Radiomic Analysis of Intrahepatic Cholangiocarcinoma: Non-Invasive Prediction of Pathology Data: A Multicenter Study to Develop a Clinical–Radiomic Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Endpoints
2.2. Imaging Acquisition, Tumor Segmentation, and Radiomic Features Extraction
2.3. Statistical Analyses
3. Results
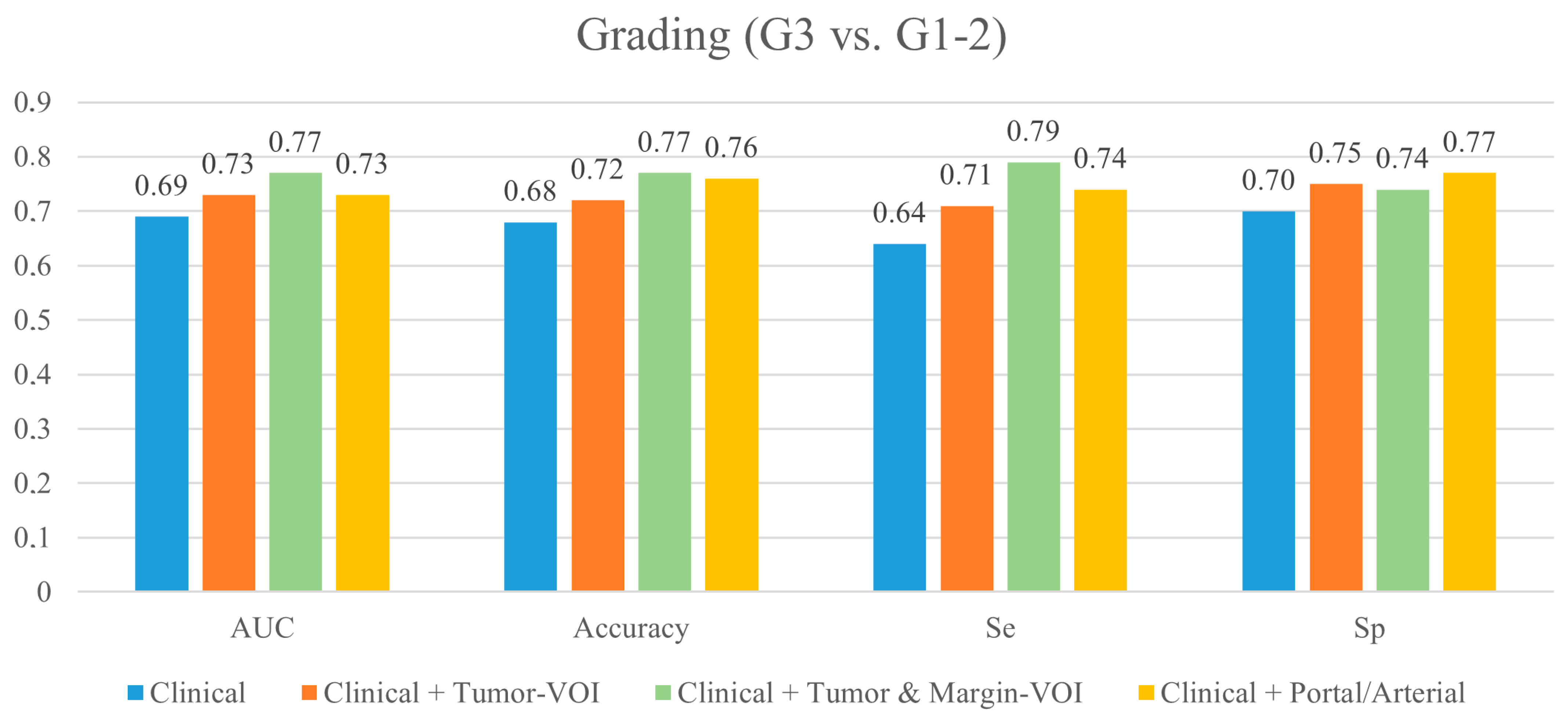
3.1. Prediction of Tumor Grading (G3 vs. G1-2)
3.2. Prediction of Microscopic Vascular Invasion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef]
- Kelley, R.K.; Bridgewater, J.; Gores, G.J.; Zhu, A.X. Systemic therapies for intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 72, 353–363. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Gorgen, A.; Roayaie, S.; Droz Dit Busset, M.; Sapisochin, G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 72, 364–377. [Google Scholar] [CrossRef]
- Bagante, F.; Spolverato, G.; Weiss, M.; Alexandrescu, S.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; Shen, F.; et al. Defining Long-Term Survivors Following Resection of Intrahepatic Cholangiocarcinoma. J. Gastrointest. Surg. 2017, 21, 1888–1897. [Google Scholar] [CrossRef]
- Conci, S.; Ruzzenente, A.; Vigano, L.; Ercolani, G.; Fontana, A.; Bagante, F.; Bertuzzo, F.; Dore, A.; Pinna, A.D.; Torzilli, G. Patterns of Distribution of Hepatic Nodules (Single, Satellites or Multifocal) in Intrahepatic Cholangiocarcinoma: Prognostic Impact After Surgery. Ann. Surg. Oncol. 2018, 25, 3719–3727. [Google Scholar] [CrossRef]
- Torzilli, G.; Vigano, L.; Fontana, A.; Procopio, F.; Terrone, A.; Cimino, M.M.; Donadon, M.; Del Fabbro, D. Oncological outcome of R1 vascular margin for mass-forming cholangiocarcinoma. A single center observational cohort analysis. HPB 2020, 22, 570–577. [Google Scholar] [CrossRef]
- Doussot, A.; Gonen, M.; Wiggers, J.K.; Groot-Koerkamp, B.; DeMatteo, R.P.; Fuks, D.; Allen, P.J.; Farges, O.; Kingham, T.P.; Regimbeau, J.M.; et al. Recurrence Patterns and Disease-Free Survival after Resection of Intrahepatic Cholangiocarcinoma: Preoperative and Postoperative Prognostic Models. J. Am. Coll. Surg. 2016, 223, 493–505. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: Systematic review and meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Song, J.; Yin, Y.; Wang, H.; Chang, Z.; Liu, Z.; Cui, L. A review of original articles published in the emerging field of radiomics. Eur. J. Radiol. 2020, 127, 108991. [Google Scholar] [CrossRef] [PubMed]
- Sollini, M.; Antunovic, L.; Chiti, A.; Kirienko, M. Towards clinical application of image mining: A systematic review on artificial intelligence and radiomics. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2656–2672. [Google Scholar] [CrossRef]
- Fiz, F.; Jayakody Arachchige, V.S.; Gionso, M.; Pecorella, I.; Selvam, A.; Wheeler, D.R.; Sollini, M.; Viganò, L. Radiomics of Biliary Tumors: A Systematic Review of Current Evidence. Diagnostics 2022, 12, 826. [Google Scholar] [CrossRef] [PubMed]
- Fiz, F.; Masci, C.; Costa, G.; Sollini, M.; Chiti, A.; Ieva, F.; Torzilli, G.; Viganò, L. PET/CT-based radiomics of mass-forming intrahepatic cholangiocarcinoma improves prediction of pathology data and survival. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3387–3400. [Google Scholar] [CrossRef] [PubMed]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuze, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallieres, M.; Abdalah, M.A.; Aerts, H.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Baheti, A.D.; Tirumani, S.H.; Shinagare, A.B.; Rosenthal, M.H.; Hornick, J.L.; Ramaiya, N.H.; Wolpin, B.M. Correlation of CT patterns of primary intrahepatic cholangiocarcinoma at the time of presentation with the metastatic spread and clinical outcomes: Retrospective study of 92 patients. Abdom. Imaging 2014, 39, 1193–1201. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D. Mixed-Effects Models in S and S-PLUS; Springer Science & Business Media: New York, NY, USA, 2006. [Google Scholar]
- Goldstein, H.; Browne, W.; Rasbash, J. Partitioning Variation in Multilevel Models. Underst. Stat. 2002, 1, 223–231. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Sahara, K.; Wu, L.; Moris, D.; Bagante, F.; Guglielmi, A.; Aldrighetti, L.; Weiss, M.; Bauer, T.W.; Alexandrescu, S.; et al. Very Early Recurrence After Liver Resection for Intrahepatic Cholangiocarcinoma: Considering Alternative Treatment Approaches. JAMA Surg. 2020, 155, 823–831. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer Nature: New York, NY, USA, 2017. [Google Scholar]
- Fiz, F.; Vigano, L.; Gennaro, N.; Costa, G.; La Bella, L.; Boichuk, A.; Cavinato, L.; Sollini, M.; Politi, L.S.; Chiti, A.; et al. Radiomics of Liver Metastases: A Systematic Review. Cancers 2020, 12, 2881. [Google Scholar] [CrossRef]
- Ji, G.W.; Zhu, F.P.; Zhang, Y.D.; Liu, X.S.; Wu, F.Y.; Wang, K.; Xia, Y.X.; Zhang, Y.D.; Jiang, W.J.; Li, X.C.; et al. A radiomics approach to predict lymph node metastasis and clinical outcome of intrahepatic cholangiocarcinoma. Eur. Radiol. 2019, 29, 3725–3735. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, P.; Liang, W.; Liu, W.; Wang, W.; Luo, C.; Wang, J.; Peng, Z.; Xing, L.; Huang, M.; et al. A radiomics approach based on support vector machine using MR images for preoperative lymph node status evaluation in intrahepatic cholangiocarcinoma. Theranostics 2019, 9, 5374–5385. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, P.; Yen, E.A.; Wan, Y.; Jiang, Y.; Cao, Z.; Niu, T. A multi-organ cancer study of the classification performance using 2D and 3D image features in radiomics analysis. A multi-organ cancer study of the classification performance using 2D and 3D image features in radiomics analysis. Phys. Med. Biol. 2019, 64, 215009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, S.; He, W.; Wei, J.; Huo, L.; Jia, N.; Lin, J.; Tang, Z.; Yuan, Y.; Tian, J.; et al. Radiomics-Based Preoperative Prediction of Lymph Node Metastasis in Intrahepatic Cholangiocarcinoma Using Contrast-Enhanced Computed Tomography. Ann. Surg. Oncol. 2022, 29, 6786–6799. [Google Scholar] [CrossRef]
- Xiang, F.; Wei, S.; Liu, X.; Liang, X.; Yang, L.; Yan, S. Radiomics Analysis of Contrast-Enhanced CT for the Preoperative Prediction of Microvascular Invasion in Mass-Forming Intrahepatic Cholangiocarcinoma. Front. Oncol. 2021, 11, 774117. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, G.; Zhang, J.; Xu, C.; Wang, X.; Xu, P. Radiomics signature on dynamic contrast-enhanced MR images: A potential imaging biomarker for prediction of microvascular invasion in mass-forming intrahepatic cholangiocarcinoma. Eur. Radiol. 2021, 31, 6846–6855. [Google Scholar] [CrossRef]
- Qian, X.; Lu, X.; Ma, X.; Zhang, Y.; Zhou, C.; Wang, F.; Shi, Y.; Zeng, M. A Multi-Parametric Radiomics Nomogram for Preoperative Prediction of Microvascular Invasion Status in Intrahepatic Cholangiocarcinoma. Front. Oncol. 2022, 12, 838701. [Google Scholar] [CrossRef]
- Peng, Y.T.; Zhou, C.Y.; Lin, P.; Wen, D.Y.; Wang, X.D.; Zhong, X.Z.; Pan, D.H.; Que, Q.; Li, X.; Chen, L.; et al. Preoperative Ultrasound Radiomics Signatures for Noninvasive Evaluation of Biological Characteristics of Intrahepatic Cholangiocarcinoma. Acad. Radiol. 2020, 27, 785–797. [Google Scholar] [CrossRef]
- King, M.J.; Hectors, S.; Lee, K.M.; Omidele, O.; Babb, J.S.; Schwartz, M.; Tabrizian, P.; Taouli, B.; Lewis, S. Outcomes assessment in intrahepatic cholangiocarcinoma using qualitative and quantitative imaging features. Cancer Imaging 2020, 20, 43. [Google Scholar] [CrossRef]
- Job, S.; Rapoud, D.; Dos Santos, A.; Gonzalez, P.; Desterke, C.; Pascal, G.; Elarouci, N.; Ayadi, M.; Adam, R.; Azoulay, D.; et al. Identification of Four Immune Subtypes Characterized by Distinct Composition and Functions of Tumor Microenvironment in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 72, 965–981. [Google Scholar] [CrossRef]
- Fabris, L.; Sato, K.; Alpini, G.; Strazzabosco, M. The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology 2021, 73, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Vigano, L.; Soldani, C.; Franceschini, B.; Cimino, M.; Lleo, A.; Donadon, M.; Roncalli, M.; Aghemo, A.; Di Tommaso, L.; Torzilli, G. Tumor-Infiltrating Lymphocytes and Macrophages in Intrahepatic Cholangiocellular Carcinoma. Impact on Prognosis after Complete Surgery. J. Gastrointest. Surg. 2019, 23, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Yugawa, K.; Itoh, S.; Iseda, N.; Kurihara, T.; Kitamura, Y.; Toshima, T.; Harada, N.; Kohashi, K.; Baba, S.; Ishigami, K.; et al. Obesity is a risk factor for intrahepatic cholangiocarcinoma progression associated with alterations of metabolic activity and immune status. Sci. Rep. 2021, 11, 5845. [Google Scholar] [CrossRef] [PubMed]
- Fernández Moro, C.; Bozóky, B.; Gerling, M. Growth patterns of colorectal cancer liver metastases and their impact on prognosis: A systematic review. BMJ Open. Gastroenterol. 2018, 5, e000217. [Google Scholar] [CrossRef] [PubMed]
- Vigano, L.; Branciforte, B.; Laurenti, V.; Costa, G.; Procopio, F.; Cimino, M.; Del Fabbro, D.; Di Tommaso, L.; Torzilli, G. The Histopathological Growth Pattern of Colorectal Liver Metastases Impacts Local Recurrence Risk and the Adequate Width of the Surgical Margin. Ann. Surg. Oncol. 2022, 29, 5515–5524. [Google Scholar] [CrossRef]
- Min, J.H.; Kim, Y.K.; Choi, S.Y.; Kang, T.W.; Lee, S.J.; Kim, J.M.; Ahn, S.; Cho, H. Intrahepatic Mass-forming Cholangiocarcinoma: Arterial Enhancement Patterns at MRI and Prognosis. Radiology 2019, 290, 691–699. [Google Scholar] [CrossRef]
- Jiao, C.Y.; Zhang, H.; Ji, G.W.; Xu, Q.; Lu, M.; Zhang, B.; Yang, Y.; Wang, X.H.; Li, X.C. CT-based clinico-radiological nomograms for prognosis prediction in patients with intrahepatic mass-forming cholangiocarcinoma: A multi-institutional study. Eur. Radiol. 2022, 32, 8326–8338. [Google Scholar] [CrossRef]
- Fujita, N.; Asayama, Y.; Nishie, A.; Ishigami, K.; Ushijima, Y.; Takayama, Y.; Okamoto, D.; Moirta, K.; Shirabe, K.; Aishima, S.; et al. Mass-forming intrahepatic cholangiocarcinoma: Enhancement patterns in the arterial phase of dynamic hepatic CT—Correlation with clinicopathological findings. Eur. Radiol. 2017, 27, 498–506. [Google Scholar] [CrossRef]
- Mosconi, C.; Cucchetti, A.; Bruno, A.; Cappelli, A.; Bargellini, I.; De Benedittis, C.; Lorenzoni, G.; Gramenzi, A.; Tarantino, F.P.; Parini, L.; et al. Radiomics of cholangiocarcinoma on pretreatment CT can identify patients who would best respond to radioembolisation. Eur. Radiol. 2020, 30, 4534–4544. [Google Scholar] [CrossRef]
- Viganò, L.; Ammirabile, A.; Zwanenburg, A. Radiomics in liver surgery: Defining the path toward clinical application. Updat. Surg. 2023, in press. [CrossRef]


| Characteristic | Number (%)—Median (Range) | Missing (Number) |
|---|---|---|
| Age, years | 67.5 (21–86) | - |
| Sex, male/female | 120 (49.2%):124 (50.8%) | - |
| HBV infection | 19 (7.8%) | 1 |
| HCV infection | 27 (11.1%) | 1 |
| Liver cirrhosis | 26 (10.7%) | - |
| Tumor diameter, mm | 50 (10–270) | - |
| Solitary tumor | 206 (84.4%) | - |
| Tumor pattern | ||
| Type 1 | 151 (61.9%) | - |
| Type 2 | 61 (25.0%) | - |
| Type 3 | 32 (13.1%) | - |
| Ca 19.9, U/mL | 29 (0.2–67,456.3) | 30 |
| Ca 19.9 ≥ 55 U/mL | 74 (30.3%) | 30 |
| Preoperative chemotherapy | 26 (10.7%) | - |
| Partial response | 13 (50%) | 3 |
| Stable disease | 8 (30.8%) | |
| Disease progression | 2 (7.7%) | |
| Major hepatectomy | 128 (52.5%) | - |
| Tumor grading, G3 | 82 (33.6%) | - |
| Microscopic vascular invasion | 139 (57%) | - |
| Parameter | Odds Ratio | Lower Bound | Upper Bound | p-Value |
|---|---|---|---|---|
| Model with preoperative clinical data | ||||
| Age (years) | 1.080 | 0.786 | 1.480 | 0.638 |
| Sex | 1.550 | 0.837 | 2.850 | 0.164 |
| HBV | 0.614 | 0.177 | 2.120 | 0.441 |
| HCV | 1.690 | 0.647 | 4.440 | 0.283 |
| CA 19-9 (ng/mL) | 1.110 | 0.803 | 1.530 | 0.528 |
| Preoperative chemotherapy | 1.310 | 0.505 | 3.380 | 0.582 |
| Major hepatectomy | 1.490 | 0.754 | 2.940 | 0.251 |
| Cirrhosis | 0.947 | 0.348 | 2.580 | 0.916 |
| Tumor pattern | ||||
| Type 1 | 1 | - | - | - |
| Type 2 | 1.200 | 0.541 | 2.640 | 0.658 |
| Type 3 | 1.800 | 0.354 | 9.160 | 0.478 |
| Tumor size (mm) | 1.230 | 0.889 | 1.690 | 0.214 |
| Single nodule | 1.440 | 0.318 | 6.470 | 0.638 |
| Model with preoperative clinical data + Tumor-VOI radiomics (portal phase) | ||||
| Portal_Tumor_GLRLM_SRHGE | 0.733 | 0.556 | 0.966 | 0.027 |
| Model with preoperative clinical data + Tumor- and Margin-VOI radiomics (portal phase) | ||||
| Major hepatectomy | 1.661 | 1.132 | 2.438 | 0.010 |
| Portal_Tumor_HUmin | 1.521 | 0.944 | 2.453 | 0.085 |
| Portal_Tumor_GLRLM_SRHGE | 0.672 | 0.497 | 0.908 | 0.010 |
| Portal_Margin_NGLDM_Busyness | 0.644 | 0.442 | 0.938 | 0.022 |
| Portal_Margin_GLZLM_ZLNU | 2.050 | 1.284 | 3.274 | 0.003 |
| Clinical vs. Tumor-VOI | Clinical vs. Tumor- and Margin-VOI | Tumor-VOI vs. Tumor- and Margin-VOI | |
|---|---|---|---|
| Tumor Grading | |||
| Accuracy | 0.007 | <0.001 | <0.001 |
| Specificity | 0.035 | <0.001 | 0.037 |
| Sensitivity | 0.028 | <0.001 | <0.001 |
| Precision | 0.003 | <0.001 | 0.118 |
| Pr AUC | <0.001 | <0.001 | <0.001 |
| Roc AUC | 0.476 | <0.001 | <0.001 |
| Parameter | Odds Ratio | Lower Bound | Upper Bound | p-Value |
|---|---|---|---|---|
| Model with preoperative clinical data | ||||
| Age (years) | 0.992 | 0.987 | 0.998 | 0.007 |
| CA 19-9 (ng/mL) | 1.001 | 1.000 | 1.001 | 0.056 |
| Major hepatectomy | 3.296 | 1.934 | 5.617 | <0.001 |
| Model with preoperative clinical data + Tumor-VOI radiomics (portal phase) | ||||
| Major hepatectomy | 3.277 | 2.180 | 4.925 | <0.001 |
| Portal_Tumor_HUmin | 0.496 | 0.307 | 0.800 | 0.004 |
| Portal_Tumor_GLRLM_SRHGE | 0.673 | 0502 | 0.902 | 0.008 |
| Model with preoperative clinical data + Tumor- and Margin-VOI radiomics (portal phase) | ||||
| Major hepatectomy | 2.760 | 1.820 | 4.186 | <0.001 |
| Portal_Margin_HUQ2 | 0.651 | 0.460 | 0.922 | 0.015 |
| Portal_Margin_Shape_Sphericity | 0.560 | 0.408 | 0.768 | <0.001 |
| Portal_Margin_GLCM_Correlation | 1.542 | 1.112 | 2.139 | 0.009 |
| Portal_Margin_NGLDM_Contrast | 1.436 | 0.924 | 2.231 | 0.107 |
| Portal_Margin_GLZLM_SZHGE | 1.636 | 1.043 | 2.567 | 0.032 |
| Clinical vs. Tumor-VOI | Clinical vs. Tumor- and Margin-VOI | Tumor-VOI vs. Tumor- and Margin-VOI | |
|---|---|---|---|
| Microscopic vascular invasion | |||
| Accuracy | 0.295 | <0.001 | 0.001 |
| Specificity | 0.072 | <0.001 | 0.025 |
| Sensitivity | 0.152 | <0.001 | 0.008 |
| Precision | 0.081 | 0.003 | 0.087 |
| Pr AUC | 0.014 | 0.008 | 0.212 |
| Roc AUC | 0.007 | <0.001 | 0.112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiz, F.; Rossi, N.; Langella, S.; Ruzzenente, A.; Serenari, M.; Ardito, F.; Cucchetti, A.; Gallo, T.; Zamboni, G.; Mosconi, C.; et al. Radiomic Analysis of Intrahepatic Cholangiocarcinoma: Non-Invasive Prediction of Pathology Data: A Multicenter Study to Develop a Clinical–Radiomic Model. Cancers 2023, 15, 4204. https://doi.org/10.3390/cancers15174204
Fiz F, Rossi N, Langella S, Ruzzenente A, Serenari M, Ardito F, Cucchetti A, Gallo T, Zamboni G, Mosconi C, et al. Radiomic Analysis of Intrahepatic Cholangiocarcinoma: Non-Invasive Prediction of Pathology Data: A Multicenter Study to Develop a Clinical–Radiomic Model. Cancers. 2023; 15(17):4204. https://doi.org/10.3390/cancers15174204
Chicago/Turabian StyleFiz, Francesco, Noemi Rossi, Serena Langella, Andrea Ruzzenente, Matteo Serenari, Francesco Ardito, Alessandro Cucchetti, Teresa Gallo, Giulia Zamboni, Cristina Mosconi, and et al. 2023. "Radiomic Analysis of Intrahepatic Cholangiocarcinoma: Non-Invasive Prediction of Pathology Data: A Multicenter Study to Develop a Clinical–Radiomic Model" Cancers 15, no. 17: 4204. https://doi.org/10.3390/cancers15174204
APA StyleFiz, F., Rossi, N., Langella, S., Ruzzenente, A., Serenari, M., Ardito, F., Cucchetti, A., Gallo, T., Zamboni, G., Mosconi, C., Boldrini, L., Mirarchi, M., Cirillo, S., De Bellis, M., Pecorella, I., Russolillo, N., Borzi, M., Vara, G., Mele, C., ... Viganò, L. (2023). Radiomic Analysis of Intrahepatic Cholangiocarcinoma: Non-Invasive Prediction of Pathology Data: A Multicenter Study to Develop a Clinical–Radiomic Model. Cancers, 15(17), 4204. https://doi.org/10.3390/cancers15174204















