. J. Med. 1988, 319, 1676–1680. [Google Scholar] [CrossRef]
- Rohaan, M.W.; Borch, T.H.; van den Berg, J.H.; Met, Ö.; Kessels, R.; Geukes Foppen, M.H.; Stoltenborg Granhøj, J.; Nuijen, B.; Nijenhuis, C.; Jedema, I.; et al. Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2022, 387, 2113–2125. [Google Scholar] [CrossRef]
- Sarnaik, A.A.; Hamid, O.; Khushalani, N.I.; Lewis, K.D.; Medina, T.; Kluger, H.M.; Thomas, S.S.; Domingo-Musibay, E.; Pavlick, A.C.; Whitman, E.D.; et al. Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 2656–2666. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Restifo, N.P.; Yang, J.C.; Morgan, R.A.; Dudley, M.E. Adoptive Cell Transfer: A Clinical Path to Effective Cancer Immunotherapy. Nat. Rev. Cancer 2008, 8, 299–308. [Google Scholar] [CrossRef]
- Goff, S.L.; Dudley, M.E.; Citrin, D.E.; Somerville, R.P.; Wunderlich, J.R.; Danforth, D.N.; Zlott, D.A.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; et al. Randomized, Prospective Evaluation Comparing Intensity of Lymphodepletion Before Adoptive Transfer of Tumor-Infiltrating Lymphocytes for Patients With Metastatic Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 2389–2397. [Google Scholar] [CrossRef]
- van den Berg, J.H.; Heemskerk, B.; van Rooij, N.; Gomez-Eerland, R.; Michels, S.; van Zon, M.; de Boer, R.; Bakker, N.A.M.; Jorritsma-Smit, A.; van Buuren, M.M.; et al. Tumor Infiltrating Lymphocytes (TIL) Therapy in Metastatic Melanoma: Boosting of Neoantigen-Specific T Cell Reactivity and Long-Term Follow-Up. J. Immunother. Cancer 2020, 8, e000848. [Google Scholar] [CrossRef] [PubMed]
- Maurer, D.; Butterfield, L.H.; Vujanovic, L. Melanoma Vaccines: Clinical Status and Immune Endpoints. Melanoma Res. 2019, 29, 109–118. [Google Scholar] [CrossRef]
- Nestle, F.O.; Alijagic, S.; Gilliet, M.; Sun, Y.; Grabbe, S.; Dummer, R.; Burg, G.; Schadendorf, D. Vaccination of Melanoma Patients with Peptide- or Tumor Lysate-Pulsed Dendritic Cells. Nat. Med. 1998, 4, 328–332. [Google Scholar] [CrossRef]
- Dreno, B.; Thompson, J.F.; Smithers, B.M.; Santinami, M.; Jouary, T.; Gutzmer, R.; Levchenko, E.; Rutkowski, P.; Grob, J.-J.; Korovin, S.; et al. MAGE-A3 Immunotherapeutic as Adjuvant Therapy for Patients with Resected, MAGE-A3-Positive, Stage III Melanoma (DERMA): A Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2018, 19, 916–929. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An Immunogenic Personal Neoantigen Vaccine for Patients with Melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef]
- AACR Annual Meeting 2023 Itinerary Planner|Presentation. Available online: https://www.abstractsonline.com/pp8/#!/10828/presentation/10243 (accessed on 24 April 2023).
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An RNA Vaccine Drives Immunity in Checkpoint-Inhibitor-Treated Melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef]
- Khattak, A.; Carlino, M.; Meniawy, T.; Ansstas, G.; Medina, T.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Abstract CT001: A Personalized Cancer Vaccine, MRNA-4157, Combined with Pembrolizumab versus Pembrolizumab in Patients with Resected High-Risk Melanoma: Efficacy and Safety Results from the Randomized, Open-Label Phase 2 MRNA-4157-P201/Keynote-942 Trial. Cancer Res. 2023, 83 (Suppl. 8), CT001. [Google Scholar] [CrossRef]
- Kjeldsen, J.W.; Lorentzen, C.L.; Martinenaite, E.; Ellebaek, E.; Donia, M.; Holmstroem, R.B.; Klausen, T.W.; Madsen, C.O.; Ahmed, S.M.; Weis-Banke, S.E.; et al. Phase 1/2 Trial of an Immune-Modulatory Vaccine against IDO/PD-L1 in Combination with Nivolumab in Metastatic Melanoma. Nat. Med. 2021, 27, 2212–2223. [Google Scholar] [CrossRef]
- Long, G.V.; Dummer, R.; Hamid, O.; Gajewski, T.F.; Caglevic, C.; Dalle, S.; Arance, A.; Carlino, M.S.; Grob, J.-J.; Kim, T.M.; et al. Epacadostat plus Pembrolizumab versus Placebo plus Pembrolizumab in Patients with Unresectable or Metastatic Melanoma (ECHO-301/KEYNOTE-252): A Phase 3, Randomised, Double-Blind Study. Lancet Oncol. 2019, 20, 1083–1097. [Google Scholar] [CrossRef]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Combined BRAF and MEK Inhibition versus BRAF Inhibition Alone in Melanoma. N. Engl. J. Med. 2014, 371, 1877–1888. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Tarhini, A.A.; Cohen, G.I.; Truong, T.-G.; Moon, H.H.; Davar, D.; O’Rourke, M.; Stephenson, J.J.; et al. Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients with Advanced BRAF-Mutant Melanoma: The DREAMseq Trial—ECOG-ACRIN EA6134. J. Clin. Oncol. 2023, 41, 186–197. [Google Scholar] [CrossRef]
- Boni, A.; Cogdill, A.P.; Dang, P.; Udayakumar, D.; Njauw, C.-N.J.; Sloss, C.M.; Ferrone, C.R.; Flaherty, K.T.; Lawrence, D.P.; Fisher, D.E.; et al. Selective BRAFV600E Inhibition Enhances T-Cell Recognition of Melanoma without Affecting Lymphocyte Function. Cancer Res. 2010, 70, 5213–5219. [Google Scholar] [CrossRef]
- Frederick, D.T.; Piris, A.; Cogdill, A.P.; Cooper, Z.A.; Lezcano, C.; Ferrone, C.R.; Mitra, D.; Boni, A.; Newton, L.P.; Liu, C.; et al. BRAF Inhibition Is Associated with Enhanced Melanoma Antigen Expression and a More Favorable Tumor Microenvironment in Patients with Metastatic Melanoma. Clin. Cancer Res. 2013, 19, 1225–1231. [Google Scholar] [CrossRef]
- Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Atezolizumab, Vemurafenib, and Cobimetinib as First-Line Treatment for Unresectable Advanced BRAFV600 Mutation-Positive Melanoma (IMspire150): Primary Analysis of the Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2020, 395, 1835–1844. [Google Scholar] [CrossRef]
- Ribas, A.; Hodi, F.S.; Lawrence, D.; Atkinson, V.; Agarwal, S.; Carlino, M.S.; Fisher, R.; Long, G.V.; Miller, W.H.; Huang, Y.; et al. KEYNOTE-022 Update: Phase 1 Study of First-Line Pembrolizumab (Pembro) plus Dabrafenib (D) and Trametinib (T) for BRAF-Mutant Advanced Melanoma. Ann. Oncol. 2017, 28, v430. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Di Giacomo, A.M.; Del Vecchio, M.; Atkinson, V.; Schmidt, H.; Schachter, J.; Queirolo, P.; Long, G.V.; Stephens, R.; Svane, I.M.; et al. KEYNOTE-022 Part 3: A Randomized, Double-Blind, Phase 2 Study of Pembrolizumab, Dabrafenib, and Trametinib in BRAF-Mutant Melanoma. J. Immunother. Cancer 2020, 8, e001806. [Google Scholar] [CrossRef]
- Dummer, R.; Long, G.V.; Robert, C.; Tawbi, H.A.; Flaherty, K.T.; Ascierto, P.A.; Nathan, P.D.; Rutkowski, P.; Leonov, O.; Dutriaux, C.; et al. Randomized Phase III Trial Evaluating Spartalizumab Plus Dabrafenib and Trametinib for BRAF V600-Mutant Unresectable or Metastatic Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 1428–1438. [Google Scholar] [CrossRef]
- Chauvin, J.-M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; Kirkwood, J.M.; Chen, T.T.; Maurer, M.; Korman, A.J.; et al. TIGIT and PD-1 Impair Tumor Antigen-Specific CD8+ T Cells in Melanoma Patients. J. Clin. Investig. 2015, 125, 2046–2058. [Google Scholar] [CrossRef]
- Dummer, R.; Robert, C.; Scolyer, R.A.; Taube, J.M.; Tetzlaff, M.T.; Hill, A.; Grob, J.-J.; Portnoy, D.C.; Lebbe, C.; Khattak, M.A.; et al. Abstract CT002: KEYMAKER-U02 Substudy 02C: Neoadjuvant Pembrolizumab (Pembro) + Vibostolimab (Vibo) or Gebasaxturev (Geba) or Pembro Alone Followed by Adjuvant Pembro for Stage IIIB-D Melanoma. Cancer Res. 2023, 83 (Suppl. 8), CT002. [Google Scholar] [CrossRef]
- Luke, J.J.; Rutkowski, P.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Chiarion-Sileni, V.; de la Cruz Merino, L.; Khattak, M.A.; Schadendorf, D.; Long, G.V.; et al. Pembrolizumab versus Placebo as Adjuvant Therapy in Completely Resected Stage IIB or IIC Melanoma (KEYNOTE-716): A Randomised, Double-Blind, Phase 3 Trial. Lancet Lond. Engl. 2022, 399, 1718–1729. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Long, G.V.; Vecchio, M.D.; Weber, J.; Hoeller, C.; Grob, J.-J.; Mohr, P.; Grabbe, S.; Dutriaux, C.; Chiarion-Sileni, V.; Mackiewicz, J.; et al. Adjuvant Therapy with Nivolumab versus Placebo in Patients with Resected Stage IIB/C Melanoma (CheckMate 76K). SKIN J. Cutan. Med. 2023, 7, s163. [Google Scholar] [CrossRef]
- Weber, J.S.; Schadendorf, D.; Del Vecchio, M.; Larkin, J.; Atkinson, V.; Schenker, M.; Pigozzo, J.; Gogas, H.; Dalle, S.; Meyer, N.; et al. Adjuvant Therapy of Nivolumab Combined With Ipilimumab Versus Nivolumab Alone in Patients With Resected Stage IIIB-D or Stage IV Melanoma (CheckMate 915). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, L.; Livingstone, E.; Hassel, J.C.; Fluck, M.; Eigentler, T.; Loquai, C.; Haferkamp, S.; Gutzmer, R.; Meier, F.; Mohr, P.; et al. Adjuvant Nivolumab plus Ipilimumab or Nivolumab Monotherapy versus Placebo in Patients with Resected Stage IV Melanoma with No Evidence of Disease (IMMUNED): A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet 2020, 395, 1558–1568. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant Ipilimumab versus Placebo after Complete Resection of Stage III Melanoma: Long-Term Follow-up Results of the European Organisation for Research and Treatment of Cancer 18071 Double-Blind Phase 3 Randomised Trial. Eur. J. Cancer 2019, 119, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Meshcheryakov, A.; Khattak, A.; et al. Five-Year Analysis of Adjuvant Pembrolizumab or Placebo in Stage III Melanoma. NEJM Evid. 2022, 1, EVIDoa2200214. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.-G.; et al. Neoadjuvant–Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef]
- Reijers, I.L.M.; Menzies, A.M.; van Akkooi, A.C.J.; Versluis, J.M.; van den Heuvel, N.M.J.; Saw, R.P.M.; Pennington, T.E.; Kapiteijn, E.; van der Veldt, A.A.M.; Suijkerbuijk, K.P.M.; et al. Personalized Response-Directed Surgery and Adjuvant Therapy after Neoadjuvant Ipilimumab and Nivolumab in High-Risk Stage III Melanoma: The PRADO Trial. Nat. Med. 2022, 28, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Amaria, R.N.; Postow, M.; Burton, E.M.; Tetzlaff, M.T.; Ross, M.I.; Torres-Cabala, C.; Glitza, I.C.; Duan, F.; Milton, D.R.; Busam, K.; et al. Neoadjuvant Relatlimab and Nivolumab in Resectable Melanoma. Nature 2022, 611, 155–160. [Google Scholar] [CrossRef]
- Rozeman, E.A.; Menzies, A.M.; van Akkooi, A.C.J.; Adhikari, C.; Bierman, C.; van de Wiel, B.A.; Scolyer, R.A.; Krijgsman, O.; Sikorska, K.; Eriksson, H.; et al. Identification of the Optimal Combination Dosing Schedule of Neoadjuvant Ipilimumab plus Nivolumab in Macroscopic Stage III Melanoma (OpACIN-Neo): A Multicentre, Phase 2, Randomised, Controlled Trial. Lancet Oncol. 2019, 20, 948–960. [Google Scholar] [CrossRef]
- Liu, J.; Blake, S.J.; Yong, M.C.R.; Harjunpää, H.; Ngiow, S.F.; Takeda, K.; Young, A.; O’Donnell, J.S.; Allen, S.; Smyth, M.J.; et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov. 2016, 6, 1382–1399. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.W.; Lijnsvelt, J.; Pulleman, S.; Scolyer, R.A.; Menzies, A.M.; Van Akkooi, A.C.J.; van Houdt, W.J.; Shannon, K.F.; Pennington, T.; Suijkerbuijk, K.; et al. The NADINA Trial: A Multicenter, Randomised, Phase 3 Trial Comparing the Efficacy of Neoadjuvant Ipilimumab plus Nivolumab with Standard Adjuvant Nivolumab in Macroscopic Resectable Stage III Melanoma. J. Clin. Oncol. 2022, 40 (Suppl. 16), TPS9605. [Google Scholar] [CrossRef]
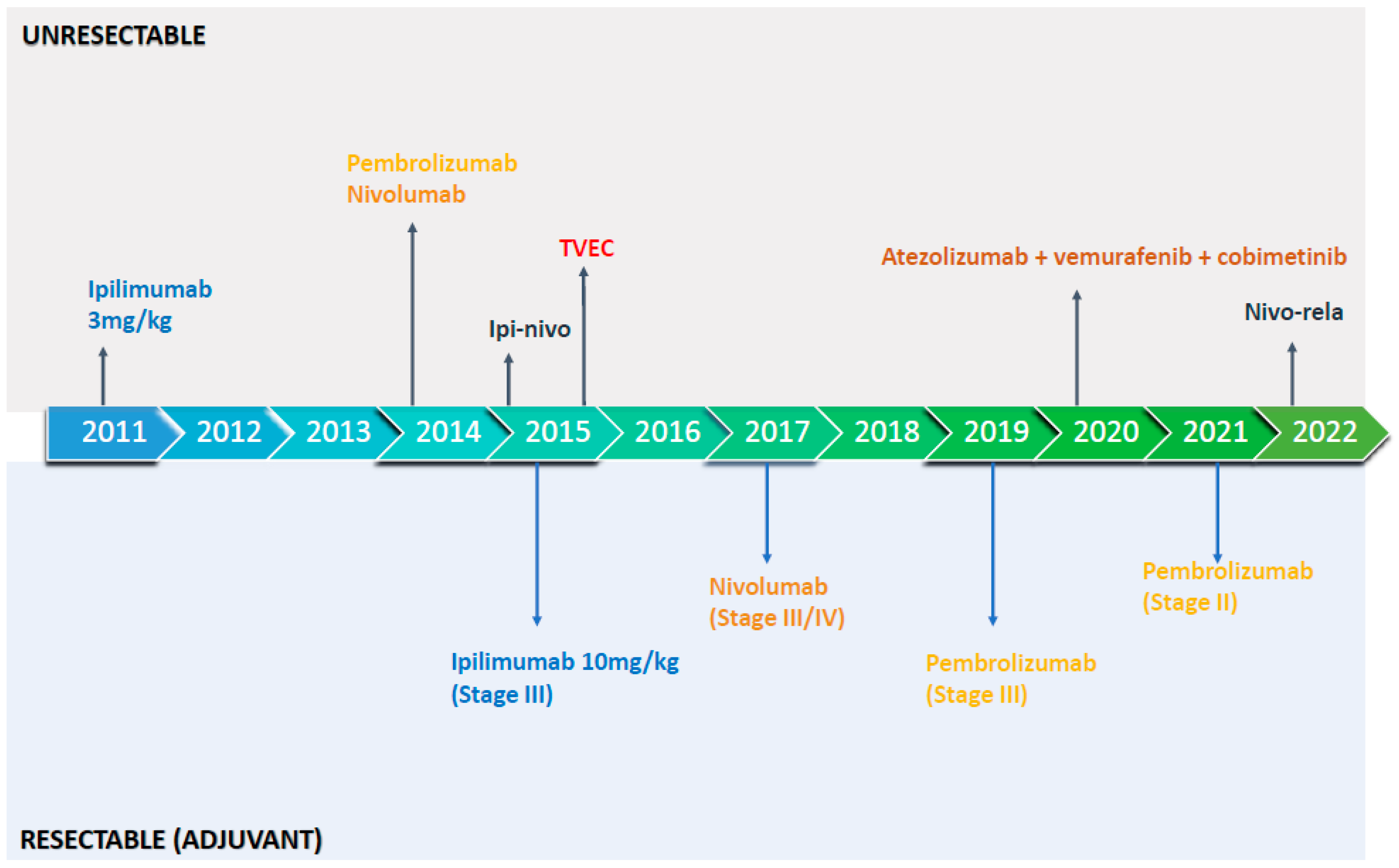
| Metastatic/ Unresectable Trials | Active Treatment Arm (Randomization) | Control Arm | Primary Endpoint/s | OS Data | Treatment-Related Grade ≥ 3 Toxicity |
|---|---|---|---|---|---|
| Checkmate 066 | Nivolumab 3 mg/kg Q2 weeks (1:1) | Dacarbazine 1000 mg/m2 Q3 weeks | OS | 5-years OS: 39% vs. 17% | 11.7 nivolumab vs. 17.6% dacarbazine |
| KEYNOTE 006 | Pembrolizumab 10 mg/kg Q2 weeks or Q3 week (1:1:1) | Ipilimumab 3 mg/kg Q3 weeks × 4 doses | PFS and OS Median PFS: 8.4 months in combined pembro groups vs. 3.4 months in ipi group | 5-years OS: 38.7% vs. 31% | 17% pembro arms vs. 20% ipi |
| Checkmate 067 | Ipilimumab (3 mg/kg) and nivolumab (1 mg/kg) Q3 weeks × 4 cycles followed by nivolumab maintenance or nivolumab 3 mg/kg (1:1:1) | Ipilimumab 3 mg/kg Q3 weeks × 4 doses | PFS and OS Median PFS 11.5 months ipi-nivo vs. 2.9 months for ipi alone | 7.5-years OS: 48% ipi-nivo vs. 42% nivo vs. 22% ipi | 59% ipi-nivo vs. 21% nivo vs. 28% ipi |
| RELATIVITY 047 | Nivolumab-relatlimab (fixed dose, 480 mg nivo and 160 mg of rela) IV Q4 weeks (1:1) | Nivolumab | PFS: Median PFS 10.1 months vs. 4.6 months; 2-year PFS 39% vs. 29% | 3-years OS: 56% vs. 48% ** | 21% nivo-rela vs. 11% nivo |
| IMspire150 | Atezolizumab 840 mg IV Q2 weeks, vemurafenib 720 mg PO BID for 28 days, and cobimetinib 60 mg PO QD for 21 days (7 days off) | Vemurafenib 960 mg PO BID for 28 days and cobimetinib 60 mg QD for 21 days (7 days off) | PFS: Median PFS 15.1 months vs. 10.6 months | Median OS: 39 months vs. 25.8 months ** | 79% atezo-containing arm vs. 73% doublet |
| OPTiM | Talimogene laherparepvec intra-tumoral (2:1) | Subcutaneous recombinant GM-CSF | Durable response rate: 19.3 vs. 1.4% | Median OS: 23.3 months vs. 18.9 months | 11.3% vs. 4.7% |
| Adjuvant Trials | Active Treatment Arm (Randomization) | Control Arm | Melanoma Stage * | Updated RFS/EFS | Treatment-Related Grade ≥ 3 Toxicity |
|---|---|---|---|---|---|
| Checkmate 238 | Nivolumab 3 mg/kg Q2 weeks for 1 year (1:1) | Ipilimumab 10 mg/kg Q3 week four doses and then every 12 weeks for 1 year | IIIB, IIIC, or IV | 4 years RFS: 51.7% nivolumab vs. 41.2% ipilimumab | 14.4 nivolumab vs. 45.9% ipilimumab |
| KEYNOTE 054 | Pembrolizumab 200 mg Q3 week × 18 doses (1:1) | Placebo | IIIA (limited to lymph node metastasis of >1 mm) or stage IIIB or IIIC disease with no in-transit metastases | 3-years RFS: 63.7% pembrolizumab vs. 44.1% placebo | 14.7% pembrolizumab vs. 3.4% placebo |
| KEYNOTE 716 | Pembrolizumab 200 mg Q3 week × 17 doses (1:1) | Placebo | IIB/C (TNM stage T3b or T4 with a negative sentinel lymph node biopsy) | 2-years RFS: 81.2% pembrolizumab vs. 72.8% placebo | 17% pembrolizumab vs. 5% placebo |
| Checkmate 76k | Nivolumab 480 mg Q4 weeks for 1 year (2:1) | Placebo | IIB/C (TNM stage T3b or T4 with a negative sentinel lymph node biopsy) | 1-years RFS: 89% vs. 79% placebo | 22% nivolumab vs. 12% placebo |
| SWOG1801 ** | Peri-operative pembrolizumab: 3 cycles of neoadjuvant pembrolizumab 200 mg Q3 week followed by 15 cycles of adjuvant pembrolizumab (1:1) | Adjuvant pembrolizumab 200 mg Q3 week for 18 doses | IIIB/C/D and resectable IV | 2-years EFS: 72% for peri-operative pembrolizumab vs. 49% adjuvant pembrolizumab | 7% in neoadjuvant phase and 12% adjuvant phase for peri-operative pembrolizumab vs.: 14% adjuvant pembrolizumab only |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mooradian, M.J.; Sullivan, R.J. Immunotherapy in Melanoma: Recent Advancements and Future Directions. Cancers 2023, 15, 4176. https://doi.org/10.3390/cancers15164176
Mooradian MJ, Sullivan RJ. Immunotherapy in Melanoma: Recent Advancements and Future Directions. Cancers. 2023; 15(16):4176. https://doi.org/10.3390/cancers15164176
Chicago/Turabian StyleMooradian, Meghan J., and Ryan J. Sullivan. 2023. "Immunotherapy in Melanoma: Recent Advancements and Future Directions" Cancers 15, no. 16: 4176. https://doi.org/10.3390/cancers15164176
APA StyleMooradian, M. J., & Sullivan, R. J. (2023). Immunotherapy in Melanoma: Recent Advancements and Future Directions. Cancers, 15(16), 4176. https://doi.org/10.3390/cancers15164176




