Proton Therapy in the Adolescent and Young Adult Population
Abstract
Simple Summary
Abstract
1. Introduction
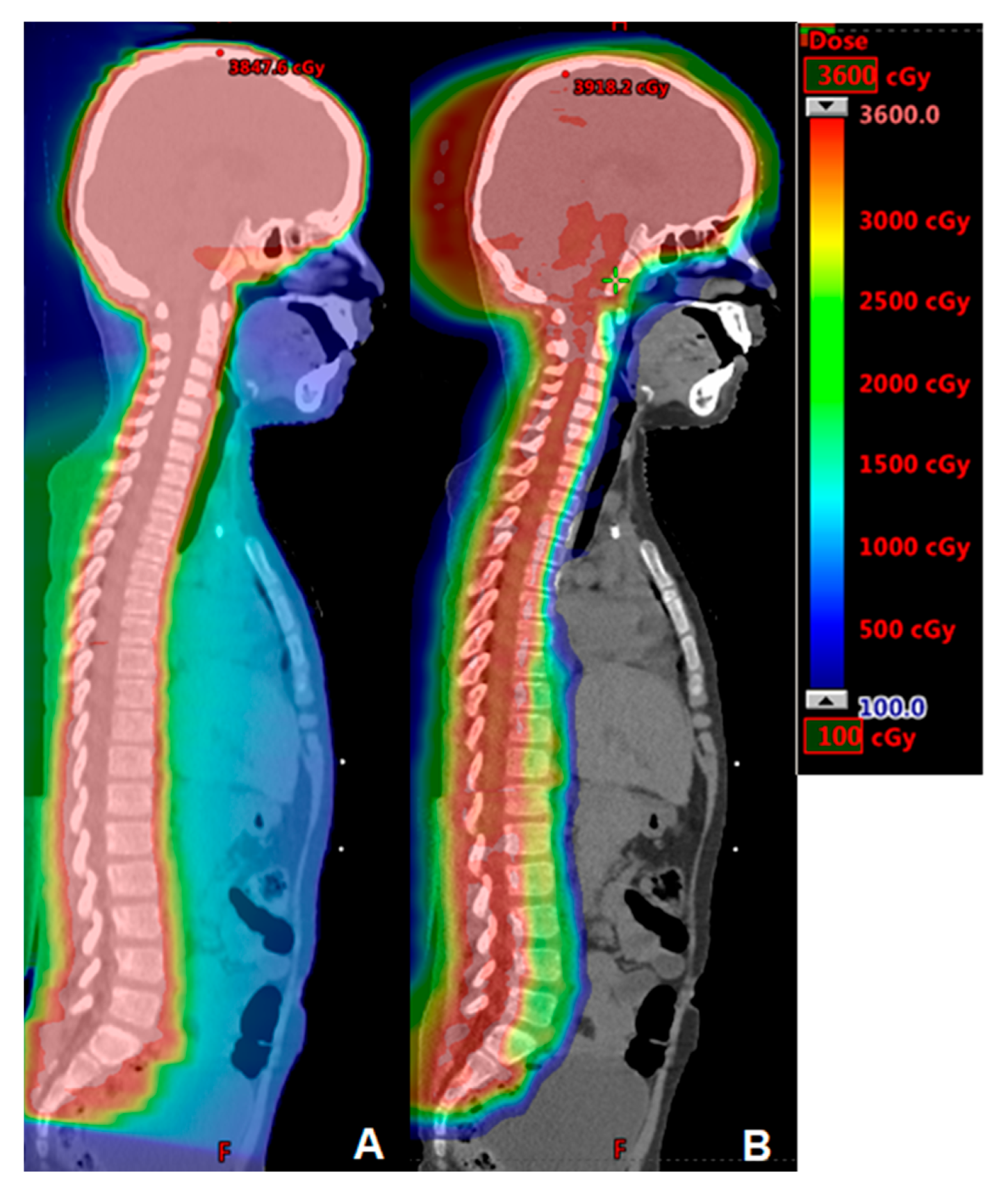
2. Central Nervous System Tumors
3. Sarcoma
4. Breast Cancer
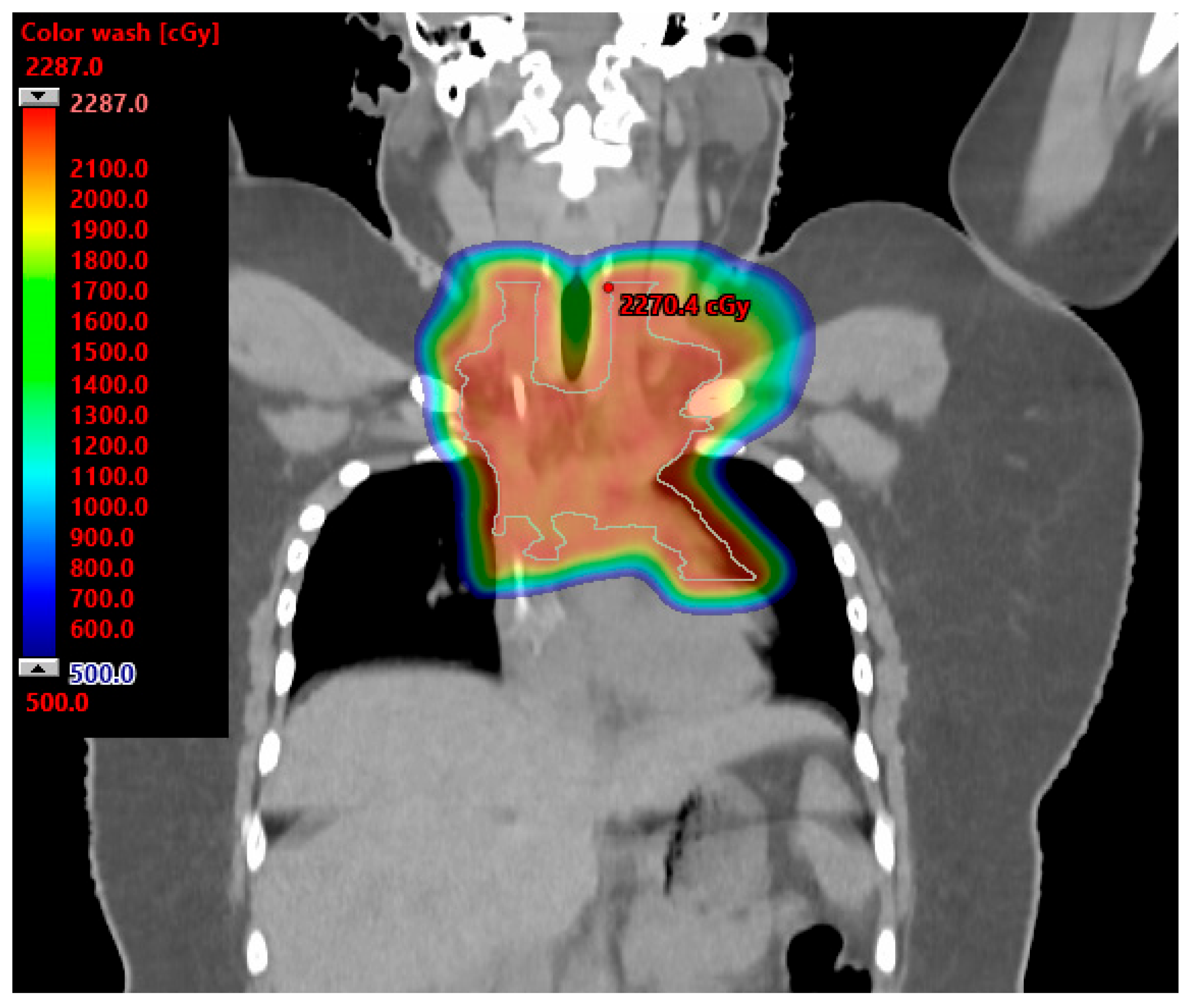
5. Hodgkin Lymphoma
6. Miscellaneous Cancers
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alvarez, E.; Keegan, T.; Johnston, E.E.; Haile, R.; Sanders, L.; Saynina, O.; Chamberlain, L.J. Adolescent and young adult oncology patients: Disparities in access to specialized cancer centers. Cancer 2017, 123, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Keegan, T.H.M.; Li, Q.; Steele, A.; Alvarez, E.M.; Brunson, A.; Flowers, C.R.; Glaser, S.L.; Wun, T. Sociodemographic disparities in the occurrence of medical conditions among adolescent and young adult Hodgkin lymphoma survivors. Cancer Causes Control 2018, 29, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.R.; Stoltzfus, K.C.; Tchelebi, L.T.; Trifiletti, D.M.; Lehrer, E.J.; Rao, P.; Bleyer, A.; Zaorsky, N.G. Trends in Cancer Incidence in US Adolescents and Young Adults, 1973–2015. JAMA Netw. Open 2020, 3, e2027738. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E. Proton beam therapy in pediatric oncology. Cancer J. 2009, 15, 298–305. [Google Scholar] [CrossRef]
- Ojerholm, E.; Hill-Kayser, C.E. Insurance coverage decisions for pediatric proton therapy. Pediatr. Blood Cancer 2018, 65, e26729. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Habrand, J.L.; Hoppe, B.S.; Hill Kayser, C.; Laack, N.N.; Langendijk, J.A.; MacDonald, S.M.; McGovern, S.L.; Pater, L.; Perentesis, J.P.; et al. Proton therapy for pediatric malignancies: Fact, figures and costs. A joint consensus statement from the pediatric subcommittee of PTCOG, PROS and EPTN. Radiother. Oncol. 2018, 128, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.; Budd, T.; Montello, M. Adolescents and young adults with cancer: The scope of the problem and criticality of clinical trials. Cancer 2006, 107, 1645–1655. [Google Scholar] [CrossRef]
- Close, A.G.; Dreyzin, A.; Miller, K.D.; Seynnaeve, B.K.N.; Rapkin, L.B. Adolescent and young adult oncology-past, present, and future. CA Cancer J. Clin. 2019, 69, 485–496. [Google Scholar] [CrossRef]
- Moke, D.J.; Tsai, K.; Hamilton, A.S.; Hwang, A.; Liu, L.; Freyer, D.R.; Deapen, D. Emerging Cancer Survival Trends, Disparities, and Priorities in Adolescents and Young Adults: A California Cancer Registry-Based Study. JNCI Cancer Spectr. 2019, 3, pkz031. [Google Scholar] [CrossRef]
- Verma, V.; Simone, C.B., 2nd; Mishra, M.V. Quality of Life and Patient-Reported Outcomes Following Proton Radiation Therapy: A Systematic Review. J. Natl. Cancer Inst. 2018, 110, 341–353. [Google Scholar] [CrossRef]
- Doyen, J.; Falk, A.T.; Floquet, V.; Herault, J.; Hannoun-Levi, J.M. Proton beams in cancer treatments: Clinical outcomes and dosimetric comparisons with photon therapy. Cancer Treat. Rev. 2016, 43, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.J.; Livingston, J.A.; Ning, M.S.; Valdez, I.D.; Wages, C.A.; McAleer, M.F.; Paulino, A.C.; Grosshans, D.R.; Woodhouse, K.D.; Tao, R.; et al. Young Adult Populations Face Yet Another Barrier to Care with Insurers: Limited Access to Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; de Blank, P.M.; Finlay, J.L.; Gurney, J.G.; McKean-Cowdin, R.; Stearns, D.S.; Wolff, J.E.; Liu, M.; Wolinsky, Y.; et al. American Brain Tumor Association Adolescent and Young Adult Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-Oncology 2016, 18 (Suppl. S1), i1–i50. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, J.; Sun, C.L.; Kang, T.; Wyatt, L.; D’Appuzzo, M.; Bhatia, S. Impact of treatment site in adolescents and young adults with central nervous system tumors. J. Natl. Cancer Inst. 2014, 106, dju166. [Google Scholar] [CrossRef] [PubMed]
- Byskov, C.S.; Hansen, C.R.; Dahlrot, R.H.; Haldbo-Classen, L.; Haslund, C.A.; Kjaer-Kristoffersen, F.; Kristensen, T.O.; Lassen-Ramshad, Y.; Lukacova, S.; Muhic, A.; et al. Treatment plan comparison of proton vs photon radiotherapy for lower-grade gliomas. Phys. Imaging Radiat. Oncol. 2021, 20, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.P.; Powell, S.; Zelko, F.; Hartsell, W.; Goldman, S.; Fangusaro, J.; Lulla, R.R.; Smiley, N.P.; Chang, J.H.; Gondi, V. Improved neuropsychological outcomes following proton therapy relative to X-ray therapy for pediatric brain tumor patients. Neuro-Oncology 2019, 21, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Kahalley, L.S.; Ris, M.D.; Grosshans, D.R.; Okcu, M.F.; Paulino, A.C.; Chintagumpala, M.; Moore, B.D.; Guffey, D.; Minard, C.G.; Stancel, H.H.; et al. Comparing Intelligence Quotient Change after Treatment with Proton Versus Photon Radiation Therapy for Pediatric Brain Tumors. J. Clin. Oncol. 2016, 34, 1043–1049. [Google Scholar] [CrossRef]
- Antonini, T.N.; Ris, M.D.; Grosshans, D.R.; Mahajan, A.; Okcu, M.F.; Chintagumpala, M.; Paulino, A.; Child, A.E.; Orobio, J.; Stancel, H.H.; et al. Attention, processing speed, and executive functioning in pediatric brain tumor survivors treated with proton beam radiation therapy. Radiother. Oncol. 2017, 124, 89–97. [Google Scholar] [CrossRef]
- Vatner, R.E.; Niemierko, A.; Misra, M.; Weyman, E.A.; Goebel, C.P.; Ebb, D.H.; Jones, R.M.; Huang, M.S.; Mahajan, A.; Grosshans, D.R.; et al. Endocrine Deficiency as a Function of Radiation Dose to the Hypothalamus and Pituitary in Pediatric and Young Adult Patients with Brain Tumors. J. Clin. Oncol. 2018, 36, 2854–2862. [Google Scholar] [CrossRef]
- Eaton, B.R.; Esiashvili, N.; Kim, S.; Patterson, B.; Weyman, E.A.; Thornton, L.T.; Mazewski, C.; MacDonald, T.J.; Ebb, D.; MacDonald, S.M.; et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro-Oncology 2016, 18, 881–887. [Google Scholar] [CrossRef]
- Aldrich, K.D.; Horne, V.E.; Bielamowicz, K.; Sonabend, R.Y.; Scheurer, M.E.; Paulino, A.C.; Mahajan, A.; Chintagumpala, M.; Okcu, M.F.; Brown, A.L. Comparison of hypothyroidism, growth hormone deficiency, and adrenal insufficiency following proton and photon radiotherapy in children with medulloblastoma. J. Neuro-Oncol. 2021, 155, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Marta, G.N.; Murphy, E.; Chao, S.; Yu, J.S.; Suh, J.H. The incidence of second brain tumors related to cranial irradiation. Expert. Rev. Anticancer Ther. 2015, 15, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.S.; Aghi, M.K.; Muzikansky, A.; Shih, H.A.; Barker, F.G., 2nd; Curry, W.T., Jr. Outcomes and patterns of care in adult skull base chordomas from the Surveillance, Epidemiology, and End Results (SEER) database. J. Clin. Neurosci. 2014, 21, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Na’ara, S.; Binenbaum, Y.; Billan, S.; Sviri, G.; Cohen, J.T.; Gil, Z. Treatment and Outcome of Patients with Skull Base Chordoma: A Meta-analysis. J. Neurol. Surg. B Skull Base 2014, 75, 383–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zorlu, F.; Gurkaynak, M.; Yildiz, F.; Oge, K.; Atahan, I.L. Conventional external radiotherapy in the management of clivus chordomas with overt residual disease. Neurol. Sci. 2000, 21, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Bugoci, D.M.; Girvigian, M.R.; Chen, J.C.; Miller, M.M.; Rahimian, J. Photon-based fractionated stereotactic radiotherapy for postoperative treatment of skull base chordomas. Am. J. Clin. Oncol. 2013, 36, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Debus, J.; Schulz-Ertner, D.; Schad, L.; Essig, M.; Rhein, B.; Thillmann, C.O.; Wannenmacher, M. Stereotactic fractionated radiotherapy for chordomas and chondrosarcomas of the skull base. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 591–596. [Google Scholar] [CrossRef]
- Ares, C.; Hug, E.B.; Lomax, A.J.; Bolsi, A.; Timmermann, B.; Rutz, H.P.; Schuller, J.C.; Pedroni, E.; Goitein, G. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: First long-term report. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1111–1118. [Google Scholar] [CrossRef]
- Nie, M.; Chen, L.; Zhang, J.; Qiu, X. Pure proton therapy for skull base chordomas and chondrosarcomas: A systematic review of clinical experience. Front. Oncol. 2022, 12, 1016857. [Google Scholar] [CrossRef]
- Palm, R.F.; Oliver, D.E.; Yang, G.Q.; Abuodeh, Y.; Naghavi, A.O.; Johnstone, P.A.S. The role of dose escalation and proton therapy in perioperative or definitive treatment of chondrosarcoma and chordoma: An analysis of the National Cancer Data Base. Cancer 2019, 125, 642–651. [Google Scholar] [CrossRef]
- Holtzman, A.L.; Rotondo, R.L.; Rutenberg, M.S.; Indelicato, D.J.; De Leo, A.; Rao, D.; Patel, J.; Morris, C.G.; Mendenhall, W.M. Clinical Outcomes Following Dose-Escalated Proton Therapy for Skull-Base Chordoma. Int. J. Part. Ther. 2021, 8, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Malyapa, R.; Albertini, F.; Bolsi, A.; Kliebsch, U.; Walser, M.; Pica, A.; Combescure, C.; Lomax, A.J.; Schneider, R. Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother. Oncol. 2016, 120, 169–174. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Brown, P.D.; Foote, R.L. Protons vs Photons for Brain and Skull Base Tumors. Semin. Radiat. Oncol. 2018, 28, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Indelicato, D.J.; Keole, S.R.; Shahlaee, A.H.; Morris, C.G.; Gibbs, C.P., Jr.; Scarborough, M.T.; Pincus, D.W.; Marcus, R.B., Jr. Spinal and paraspinal Ewing tumors. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Yock, T.I.; Krailo, M.; Fryer, C.J.; Donaldson, S.S.; Miser, J.S.; Chen, Z.; Bernstein, M.; Laurie, F.; Gebhardt, M.C.; Grier, H.E.; et al. Local control in pelvic Ewing sarcoma: Analysis from INT-0091—A report from the Children’s Oncology Group. J. Clin. Oncol. 2006, 24, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Indelicato, D.J.; Keole, S.R.; Shahlaee, A.H.; Shi, W.; Morris, C.G.; Gibbs, C.P., Jr.; Scarborough, M.T.; Marcus, R.B., Jr. Impact of local management on long-term outcomes in Ewing tumors of the pelvis and sacral bones: The University of Florida experience. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 41–48. [Google Scholar] [CrossRef]
- Lee, C.T.; Bilton, S.D.; Famiglietti, R.M.; Riley, B.A.; Mahajan, A.; Chang, E.L.; Maor, M.H.; Woo, S.Y.; Cox, J.D.; Smith, A.R. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: How do protons compare with other conformal techniques? Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 362–372. [Google Scholar] [CrossRef]
- Cathcart-Rake, E.J.; Ruddy, K.J.; Bleyer, A.; Johnson, R.H. Breast Cancer in Adolescent and Young Adult Women under the Age of 40 Years. JCO Oncol. Pract. 2021, 17, 305–313. [Google Scholar] [CrossRef]
- Johnson, R.H.; Anders, C.K.; Litton, J.K.; Ruddy, K.J.; Bleyer, A. Breast cancer in adolescents and young adults. Pediatr. Blood Cancer 2018, 65, e27397. [Google Scholar] [CrossRef]
- Gnerlich, J.L.; Deshpande, A.D.; Jeffe, D.B.; Sweet, A.; White, N.; Margenthaler, J.A. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J. Am. Coll. Surg. 2009, 208, 341–347. [Google Scholar] [CrossRef]
- Lalloo, F.; Varley, J.; Moran, A.; Ellis, D.; O’Dair, L.; Pharoah, P.; Antoniou, A.; Hartley, R.; Shenton, A.; Seal, S.; et al. BRCA1, BRCA2 and TP53 mutations in very early-onset breast cancer with associated risks to relatives. Eur. J. Cancer 2006, 42, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Voogd, A.C.; Nielsen, M.; Peterse, J.L.; Blichert-Toft, M.; Bartelink, H.; Overgaard, M.; van Tienhoven, G.; Andersen, K.W.; Sylvester, R.J.; van Dongen, J.A.; et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: Pooled results of two large European randomized trials. J. Clin. Oncol. 2001, 19, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.L.; Day, C.N.; Hoskin, T.L.; Habermann, E.B.; Boughey, J.C. Adolescents and Young Adults with Breast Cancer have More Aggressive Disease and Treatment Than Patients in Their Forties. Ann. Surg. Oncol. 2019, 26, 3920–3930. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Bronnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; McGale, P.; Bronnum, D.; Correa, C.; Cutter, D.; Duane, F.K.; Gigante, B.; Jensen, M.B.; Lorenzen, E.; Rahimi, K.; et al. Cardiac Structure Injury after Radiotherapy for Breast Cancer: Cross-Sectional Study with Individual Patient Data. J. Clin. Oncol. 2018, 36, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaard, V.A.; Ta, B.D.; van der Schaaf, A.; Bouma, A.B.; Middag, A.M.; Bantema-Joppe, E.J.; van Dijk, L.V.; van Dijk-Peters, F.B.; Marteijn, L.A.; de Bock, G.H.; et al. Validation and Modification of a Prediction Model for Acute Cardiac Events in Patients with Breast Cancer Treated with Radiotherapy Based on Three-Dimensional Dose Distributions to Cardiac Substructures. J. Clin. Oncol. 2017, 35, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Wennstig, A.K.; Garmo, H.; Isacsson, U.; Gagliardi, G.; Rintela, N.; Lagerqvist, B.; Holmberg, L.; Blomqvist, C.; Sund, M.; Nilsson, G. The relationship between radiation doses to coronary arteries and location of coronary stenosis requiring intervention in breast cancer survivors. Radiat. Oncol. 2019, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Cuaron, J.J.; Chon, B.; Tsai, H.; Goenka, A.; DeBlois, D.; Ho, A.; Powell, S.; Hug, E.; Cahlon, O. Early toxicity in patients treated with postoperative proton therapy for locally advanced breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 284–291. [Google Scholar] [CrossRef]
- Flejmer, A.M.; Edvardsson, A.; Dohlmar, F.; Josefsson, D.; Nilsson, M.; Witt Nystrom, P.; Dasu, A. Respiratory gating for proton beam scanning versus photon 3D-CRT for breast cancer radiotherapy. Acta Oncol. 2016, 55, 577–583. [Google Scholar] [CrossRef]
- Jimenez, R.B.; Goma, C.; Nyamwanda, J.; Kooy, H.M.; Halabi, T.; Napolitano, B.N.; McBride, S.M.; Taghian, A.G.; Lu, H.M.; MacDonald, S.M. Intensity modulated proton therapy for postmastectomy radiation of bilateral implant reconstructed breasts: A treatment planning study. Radiother. Oncol. 2013, 107, 213–217. [Google Scholar] [CrossRef]
- MacDonald, S.M.; Jimenez, R.; Paetzold, P.; Adams, J.; Beatty, J.; DeLaney, T.F.; Kooy, H.; Taghian, A.G.; Lu, H.M. Proton radiotherapy for chest wall and regional lymphatic radiation; dose comparisons and treatment delivery. Radiat. Oncol. 2013, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, M.; Hug, E.B.; Pankuch, M.; Fang, C.; McNeeley, S.; Mao, L.; Lavilla, M.; Schmidt, S.L.; Ward, C.; Cahlon, O.; et al. Proton Therapy for Local-regionally Advanced Breast Cancer Maximizes Cardiac Sparing. Int. J. Part. Ther. 2015, 1, 827–844. [Google Scholar] [CrossRef]
- Gokula, K.; Earnest, A.; Wong, L.C. Meta-analysis of incidence of early lung toxicity in 3-dimensional conformal irradiation of breast carcinomas. Radiat. Oncol. 2013, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.A.; Dagan, R.; Ho, M.W.; Rutenberg, M.; Morris, C.G.; Li, Z.; Mendenhall, N.P. Initial Report of a Prospective Dosimetric and Clinical Feasibility Trial Demonstrates the Potential of Protons to Increase the Therapeutic Ratio in Breast Cancer Compared with Photons. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.B.; Bentzen, S.M.; Deasy, J.O.; Kong, F.M.; Bradley, J.D.; Vogelius, I.S.; El Naqa, I.; Hubbs, J.L.; Lebesque, J.V.; Timmerman, R.D.; et al. Radiation dose-volume effects in the lung. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S70–S76. [Google Scholar] [CrossRef] [PubMed]
- Ranger, A.; Dunlop, A.; Hutchinson, K.; Convery, H.; Maclennan, M.K.; Chantler, H.; Twyman, N.; Rose, C.; McQuaid, D.; Amos, R.A.; et al. A Dosimetric Comparison of Breast Radiotherapy Techniques to Treat Locoregional Lymph Nodes Including the Internal Mammary Chain. Clin. Oncol. 2018, 30, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Giaj-Levra, N.; Sciascia, S.; Fiorentino, A.; Fersino, S.; Mazzola, R.; Ricchetti, F.; Roccatello, D.; Alongi, F. Radiotherapy in patients with connective tissue diseases. Lancet Oncol. 2016, 17, e109–e117. [Google Scholar] [CrossRef]
- Goodman, C.D.; Nijman, S.F.M.; Senan, S.; Nossent, E.J.; Ryerson, C.J.; Dhaliwal, I.; Qu, X.M.; Laba, J.; Rodrigues, G.B.; Palma, D.A.; et al. A Primer on Interstitial Lung Disease and Thoracic Radiation. J. Thorac. Oncol. 2020, 15, 902–913. [Google Scholar] [CrossRef]
- Sethi, R.V.; Shih, H.A.; Yeap, B.Y.; Mouw, K.W.; Petersen, R.; Kim, D.Y.; Munzenrider, J.E.; Grabowski, E.; Rodriguez-Galindo, C.; Yock, T.I.; et al. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer 2014, 120, 126–133. [Google Scholar] [CrossRef]
- Grantzau, T.; Thomsen, M.S.; Vaeth, M.; Overgaard, J. Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiother. Oncol. 2014, 111, 366–373. [Google Scholar] [CrossRef]
- Stovall, M.; Smith, S.A.; Langholz, B.M.; Boice, J.D., Jr.; Shore, R.E.; Andersson, M.; Buchholz, T.A.; Capanu, M.; Bernstein, L.; Lynch, C.F.; et al. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H.; Depauw, N.; Johnson, A.; Forman, R.B.; Lau, J.; Jimenez, R. The risk for developing a secondary cancer after breast radiation therapy: Comparison of photon and proton techniques. Radiother. Oncol. 2020, 149, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Correa, C.; Duane, F.K.; Aznar, M.C.; Anderson, S.J.; Bergh, J.; Dodwell, D.; Ewertz, M.; Gray, R.; Jagsi, R.; et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence from Modern Radiation Doses to the Lungs and Heart and from Previous Randomized Trials. J. Clin. Oncol. 2017, 35, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Mutter, R.W.; Choi, J.I.; Jimenez, R.B.; Kirova, Y.M.; Fagundes, M.; Haffty, B.G.; Amos, R.A.; Bradley, J.A.; Chen, P.Y.; Ding, X.; et al. Proton Therapy for Breast Cancer: A Consensus Statement from the Particle Therapy Cooperative Group Breast Cancer Subcommittee. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Punnett, A.; Tsang, R.W.; Hodgson, D.C. Hodgkin lymphoma across the age spectrum: Epidemiology, therapy, and late effects. Semin. Radiat. Oncol. 2010, 20, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Flerlage, J.E.; Metzger, M.L.; Bhakta, N. The management of Hodgkin lymphoma in adolescents and young adults: Burden of disease or burden of choice? Blood 2018, 132, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Milgrom, S.A.; Bakst, R.L.; Campbell, B.A. Clinical Outcomes Confirm Conjecture: Modern Radiation Therapy Reduces the Risk of Late Toxicity in Survivors of Hodgkin Lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Mulrooney, D.A.; Hyun, G.; Ness, K.K.; Ehrhardt, M.J.; Yasui, Y.; Duprez, D.; Howell, R.M.; Leisenring, W.M.; Constine, L.S.; Tonorezos, E.; et al. Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: Report from the Childhood Cancer Survivor Study cohort. Br. Med. J. 2020, 368, l6794. [Google Scholar] [CrossRef]
- Bhatia, S.; Robison, L.L.; Oberlin, O.; Greenberg, M.; Bunin, G.; Fossati-Bellani, F.; Meadows, A.T. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N. Engl. J. Med. 1996, 334, 745–751. [Google Scholar] [CrossRef]
- Ng, A.K.; Bernardo, M.V.; Weller, E.; Backstrand, K.; Silver, B.; Marcus, K.C.; Tarbell, N.J.; Stevenson, M.A.; Friedberg, J.W.; Mauch, P.M. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: Long-term risks and risk factors. Blood 2002, 100, 1989–1996. [Google Scholar] [CrossRef]
- Travis, L.B.; Hill, D.A.; Dores, G.M.; Gospodarowicz, M.; van Leeuwen, F.E.; Holowaty, E.; Glimelius, B.; Andersson, M.; Wiklund, T.; Lynch, C.F.; et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. J. Am. Med. Assoc. 2003, 290, 465–475. [Google Scholar] [CrossRef]
- Schaapveld, M.; Aleman, B.M.; van Eggermond, A.M.; Janus, C.P.; Krol, A.D.; van der Maazen, R.W.; Roesink, J.; Raemaekers, J.M.; de Boer, J.P.; Zijlstra, J.M.; et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 373, 2499–2511. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.L.; Connors, J.M.; Tyldesley, S.; Savage, K.J.; Campbell, B.A.; Zheng, Y.Y.; Hamm, J.; Pickles, T. Secondary Breast Cancer Risk by Radiation Volume in Women with Hodgkin Lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Dabaja, B.S.; Hoppe, B.S.; Plastaras, J.P.; Newhauser, W.; Rosolova, K.; Flampouri, S.; Mohan, R.; Mikhaeel, N.G.; Kirova, Y.; Specht, L.; et al. Proton therapy for adults with mediastinal lymphomas: The International Lymphoma Radiation Oncology Group guidelines. Blood 2018, 132, 1635–1646. [Google Scholar] [CrossRef]
- Asakage, T. Epidemiology and treatment of head and neck malignancies in the AYA generation. Int. J. Clin. Oncol. 2022, 27, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.N.; Howard, F.; Mahdavi, S.; Martinez, I.S.; Afghari, N.; Tran, E.; Goddard, K. Patient-Reported Outcomes in Adolescent and Young Adult Head and Neck Cancer Survivors Treated with Radiotherapy. J. Adolesc. Young Adult Oncol. 2023, 12, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sio, T.T.; Lin, H.K.; Shi, Q.; Gunn, G.B.; Cleeland, C.S.; Lee, J.J.; Hernandez, M.; Blanchard, P.; Thaker, N.G.; Phan, J.; et al. Intensity Modulated Proton Therapy Versus Intensity Modulated Photon Radiation Therapy for Oropharyngeal Cancer: First Comparative Results of Patient-Reported Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.; Garden, A.S.; Gunn, G.B.; Rosenthal, D.I.; Morrison, W.H.; Hernandez, M.; Crutison, J.; Lee, J.J.; Ye, R.; Fuller, C.D.; et al. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer—A case matched analysis. Radiother. Oncol. 2016, 120, 48–55. [Google Scholar] [CrossRef]
- Romesser, P.B.; Cahlon, O.; Scher, E.; Zhou, Y.; Berry, S.L.; Rybkin, A.; Sine, K.M.; Tang, S.; Sherman, E.J.; Wong, R.; et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother. Oncol. 2016, 118, 286–292. [Google Scholar] [CrossRef]
- Blanchard, P.; Gunn, G.B.; Lin, A.; Foote, R.L.; Lee, N.Y.; Frank, S.J. Proton therapy for head and neck cancers. Semin. Radiat. Oncol. 2018, 28, 1053–1063. [Google Scholar] [CrossRef]
- Stoneham, S.; Murray, M.; Thomas, B.; Williamson, M.; Sweeney, C.; Frazier, L. AYA testis cancer: The unment challenge. Pediatr. Blood Cnabcer 2019, 66, e27796. [Google Scholar] [CrossRef] [PubMed]
- Horwich, A.; Fossa, S.D.; Huddart, R.; Dearnaley, D.P.; Stenning, S.; Aresu, M.; Bliss, J.M.; Hall, E. Second cancer risk and mortality in men treated with radiotherapy for Stage I seminoma. Br. J. Cancer 2014, 110, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Pasalic, D.; Prajapati, S.; Ludmir, E.B.; Tang, C.; Choi, S.; Kudchadker, R.; Frank, S.J. Outcomes and Toxicities of Proton and Photon Radiation Therapy for Testicular Seminoma. Int. J. Part. Ther. 2020, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, R.; Chang, Y.; Paul, C.; Vaughn, D.J.; Christodouleas, J.P. Cancer control, toxicity, and secondary malignancy risks of proton radiation therapy for stage I-IIB testicular seminoma. Adv. Rad. Oncol. 2023, 8, 101259. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.K.; Keole, S.R. Proton Therapy in the Adolescent and Young Adult Population. Cancers 2023, 15, 4269. https://doi.org/10.3390/cancers15174269
Ahmed SK, Keole SR. Proton Therapy in the Adolescent and Young Adult Population. Cancers. 2023; 15(17):4269. https://doi.org/10.3390/cancers15174269
Chicago/Turabian StyleAhmed, Safia K., and Sameer R. Keole. 2023. "Proton Therapy in the Adolescent and Young Adult Population" Cancers 15, no. 17: 4269. https://doi.org/10.3390/cancers15174269
APA StyleAhmed, S. K., & Keole, S. R. (2023). Proton Therapy in the Adolescent and Young Adult Population. Cancers, 15(17), 4269. https://doi.org/10.3390/cancers15174269




