The Role of GAB1 in Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Participation of GAB1 in Cell Signal Transduction
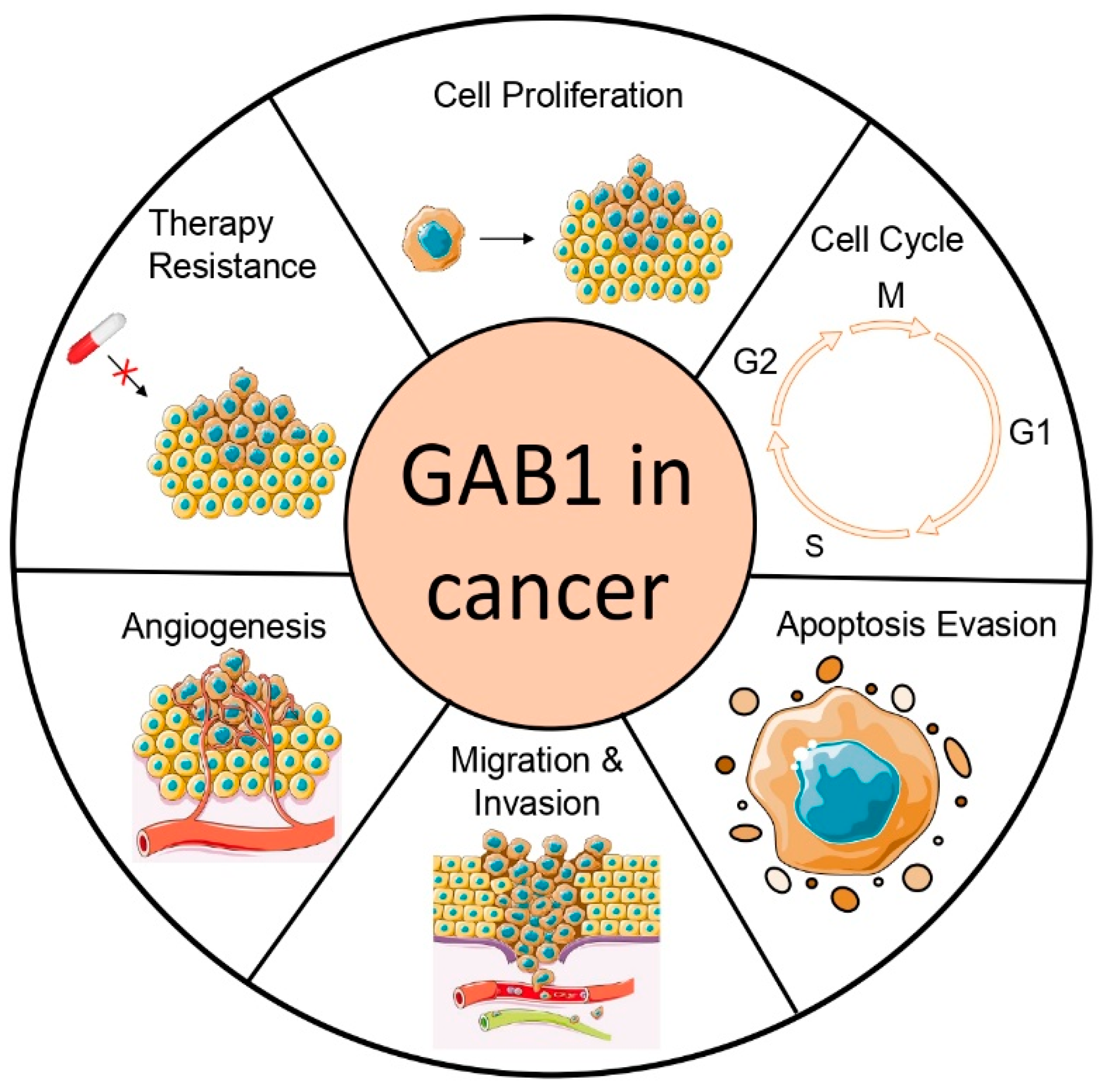
3. Role of GAB1 in Cancer
3.1. GAB1 in Proliferation and Apoptosis
3.2. GAB1 in Angiogenesis
3.3. GAB1 in Tumor Migration and Invasion
3.4. GAB1 in Resistance to Tumor Therapy
3.5. GAB1 Inhibitors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- White, M.F.; Yenush, L. The IRS-Signaling System: A Network of Docking Proteins That Mediate Insulin and Cytokine Action. Curr. Top. Microbiol. Immunol. 1998, 228, 179–208. [Google Scholar] [CrossRef] [PubMed]
- Hibi, M.; Hirano, T. Gab-Family Adapter Molecules in Signal Transduction of Cytokine and Growth Factor Receptors, and T and B Cell Antigen Receptors. Leuk. Lymphoma 2000, 37, 299–307. [Google Scholar] [CrossRef]
- Huyer, G.; Alexander, D.R. Immune Signalling: SHP-2 Docks at Multiple Ports. Curr. Biol. 1999, 9, 129–132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gu, H.; Neel, B.G. The “Gab” in Signal Transduction. Trends Cell Biol. 2003, 13, 122–130. [Google Scholar] [CrossRef]
- Holgado-Madruga, M.; Emlet, D.R.; Moscatello, D.K.; Godwin, A.K.; Wong, A.J. A Grb2-Associated Docking Protein in EGF- and Insulin-Receptor Signalling. Nature 1996, 379, 560–564. [Google Scholar] [CrossRef]
- Gu, H.; Pratt, J.C.; Burakoff, S.J.; Neel, B.G. Cloning of P97/Gab2, the Major SHP2-Binding Protein in Hematopoietic Cells, Reveals a Novel Pathway for Cytokine-Induced Gene Activation. Mol. Cell 1998, 2, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Wolf, I.; Jenkins, B.J.; Liu, Y.; Seiffert, M.; Custodio, J.M.; Young, P.; Rohrschneider, L.R. Gab3, a New DOS/Gab Family Member, Facilitates Macrophage Differentiation. Mol. Cell. Biol. 2002, 22, 231–244. [Google Scholar] [CrossRef]
- Raabe, T.; Riesgo-Escovar, J.; Liu, X.; Bausenwein, B.S.; Deak, P.; Maröy, P.; Hafen, E. DOS, a Novel Pleckstrin Homology Domain–Containing Protein Required for Signal Transduction between Sevenless and Ras1 in Drosophila. Cell 1996, 85, 911–920. [Google Scholar] [CrossRef][Green Version]
- Herbst, R.; Carroll, P.M.; Allard, J.D.; Schilling, J.; Raabe, T.; Simon, M.A. Daughter of Sevenless Is a Substrate of the Phosphotyrosine Phosphatase Corkscrew and Functions during Sevenless Signaling. Cell 1996, 85, 899–909. [Google Scholar] [CrossRef]
- Schutzman, J.L.; Borland, C.Z.; Newman, J.C.; Robinson, M.K.; Kokel, M.; Stern, M.J. The Caenorhabditis Elegans EGL-15 Signaling Pathway Implicates a DOS-like Multisubstrate Adaptor Protein in Fibroblast Growth Factor Signal Transduction. Mol. Cell. Biol. 2001, 21, 8104–8116. [Google Scholar] [CrossRef]
- Liu, Y.; Rohrschneider, L.R. The Gift of Gab. FEBS Lett. 2002, 515, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Nishida, K.; Hibi, M.; Hirano, T.; Matsuda, Y. Comparative FISH Mapping of GAB1 and Gab2 Genes in Human, Mouse and Rat. Cytogenet. Cell Genet. 2001, 94, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Masaki, T.; Arita, Y.; Ishibashi, T.; Inagaki, T.; Okazawa, M.; Oka, T.; Shioyama, W.; Yamauchi-Takihara, K.; Komuro, I.; et al. Molecular Characterization of Striated Muscle-Specific GAB1 Isoform as a Critical Signal Transducer for Neuregulin-1/ErbB Signaling in Cardiomyocytes. PLoS ONE 2016, 11, e0166710. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Holgado-Madruga, M.; Maroun, C.; Fixman, E.D.; Kamikura, D.; Fournier, T.; Charest, A.; Tremblay, M.L.; Wong, A.J.; Park, M. Association of the Multisubstrate Docking Protein GAB1 with the Hepatocyte Growth Factor Receptor Requires a Functional Grb2 Binding Site Involving Tyrosine 1356. J. Biol. Chem. 1997, 272, 20811–20819. [Google Scholar] [CrossRef]
- Osawa, M.; Itoh, S.; Ohta, S.; Huang, Q.; Berk, B.C.; Marmarosh, N.-L.; Che, W.; Ding, B.; Yan, C.; Abe, J. ERK1/2 Associates with the c-Met-Binding Domain of Growth Factor Receptor-Bound Protein 2 (Grb2)-Associated Binder-1 (GAB1): Role in ERK1/2 and Early Growth Response Factor-1 (Egr-1) Nuclear Accumulation. J. Biol. Chem. 2004, 279, 29691–29699. [Google Scholar] [CrossRef]
- Itoh, M.; Yoshida, Y.; Nishida, K.; Narimatsu, M.; Hibi, M.; Hirano, T. Role of GAB1 in Heart, Placenta, and Skin Development and Growth Factor- and Cytokine-Induced Extracellular Signal-Regulated Kinase Mitogen-Activated Protein Kinase Activation. Mol. Cell. Biol. 2000, 20, 3695–3704. [Google Scholar] [CrossRef]
- Sachs, M.; Brohmann, H.; Zechner, D.; Müller, T.; Hülsken, J.; Walther, I.; Schaeper, U.; Birchmeier, C.; Birchmeier, W. Essential Role of GAB1 for Signaling by the C-Met Receptor in Vivo. J. Cell Biol. 2000, 150, 1375–1384. [Google Scholar] [CrossRef]
- Lamorte, L.; Kamikura, D.M.; Park, M. A Switch from P130Cas/Crk to GAB1/Crk Signaling Correlates with Anchorage Independent Growth and JNK Activation in Cells Transformed by the Met Receptor Oncoprotein. Oncogene 2000, 19, 5973–5981. [Google Scholar] [CrossRef]
- Chan, P.-C.; Chen, Y.-L.; Cheng, C.-H.; Yu, K.-C.; Cary, L.A.; Shu, K.-H.; Ho, W.L.; Chen, H.-C. Src Phosphorylates Grb2-Associated Binder 1 upon Hepatocyte Growth Factor Stimulation. J. Biol. Chem. 2003, 278, 44075–44082. [Google Scholar] [CrossRef]
- Podar, K.; Mostoslavsky, G.; Sattler, M.; Tai, Y.-T.; Hayashi, T.; Catley, L.P.; Hideshima, T.; Mulligan, R.C.; Chauhan, D.; Anderson, K.C. Critical Role for Hematopoietic Cell Kinase (Hck)-Mediated Phosphorylation of GAB1 and Gab2 Docking Proteins in Interleukin 6-Induced Proliferation and Survival of Multiple Myeloma Cells. J. Biol. Chem. 2004, 279, 21658–21665. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, S.; Gao, Y.; Greer, P.A.; Tonks, N.K. HGF-Independent Regulation of MET and GAB1 by Nonreceptor Tyrosine Kinase FER Potentiates Metastasis in Ovarian Cancer. Genes Dev. 2016, 30, 1542–1557. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yuan, J.; Liu, H.; Shi, Z.; Baker, K.; Vuori, K.; Wu, J.; Feng, G.-S. Role of GAB1 in UV-Induced c-Jun NH2-Terminal Kinase Activation and Cell Apoptosis. Mol. Cell. Biol. 2004, 24, 1531–1539. [Google Scholar] [CrossRef][Green Version]
- Jin, Z.-G.; Wong, C.; Wu, J.; Berk, B.C. Flow Shear Stress Stimulates GAB1 Tyrosine Phosphorylation to Mediate Protein Kinase B and Endothelial Nitric-Oxide Synthase Activation in Endothelial Cells. J. Biol. Chem. 2005, 280, 12305–12309. [Google Scholar] [CrossRef]
- Holgado-Madruga, M.; Wong, A.J. GAB1 Is an Integrator of Cell Death versus Cell Survival Signals in Oxidative Stress. Mol. Cell. Biol. 2003, 23, 4471–4484. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.; Loot, A.E.; Mohamed, A.; Fisslthaler, B.; Boulanger, C.M.; Ceacareanu, B.; Hassid, A.; Busse, R.; Fleming, I. GAB1, SHP2, and Protein Kinase A Are Crucial for the Activation of the Endothelial NO Synthase by Fluid Shear Stress. Circ. Res. 2005, 97, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Weidner, K.M.; Di Cesare, S.; Sachs, M.; Brinkmann, V.; Behrens, J.; Birchmeier, W. Interaction between GAB1 and the C-Met Receptor Tyrosine Kinase Is Responsible for Epithelial Morphogenesis. Nature 1996, 384, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Schaeper, U.; Vogel, R.; Chmielowiec, J.; Huelsken, J.; Rosario, M.; Birchmeier, W. Distinct Requirements for GAB1 in Met and EGF Receptor Signaling in Vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 15376–15381. [Google Scholar] [CrossRef]
- Lock, L.S.; Frigault, M.M.; Saucier, C.; Park, M. Grb2-Independent Recruitment of GAB1 Requires the C-Terminal Lobe and Structural Integrity of the Met Receptor Kinase Domain. J. Biol. Chem. 2003, 278, 30083–30090. [Google Scholar] [CrossRef]
- Saucier, C.; Papavasiliou, V.; Palazzo, A.; Naujokas, M.A.; Kremer, R.; Park, M. Use of Signal Specific Receptor Tyrosine Kinase Oncoproteins Reveals That Pathways Downstream from Grb2 or Shc Are Sufficient for Cell Transformation and Metastasis. Oncogene 2002, 21, 1800–1811. [Google Scholar] [CrossRef]
- Bard-Chapeau, E.A.; Yuan, J.; Droin, N.; Long, S.; Zhang, E.E.; Nguyen, T.V.; Feng, G.-S. Concerted Functions of GAB1 and Shp2 in Liver Regeneration and Hepatoprotection. Mol. Cell. Biol. 2006, 26, 4664–4674. [Google Scholar] [CrossRef]
- Lock, L.S.; Royal, I.; Naujokas, M.A.; Park, M. Identification of an Atypical Grb2 Carboxyl-Terminal SH3 Domain Binding Site in Gab Docking Proteins Reveals Grb2-Dependent and -Independent Recruitment of GAB1 to Receptor Tyrosine Kinases. J. Biol. Chem. 2000, 275, 31536–31545. [Google Scholar] [CrossRef] [PubMed]
- Holgado-Madruga, M.; Moscatello, D.K.; Emlet, D.R.; Dieterich, R.; Wong, A.J. Grb2-Associated Binder-1 Mediates Phosphatidylinositol 3-Kinase Activation and the Promotion of Cell Survival by Nerve Growth Factor. Proc. Natl. Acad. Sci. USA 1997, 94, 12419–12424. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.A.; Falasca, M.; Zhang, Z.; Ong, S.H.; Schlessinger, J. A Novel Positive Feedback Loop Mediated by the Docking Protein GAB1 and Phosphatidylinositol 3-Kinase in Epidermal Growth Factor Receptor Signaling. Mol. Cell. Biol. 2000, 20, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Yart, A.; Laffargue, M.; Mayeux, P.; Chretien, S.; Peres, C.; Tonks, N.; Roche, S.; Payrastre, B.; Chap, H.; Raynal, P. A Critical Role for Phosphoinositide 3-Kinase Upstream of GAB1 and SHP2 in the Activation of Ras and Mitogen-Activated Protein Kinases by Epidermal Growth Factor. J. Biol. Chem. 2001, 276, 8856–8864. [Google Scholar] [CrossRef]
- Laffargue, M.; Raynal, P.; Yart, A.; Peres, C.; Wetzker, R.; Roche, S.; Payrastre, B.; Chap, H. An Epidermal Growth Factor Receptor/GAB1 Signaling Pathway Is Required for Activation of Phosphoinositide 3-Kinase by Lysophosphatidic Acid. J. Biol. Chem. 1999, 274, 32835–32841. [Google Scholar] [CrossRef]
- Ong, S.H.; Hadari, Y.R.; Gotoh, N.; Guy, G.R.; Schlessinger, J.; Lax, I. Stimulation of Phosphatidylinositol 3-Kinase by Fibroblast Growth Factor Receptors Is Mediated by Coordinated Recruitment of Multiple Docking Proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 6074–6079. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Whitman, M.; Cantley, L.C.; Erikson, R.L. Evidence That the Rous Sarcoma Virus Transforming Gene Product Phosphorylates Phosphatidylinositol and Diacylglycerol. Proc. Natl. Acad. Sci. USA 1984, 81, 2117–2121. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Whitman, M.; Downes, C.P.; Keeler, M.; Keller, T.; Cantley, L. Type I Phosphatidylinositol Kinase Makes a Novel Inositol Phospholipid, Phosphatidylinositol-3-Phosphate. Nature 1988, 332, 644–646. [Google Scholar] [CrossRef]
- Maroun, C.R.; Holgado-Madruga, M.; Royal, I.; Naujokas, M.A.; Fournier, T.M.; Wong, A.J.; Park, M. The GAB1 PH Domain Is Required for Localization of GAB1 at Sites of Cell-Cell Contact and Epithelial Morphogenesis Downstream from the Met Receptor Tyrosine Kinase. Mol. Cell. Biol. 1999, 19, 1784–1799. [Google Scholar] [CrossRef]
- Maroun, C.R.; Moscatello, D.K.; Naujokas, M.A.; Holgado-Madruga, M.; Wong, A.J.; Park, M. A Conserved Inositol Phospholipid Binding Site within the Pleckstrin Homology Domain of the GAB1 Docking Protein Is Required for Epithelial Morphogenesis. J. Biol. Chem. 1999, 274, 31719–31726. [Google Scholar] [CrossRef] [PubMed]
- Cunnick, J.M.; Mei, L.; Doupnik, C.A.; Wu, J. Phosphotyrosines 627 and 659 of GAB1 Constitute a Bisphosphoryl Tyrosine-Based Activation Motif (BTAM) Conferring Binding and Activation of SHP2. J. Biol. Chem. 2001, 276, 24380–24387. [Google Scholar] [CrossRef] [PubMed]
- Schaeper, U.; Gehring, N.H.; Fuchs, K.P.; Sachs, M.; Kempkes, B.; Birchmeier, W. Coupling of GAB1 to C-Met, Grb2, and Shp2 Mediates Biological Responses. J. Cell Biol. 2000, 149, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Cunnick, J.M.; Dorsey, J.F.; Munoz-Antonia, T.; Mei, L.; Wu, J. Requirement of SHP2 Binding to Grb2-Associated Binder-1 for Mitogen-Activated Protein Kinase Activation in Response to Lysophosphatidic Acid and Epidermal Growth Factor. J. Biol. Chem. 2000, 275, 13842–13848. [Google Scholar] [CrossRef]
- Maroun, C.R.; Naujokas, M.A.; Holgado-Madruga, M.; Wong, A.J.; Park, M. The Tyrosine Phosphatase SHP-2 Is Required for Sustained Activation of Extracellular Signal-Regulated Kinase and Epithelial Morphogenesis Downstream from the Met Receptor Tyrosine Kinase. Mol. Cell. Biol. 2000, 20, 8513–8525. [Google Scholar] [CrossRef]
- Bongartz, H.; Gille, K.; Hessenkemper, W.; Mandel, K.; Lewitzky, M.; Feller, S.M.; Schaper, F. The Multi-Site Docking Protein Grb2-Associated Binder 1 (GAB1) Enhances Interleukin-6-Induced MAPK-Pathway Activation in an SHP2-, Grb2-, and Time-Dependent Manner. Cell Commun. Signal. 2019, 17, 135. [Google Scholar] [CrossRef]
- Lock, L.S.; Maroun, C.R.; Naujokas, M.A.; Park, M. Distinct Recruitment and Function of GAB1 and Gab2 in Met Receptor-Mediated Epithelial Morphogenesis. Mol. Biol. Cell 2002, 13, 2132–2146. [Google Scholar] [CrossRef]
- Galabova-Kovacs, G.; Matzen, D.; Piazzolla, D.; Meissl, K.; Plyushch, T.; Chen, A.P.; Silva, A.; Baccarini, M. Essential Role of B-Raf in ERK Activation during Extraembryonic Development. Proc. Natl. Acad. Sci. USA 2006, 103, 1325–1330. [Google Scholar] [CrossRef]
- Shi, Z.-Q.; Yu, D.-H.; Park, M.; Marshall, M.; Feng, G.-S. Molecular Mechanism for the Shp-2 Tyrosine Phosphatase Function in Promoting Growth Factor Stimulation of Erk Activity. Mol. Cell. Biol. 2000, 20, 1526–1536. [Google Scholar] [CrossRef]
- Mattoon, D.R.; Lamothe, B.; Lax, I.; Schlessinger, J. The Docking Protein GAB1 Is the Primary Mediator of EGF-Stimulated Activation of the PI-3K/Akt Cell Survival Pathway. BMC Biol. 2004, 2, 24. [Google Scholar] [CrossRef]
- Hoeben, A.; Martin, D.; Clement, P.M.; Cools, J.; Gutkind, J.S. Role of GRB2-Associated Binder 1 in Epidermal Growth Factor Receptor-Induced Signaling in Head and Neck Squamous Cell Carcinoma. Int. J. Cancer 2013, 132, 1042–1050. [Google Scholar] [CrossRef]
- Paliouras, G.N.; Naujokas, M.A.; Park, M. Pak4, a Novel GAB1 Binding Partner, Modulates Cell Migration and Invasion by the Met Receptor. Mol. Cell. Biol. 2009, 29, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Tsuda, M.; Makino, Y.; Ichihara, S.; Sawa, H.; Minami, A.; Mochizuki, N.; Nagashima, K.; Tanaka, S. Adaptor Molecule Crk Is Required for Sustained Phosphorylation of Grb2-Associated Binder 1 and Hepatocyte Growth Factor-Induced Cell Motility of Human Synovial Sarcoma Cell Lines. Mol. Cancer Res. 2006, 4, 499–510. [Google Scholar] [CrossRef]
- Sakkab, D.; Lewitzky, M.; Posern, G.; Schaeper, U.; Sachs, M.; Birchmeier, W.; Feller, S.M. Signaling of Hepatocyte Growth Factor/Scatter Factor (HGF) to the Small GTPase Rap1 via the Large Docking Protein GAB1 and the Adapter Protein CRKL. J. Biol. Chem. 2000, 275, 10772–10778. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xue, B.; Umitsu, M.; Ikura, M.; Muthuswamy, S.K.; Neel, B.G. The Signaling Adaptor GAB1 Regulates Cell Polarity by Acting as a PAR Protein Scaffold. Mol. Cell 2012, 47, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Gual, P.; Giordano, S.; Williams, T.A.; Rocchi, S.; Van Obberghen, E.; Comoglio, P.M. Sustained Recruitment of Phospholipase C-Gamma to GAB1 Is Required for HGF-Induced Branching Tubulogenesis. Oncogene 2000, 19, 1509–1518. [Google Scholar] [CrossRef]
- Kapoor, G.S.; Zhan, Y.; Johnson, G.R.; O’Rourke, D.M. Distinct Domains in the SHP-2 Phosphatase Differentially Regulate Epidermal Growth Factor Receptor/NF-KappaB Activation through GAB1 in Glioblastoma Cells. Mol. Cell. Biol. 2004, 24, 823–836. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chan, P.-C.; Li, J.-R.; Chen, C.-J.; Shieh, J.-J.; Fu, Y.-C.; Chen, H.-C.; Wu, M.-J. GAB1 Is Essential for Membrane Translocation, Activity and Integrity of MTORCs after EGF Stimulation in Urothelial Cell Carcinoma. Oncotarget 2015, 6, 1478–1489. [Google Scholar] [CrossRef][Green Version]
- Huber, M.; Helgason, C.D.; Damen, J.E.; Liu, L.; Humphries, R.K.; Krystal, G. The Src Homology 2-Containing Inositol Phosphatase (SHIP) Is the Gatekeeper of Mast Cell Degranulation. Proc. Natl. Acad. Sci. USA 1998, 95, 11330–11335. [Google Scholar] [CrossRef]
- Wöhrle, F.U.; Daly, R.J.; Brummer, T. Function, Regulation and Pathological Roles of the Gab/DOS Docking Proteins. Cell Commun. Signal. 2009, 7, 22. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Tsiaras, W.G.; Araki, T.; Wen, G.; Minichiello, L.; Klein, R.; Neel, B.G. Receptor-Specific Regulation of Phosphatidylinositol 3’-Kinase Activation by the Protein Tyrosine Phosphatase Shp2. Mol. Cell. Biol. 2002, 22, 4062–4072. [Google Scholar] [CrossRef] [PubMed]
- Montagner, A.; Yart, A.; Dance, M.; Perret, B.; Salles, J.-P.; Raynal, P. A Novel Role for GAB1 and SHP2 in Epidermal Growth Factor-Induced Ras Activation. J. Biol. Chem. 2005, 280, 5350–5360. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.F.; Liu, Z.-X.; Cantley, L.G. ERK Negatively Regulates the Epidermal Growth Factor-Mediated Interaction of GAB1 and the Phosphatidylinositol 3-Kinase. J. Biol. Chem. 2002, 277, 19382–19388. [Google Scholar] [CrossRef] [PubMed]
- Roshan, B.; Kjelsberg, C.; Spokes, K.; Eldred, A.; Crovello, C.S.; Cantley, L.G. Activated ERK2 Interacts with and Phosphorylates the Docking Protein GAB1. J. Biol. Chem. 1999, 274, 36362–36368. [Google Scholar] [CrossRef] [PubMed]
- Gual, P.; Giordano, S.; Anguissola, S.; Parker, P.J.; Comoglio, P.M. GAB1 Phosphorylation: A Novel Mechanism for Negative Regulation of HGF Receptor Signaling. Oncogene 2001, 20, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.F.; Roshan, B.; Liu, Z.X.; Cantley, L.G. ERK Regulates the Hepatocyte Growth Factor-Mediated Interaction of GAB1 and the Phosphatidylinositol 3-Kinase. J. Biol. Chem. 2001, 276, 32552–32558. [Google Scholar] [CrossRef]
- Mizutani, N.; Hikita, H.; Saito, Y.; Myojin, Y.; Sato, K.; Urabe, M.; Kurahashi, T.; Shiode, Y.; Sakane, S.; Murai, K.; et al. GAB1 in Livers with Persistent Hepatocyte Apoptosis Has an Antiapoptotic Effect and Reduces Chronic Liver Injury, Fibrosis, and Tumorigenesis. Am. J. Physiol.—Gastrointest. Liver Physiol. 2021, 320, G958–G968. [Google Scholar] [CrossRef]
- Zhou, L.; Shao, C.-Y.; Xie, Y.-J.; Wang, N.; Xu, S.-M.; Luo, B.-Y.; Wu, Z.-Y.; Ke, Y.H.; Qiu, M.; Shen, Y. GAB1 Mediates PDGF Signaling and Is Essential to Oligodendrocyte Differentiation and CNS Myelination. eLife 2020, 9, e52056. [Google Scholar] [CrossRef]
- Lu, Y.; Xiong, Y.; Huo, Y.; Han, J.; Yang, X.; Zhang, R.; Zhu, D.-S.; Klein-Hessling, S.; Li, J.; Zhang, X.; et al. Grb-2-Associated Binder 1 (GAB1) Regulates Postnatal Ischemic and VEGF-Induced Angiogenesis through the Protein Kinase A-Endothelial NOS Pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 2957–2962. [Google Scholar] [CrossRef]
- Shi, X.; Liu, Q.; Zhao, H.; Lu, J.; Huang, Y.; Ma, Y.; Xia, J.; Liu, M.; Tu, W.; Jin, L.; et al. Increased Expression of GAB1 Promotes Inflammation and Fibrosis in Systemic Sclerosis. Exp. Dermatol. 2019, 28, 1313–1320. [Google Scholar] [CrossRef]
- Holgado-Madruga, M.; Badal, S.; Wong, A.J. Abstract 314: Characterization of the Y83C GAB1 Mutation in Breast Cancer. Cancer Res. 2010, 70, 314. [Google Scholar] [CrossRef]
- Ortiz-Padilla, C.; Gallego-Ortega, D.; Browne, B.C.; Hochgräfe, F.; Caldon, C.E.; Lyons, R.J.; Croucher, D.R.; Rickwood, D.; Ormandy, C.J.; Brummer, T.; et al. Functional Characterization of Cancer-Associated GAB1 Mutations. Oncogene 2013, 32, 2696–2702. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Perret, R.; Demicco, E.G.; Gross, J.; Liu, Y.J.; Azmani, R.; Engelmann, C.; Schubart, C.; Seppet, J.; Stoehr, R.; et al. GAB1::ABL1 Fusions Define a Distinctive Soft Tissue Neoplasm, with Variable Perineurial Differentiation, and a Predilection for Children and Young Adults. Genes. Chromosomes Cancer 2023, 62, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Enrico, D.; Lacroix, L.; Chen, J.; Rouleau, E.; Scoazec, J.-Y.; Loriot, Y.; Tselikas, L.; Jovelet, C.; Planchard, D.; Gazzah, A.; et al. Oncogenic Fusions May Be Frequently Present at Resistance of EGFR Tyrosine Kinase Inhibitors in Patients with NSCLC: A Brief Report. JTO Clin. Res. Rep. 2020, 1, 100023. [Google Scholar] [CrossRef]
- Kumaran, M.; Cass, C.E.; Graham, K.; Mackey, J.R.; Hubaux, R.; Lam, W.; Yasui, Y.; Damaraju, S. Germline Copy Number Variations Are Associated with Breast Cancer Risk and Prognosis. Sci. Rep. 2017, 7, 14621. [Google Scholar] [CrossRef]
- Meng, L.-Q. Essential Role of Polymorphism of GAB1, EGFR, and EGF for the Susceptibility of Biliary Tract Cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014, 35, 12497–12508. [Google Scholar] [CrossRef]
- Wang, G.; Heij, L.R.; Liu, D.; Dahl, E.; Lang, S.A.; Ulmer, T.F.; Luedde, T.; Neumann, U.P.; Bednarsch, J. The Role of Single-Nucleotide Polymorphisms in Cholangiocarcinoma: A Systematic Review. Cancers 2022, 14, 5969. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, J.; Wu, Q.; Yan, H.; Wang, X.; Nan, C.; Wu, Z.; Chen, L.; Zhao, Z. Association of Single-Nucleotide Polymorphisms of GAB1 Gene with Susceptibility to Meningioma in a Northern Chinese Han Population. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e933444. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Ren, Y.; Yin, Z.; Quan, X.; Xue, X.; Zhou, B. Polymorphisms of Rs1347093 and Rs1397529 Are Associated with Lung Cancer Risk in Northeast Chinese Population. Oncotarget 2017, 8, 94862–94871. [Google Scholar] [CrossRef]
- Liu, H.; Li, G.; Zeng, W.; Zhang, P.; Fan, F.; Tu, Y.; Zhang, Y. Combined Detection of GAB1 and Gab2 Expression Predicts Clinical Outcome of Patients with Glioma. Med. Oncol. 2014, 31, 77. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Yang, M.; Wang, D.; Yu, L.; Guo, C.; Guo, X.; Lin, N. Identification of GRB2 and GAB1 Coexpression as an Unfavorable Prognostic Factor for Hepatocellular Carcinoma by a Combination of Expression Profile and Network Analysis. PLoS ONE 2013, 8, e85170. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liu, R. Expression of GAB1 Is Associated with Poor Prognosis of Patients with Epithelial Ovarian Cancer. Tohoku J. Exp. Med. 2016, 239, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Boetto, J.; Bielle, F.; Tran, S.; Marijon, P.; Peyre, M.; Rigau, V.; Kalamarides, M. GAB1 as a Marker of Recurrence in Anterior Skull Base Meningioma. Neurosurgery 2023, 92, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Boetto, J.; Lerond, J.; Peyre, M.; Tran, S.; Marijon, P.; Kalamarides, M.; Bielle, F. GAB1 Overexpression Identifies Hedgehog-Activated Anterior Skull Base Meningiomas. Neuropathol. Appl. Neurobiol. 2021, 47, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kang, Z.; Li, S.; Wang, C.; Zheng, X.; Cai, Z.; Pan, L.; Chen, F.; Li, W. Molecular Profile Reveals Immune-Associated Markers of Medulloblastoma for Different Subtypes. Front. Immunol. 2022, 13, 911260. [Google Scholar] [CrossRef]
- Al-Husseinawi, E.; Bui, M.M.; Ahmed, A.A. Grb2-Associated Binding Protein-1 as a Biomarker in Bone and Soft Tissue Sarcomas. Pathology 2019, 51, 610–614. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Mood, K.; Saucier, C.; Bong, Y.-S.; Lee, H.-S.; Park, M.; Daar, I.O. GAB1 Is Required for Cell Cycle Transition, Cell Proliferation, and Transformation Induced by an Oncogenic Met Receptor. Mol. Biol. Cell 2006, 17, 3717–3728. [Google Scholar] [CrossRef]
- Sang, H.; Li, T.; Li, H.; Liu, J. Down-Regulation of GAB1 Inhibits Cell Proliferation and Migration in Hilar Cholangiocarcinoma. PLoS ONE 2013, 8, e81347. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, F.; Cao, X.; Chen, C.; Zhang, X.; Zhang, X.; Lin, W.; Wang, X.; Liang, C. GAB1 Regulates SDF-1-Induced Progression via Inhibition of Apoptosis Pathway Induced by PI3K/AKT/Bcl-2/BAX Pathway in Human Chondrosarcoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Kameda, H.; Risinger, J.I.; Han, B.B.; Baek, S.J.; Barrett, J.C.; Abe, T.; Takeuchi, T.; Glasgow, W.C.; Eling, T.E. Expression of GAB1 Lacking the Pleckstrin Homology Domain Is Associated with Neoplastic Progression. Mol. Cell. Biol. 2001, 21, 6895–6905. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, J.; Kuang, Z.; Kuang, Y.; Wu, H. Knockdown of GAB1 Inhibits Cellular Proliferation, Migration, and Invasion in Human Oral Squamous Carcinoma Cells. Oncol. Res. 2018, 26, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.-Y.; Wang, D.-X.; Wang, Y.; Li, Y.; Zhang, Z.-Q.; Jiang, Q.; Luo, W.; Cao, C. MicroRNA-29a-3p Downregulation Causes GAB1 Upregulation to Promote Glioma Cell Proliferation. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 48, 450–460. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Z.; Wang, J.; Shang, R.; Zhou, L.; Wang, X.; Duan, J.; Ruan, B.; Gao, Y.; Dai, B.; et al. MicroRNA-150 Suppresses Cell Proliferation and Metastasis in Hepatocellular Carcinoma by Inhibiting the GAB1-ERK Axis. Oncotarget 2016, 7, 11595–11608. [Google Scholar] [CrossRef]
- Li, J.; Yang, N.; Tian, X.; Ouyang, L.; Jiang, M.; Zhang, S. Interference of PTK6/GAB1 Signaling Inhibits Cell Proliferation, Invasion, and Migration of Cervical Cancer Cells. Mol. Med. Rep. 2022, 26, 284. [Google Scholar] [CrossRef]
- Gillgrass, A.; Cardiff, R.D.; Sharan, N.; Kannan, S.; Muller, W.J. Epidermal Growth Factor Receptor-Dependent Activation of GAB1 Is Involved in ErbB-2-Mediated Mammary Tumor Progression. Oncogene 2003, 22, 9151–9155. [Google Scholar] [CrossRef][Green Version]
- Yamasaki, S.; Nishida, K.; Yoshida, Y.; Itoh, M.; Hibi, M.; Hirano, T. GAB1 Is Required for EGF Receptor Signaling and the Transformation by Activated ErbB2. Oncogene 2003, 22, 1546–1556. [Google Scholar] [CrossRef]
- Verma, S.; Vaughan, T.; Bunting, K.D. Gab Adapter Proteins as Therapeutic Targets for Hematologic Disease. Adv. Hematol. 2012, 2012, 380635. [Google Scholar] [CrossRef]
- Xiang, Y.-P.; Xiao, T.; Li, Q.-G.; Lu, S.-S.; Zhu, W.; Liu, Y.-Y.; Qiu, J.-Y.; Song, Z.-H.; Huang, W.; Yi, H.; et al. Y772 Phosphorylation of EphA2 Is Responsible for EphA2-Dependent NPC Nasopharyngeal Carcinoma Growth by Shp2/Erk-1/2 Signaling Pathway. Cell Death Dis. 2020, 11, 709. [Google Scholar] [CrossRef]
- An, H.-J.; Kwak, S.-Y.; Yoo, J.-O.; Kim, J.-S.; Bae, I.-H.; Park, M.-J.; Cho, M.-Y.; Kim, J.; Han, Y.-H. Novel MiR-5582-5p Functions as a Tumor Suppressor by Inducing Apoptosis and Cell Cycle Arrest in Cancer Cells through Direct Targeting of GAB1, SHC1, and CDK2. Biochim. Biophys. Acta 2016, 1862, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.-L.; Zhao, H.-J.; Song, C.-F.; Zhao, S.; Tian, Z.-S.; Zhou, J.-J. MicroRNA-383 Suppresses Pancreatic Carcinoma Development via Inhibition of GAB1 Expression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10729–10739. [Google Scholar] [CrossRef]
- Griffioen, A.W.; Dudley, A.C. Angiogenesis: A Year in Review. Angiogenesis 2021, 24, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Anti-Angiogenesis: New Concept for Therapy of Solid Tumors. Ann. Surg. 1972, 175, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Parangi, S.; O’Reilly, M.; Christofori, G.; Holmgren, L.; Grosfeld, J.; Folkman, J.; Hanahan, D. Antiangiogenic Therapy of Transgenic Mice Impairs de Novo Tumor Growth. Proc. Natl. Acad. Sci. USA 1996, 93, 2002–2007. [Google Scholar] [CrossRef]
- Li, T.; Kang, G.; Wang, T.; Huang, H. Tumor Angiogenesis and Anti-Angiogenic Gene Therapy for Cancer. Oncol. Lett. 2018, 16, 687–702. [Google Scholar] [CrossRef]
- Dor, Y.; Porat, R.; Keshet, E. Vascular Endothelial Growth Factor and Vascular Adjustments to Perturbations in Oxygen Homeostasis. Am. J. Physiol. Cell Physiol. 2001, 280, C1367–C1374. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, W.; Ha, C.H.; Kim, J.Y.; Wong, C.; Redmond, E.M.; Hamik, A.; Jain, M.K.; Feng, G.-S.; Jin, Z.G. Endothelial Grb2-Associated Binder 1 Is Crucial for Postnatal Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1016–1023. [Google Scholar] [CrossRef]
- Li, T.; Tian, Y.; Ren, W.; Chen, P.; Luo, M.; Sang, H. GAB1 Regulates Invadopodia and Autocrine VEGF through SHP2/ERK1/2 in Hilar Cholangiocarcinoma Cells. Am. J. Transl. Res. 2022, 14, 8934–8946. [Google Scholar]
- Takeuchi, S.; Wang, W.; Li, Q.; Yamada, T.; Kita, K.; Donev, I.S.; Nakamura, T.; Matsumoto, K.; Shimizu, E.; Nishioka, Y.; et al. Dual Inhibition of Met Kinase and Angiogenesis to Overcome HGF-Induced EGFR-TKI Resistance in EGFR Mutant Lung Cancer. Am. J. Pathol. 2012, 181, 1034–1043. [Google Scholar] [CrossRef]
- Wang, W.; Xu, S.; Yin, M.; Jin, Z.G. Essential Roles of GAB1 Tyrosine Phosphorylation in Growth Factor-Mediated Signaling and Angiogenesis. Int. J. Cardiol. 2015, 181, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Tumor Metastasis: Mechanistic Insights and Clinical Challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Ma, D.-N.; Chen, Q.-D.; Zhang, J.-J.; Tian, Y.-R.; Wang, Z.-C.; Cai, H.; Lin, Y.; Sun, H.-C. MicroRNA-200a Inhibits Cell Growth and Metastasis by Targeting Foxa2 in Hepatocellular Carcinoma. J. Cancer 2017, 8, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Weng, C.; Dong, H.; Li, S.; Chen, G.; Xu, Z. MicroRNA-409-3p Suppresses Colorectal Cancer Invasion and Metastasis Partly by Targeting GAB1 Expression. Int. J. Cancer 2015, 137, 2310–2322. [Google Scholar] [CrossRef]
- Sang, H.; Li, T.; Li, H.; Liu, J. GAB1 Regulates Proliferation and Migration through the PI3K/Akt Signaling Pathway in Intrahepatic Cholangiocarcinoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 8367–8377. [Google Scholar] [CrossRef]
- Song, P.; Xu, H.; He, Y.; Sun, J.; Xu, Z.; Huang, P.; Ge, M.; Zhang, X.; Ke, Y.; Cheng, H. GAB1 Is Upregulated to Promote Anaplastic Thyroid Cancer Cell Migration through AKT-MDR1. Biochem. Biophys. Res. Commun. 2022, 607, 36–43. [Google Scholar] [CrossRef]
- Murray, D.W.; Didier, S.; Chan, A.; Paulino, V.; Van Aelst, L.; Ruggieri, R.; Tran, N.L.; Byrne, A.T.; Symons, M. Guanine Nucleotide Exchange Factor Dock7 Mediates HGF-Induced Glioblastoma Cell Invasion via Rac Activation. Br. J. Cancer 2014, 110, 1307–1315. [Google Scholar] [CrossRef]
- Seda, V.; Vojackova, E.; Ondrisova, L.; Kostalova, L.; Sharma, S.; Loja, T.; Mladonicka Pavlasova, G.; Zicha, D.; Kudlickova Peskova, M.; Krivanek, J.; et al. FoxO1-GAB1 Axis Regulates Homing Capacity and Tonic AKT Activity in Chronic Lymphocytic Leukemia. Blood 2021, 138, 758–772. [Google Scholar] [CrossRef]
- Wang, X.; Peng, J.; Yang, Z.; Zhou, P.-J.; An, N.; Wei, L.; Zhu, H.H.; Lu, J.; Fang, Y.-X.; Gao, W.-Q. Elevated Expression of GAB1 Promotes Breast Cancer Metastasis by Dissociating the PAR Complex. J. Exp. Clin. Cancer Res. 2019, 38, 27. [Google Scholar] [CrossRef]
- Gonzalez, D.M.; Medici, D. Signaling Mechanisms of the Epithelial-Mesenchymal Transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, R.; Tsuda, M.; Wang, L.; Maishi, N.; Abe, T.; Kimura, T.; Tanino, M.; Nishihara, H.; Hida, K.; Ohba, Y.; et al. Adaptor Protein CRK Induces Epithelial-Mesenchymal Transition and Metastasis of Bladder Cancer Cells through HGF/c-Met Feedback Loop. Cancer Sci. 2015, 106, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Lennon, F.E.; Mirzapoiazova, T.; Mambetsariev, B.; Poroyko, V.A.; Salgia, R.; Moss, J.; Singleton, P.A. The Mu Opioid Receptor Promotes Opioid and Growth Factor-Induced Proliferation, Migration and Epithelial Mesenchymal Transition (EMT) in Human Lung Cancer. PLoS ONE 2014, 9, e91577. [Google Scholar] [CrossRef] [PubMed]
- Buonato, J.M.; Lan, I.S.; Lazzara, M.J. EGF Augments TGFβ-Induced Epithelial-Mesenchymal Transition by Promoting SHP2 Binding to GAB1. J. Cell Sci. 2015, 128, 3898–3909. [Google Scholar] [CrossRef]
- De Wever, O.; Mareel, M. Role of Tissue Stroma in Cancer Cell Invasion. J. Pathol. 2003, 200, 429–447. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental Regulation of Metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef]
- Cardoso, A.P.; Pinto, M.L.; Pinto, A.T.; Oliveira, M.I.; Pinto, M.T.; Gonçalves, R.; Relvas, J.B.; Figueiredo, C.; Seruca, R.; Mantovani, A.; et al. Macrophages Stimulate Gastric and Colorectal Cancer Invasion through EGFR Y(1086), c-Src, Erk1/2 and Akt Phosphorylation and SmallGTPase Activity. Oncogene 2014, 33, 2123–2133. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour Heterogeneity and Resistance to Cancer Therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Chen, H.; Lin, C.; Peng, T.; Hu, C.; Lu, C.; Li, L.; Wang, Y.; Han, R.; Feng, M.; Sun, F.; et al. Metformin Reduces HGF-Induced Resistance to Alectinib via the Inhibition of GAB1. Cell Death Dis. 2020, 11, 111. [Google Scholar] [CrossRef]
- Adachi, Y.; Watanabe, K.; Kita, K.; Kitai, H.; Kotani, H.; Sato, Y.; Inase, N.; Yano, S.; Ebi, H. Resistance Mediated by Alternative Receptor Tyrosine Kinases in FGFR1-Amplified Lung Cancer. Carcinogenesis 2017, 38, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Caenepeel, S.; Cooke, K.; Wadsworth, S.; Huang, G.; Robert, L.; Moreno, B.H.; Parisi, G.; Cajulis, E.; Kendall, R.; Beltran, P.; et al. MAPK Pathway Inhibition Induces MET and GAB1 Levels, Priming BRAF Mutant Melanoma for Rescue by Hepatocyte Growth Factor. Oncotarget 2017, 8, 17795–17809. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, S.; Yamaoka, T.; Ishikawa, F.; Ohmori, T.; Ando, K.; Kusumoto, S.; Kishino, Y.; Manabe, R.; Hasebe, Y.; Sagara, H.; et al. Diverse Mechanisms of Resistance against Osimertinib, a Third-Generation EGFR-TKI, in Lung Adenocarcinoma Cells with an EGFR-Activating Mutation. Cells 2022, 11, 2201. [Google Scholar] [CrossRef]
- Yamada, T.; Takeuchi, S.; Kita, K.; Bando, H.; Nakamura, T.; Matsumoto, K.; Yano, S. Hepatocyte Growth Factor Induces Resistance to Anti-Epidermal Growth Factor Receptor Antibody in Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2012, 7, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Kurupi, R.; Floros, K.V.; Jacob, S.; Chawla, A.T.; Cai, J.; Hu, B.; Puchalapalli, M.; Coon, C.M.; Khatri, R.; Crowther, G.S.; et al. Pharmacologic Inhibition of SHP2 Blocks Both PI3K and MEK Signaling in Low-Epiregulin HNSCC via GAB1. Cancer Res. Commun. 2022, 2, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Pennacchietti, S.; Cazzanti, M.; Bertotti, A.; Rideout, W.M., 3rd; Han, M.; Gyuris, J.; Perera, T.; Comoglio, P.M.; Trusolino, L.; Michieli, P. Microenvironment-Derived HGF Overcomes Genetically Determined Sensitivity to Anti-MET Drugs. Cancer Res. 2014, 74, 6598–6609. [Google Scholar] [CrossRef] [PubMed]
- Malash, I.; Mansour, O.; Gaafar, R.; Shaarawy, S.; Abdellateif, M.S.; Ahmed, O.S.; Zekri, A.-R.N.; Bahnassy, A. Her2/EGFR-PDGFR Pathway Aberrations Associated with Tamoxifen Response in Metastatic Breast Cancer Patients. J. Egypt. Natl. Canc. Inst. 2022, 34, 31. [Google Scholar] [CrossRef]
- Podar, K.; Tai, Y.-T.; Abtahi, D.; Raab, M.S.; Yasui, H.; Ishitsuka, K.; Shiraishi, N.; Catley, L.P.; Sattler, M.; Raje, N.; et al. Targeting Src-Family Kinase Activation and Downstream Molecular Events to Overcome MM Cell Growth, Survival, and Drug-Resistance. Blood 2005, 106, 1583. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- De Bacco, F.; Luraghi, P.; Medico, E.; Reato, G.; Girolami, F.; Perera, T.; Gabriele, P.; Comoglio, P.M.; Boccaccio, C. Induction of MET by Ionizing Radiation and Its Role in Radioresistance and Invasive Growth of Cancer. J. Natl. Cancer Inst. 2011, 103, 645–661. [Google Scholar] [CrossRef]
- Chen, L.; Du-Cuny, L.; Moses, S.; Dumas, S.; Song, Z.; Rezaeian, A.H.; Lin, H.-K.; Meuillet, E.J.; Zhang, S. Novel Inhibitors Induce Large Conformational Changes of GAB1 Pleckstrin Homology Domain and Kill Breast Cancer Cells. PLoS Comput. Biol. 2015, 11, e1004021. [Google Scholar] [CrossRef] [PubMed][Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Baena, M.J.; Cordero-Pérez, F.J.; Pérez-Losada, J.; Holgado-Madruga, M. The Role of GAB1 in Cancer. Cancers 2023, 15, 4179. https://doi.org/10.3390/cancers15164179
Pérez-Baena MJ, Cordero-Pérez FJ, Pérez-Losada J, Holgado-Madruga M. The Role of GAB1 in Cancer. Cancers. 2023; 15(16):4179. https://doi.org/10.3390/cancers15164179
Chicago/Turabian StylePérez-Baena, Manuel Jesús, Francisco Josué Cordero-Pérez, Jesús Pérez-Losada, and Marina Holgado-Madruga. 2023. "The Role of GAB1 in Cancer" Cancers 15, no. 16: 4179. https://doi.org/10.3390/cancers15164179
APA StylePérez-Baena, M. J., Cordero-Pérez, F. J., Pérez-Losada, J., & Holgado-Madruga, M. (2023). The Role of GAB1 in Cancer. Cancers, 15(16), 4179. https://doi.org/10.3390/cancers15164179






