Anti-Inflammatory and Immune Properties of Polyunsaturated Fatty Acids (PUFAs) and Their Impact on Colorectal Cancer (CRC) Prevention and Treatment
Abstract
:Simple Summary
Abstract
1. Background
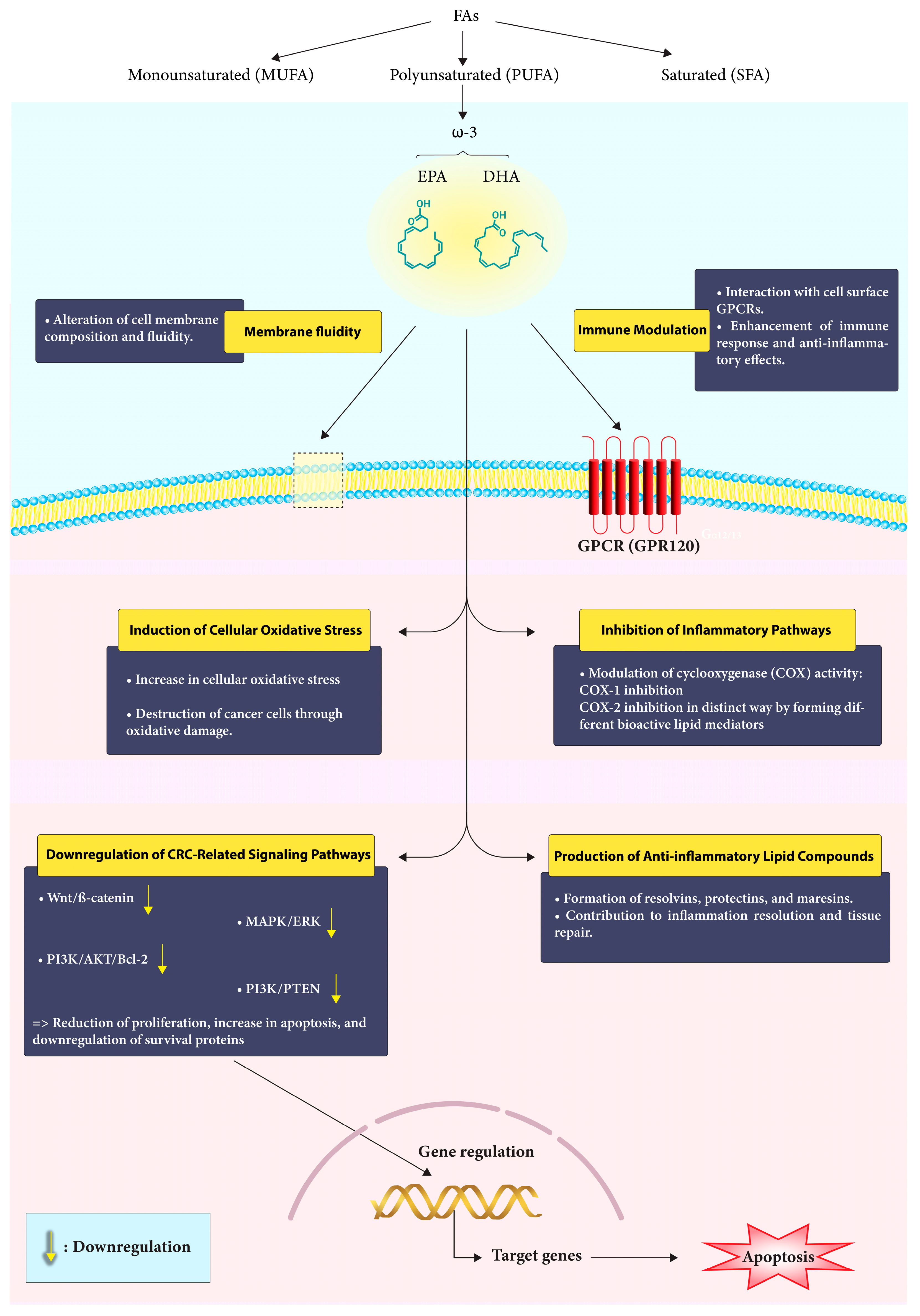
2. Polyunsaturated Fatty Acids (PUFAs)
3. CRC Prevention
4. Effects of ω-3 PUFAs on CRC Prophylaxis
4.1. Diet and ω-3 Content
4.2. The Role of ω-3 and Fiber Intake in CRC Prophylaxis
4.3. The Role of Fish Oil (FO)
4.4. Combining EPA and Aspirin
5. The Impact of ω-3 on CRC-Related Molecular Signaling Pathways
6. Role of ω-3 FAs in Patients with An Established CRC Diagnosis
6.1. Perioperative ω-3 FAs and Postoperative Outcomes in CRC Patients
6.2. Cancer Cachexia
6.3. Ω3 FAs: An Adjunct to Chemotherapy?
7. Immune Modulation by ω-3 FAs in CRC
8. Adverse Effects of ω-3 FAs
9. Future Perspective
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Miccadei, S.; Masella, R.; Mileo, A.M.; Gessani, S. ω3 Polyunsaturated fatty acids as immunomodulators in colorectal cancer: New potential role in adjuvant therapies. Front. Immunol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Zhang, J.N.; Zhang, G.D. Cytochrome P450 monooxygenase-mediated eicosanoid pathway: A potential mechanistic linkage between dietary fatty acid consumption and colon cancer risk. Food Sci. Hum. Wellness 2019, 8, 337–343. [Google Scholar] [CrossRef]
- Stubbs, C.D.; Smith, A.D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim. Et Biophys. Acta (BBA)-Rev. Biomembr. 1984, 779, 89–137. [Google Scholar] [CrossRef]
- Arima, K.; Zhong, R.; Ugai, T.; Zhao, M.; Haruki, K.; Akimoto, N.; Lau, M.C.; Okadome, K.; Mehta, R.S.; Väyrynen, J.P. Western-Style diet, PKs island-carrying Escherichia coli, and colorectal cancer: Analyses from two large prospective cohort studies. Gastroenterology 2022, 163, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Weisburger, J.H. Causes, relevant mechanisms, and prevention of large bowel cancer. Semin Oncol. 1991, 18, 316–336. [Google Scholar]
- Reddy, B.S.; Maeura, Y. Tumor promotion by dietary fat in azoxymethane-induced colon carcinogenesis in female F344 rats: Influence of amount and source of dietary fat. J. Natl. Cancer Inst. 1984, 72, 745–750. [Google Scholar]
- Reddy, B.S.; Maruyama, H. Effect of dietary fish oil on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer Res. 1986, 46, 3367–3370. [Google Scholar]
- Nelson, R.; Tanure, J.; Andrianopoulos, G.; Souza, G.; Lands, W. A comparison of dietary fish oil and corn oil in experimental colorectal carcinogenesis. Nutr. Cancer 1988, 11, 215–220. [Google Scholar] [CrossRef]
- Lindner, M.A. A fish oil diet inhibits colon cancer in mice. Nutr. Cancer 1991, 15, 1–11. [Google Scholar] [CrossRef]
- Waitzberg, D.L.; Torrinhas, R.S. Fish oil lipid emulsions and immune response: What clinicians need to know. Nutr. Clin. Pr. 2009, 24, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Serhan, C.N. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol. Asp. Med. 2017, 58, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Klek, S. Omega-3 fatty acids in modern parenteral nutrition: A review of the current evidence. J. Clin. Med. 2016, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Khaddaj-Mallat, R.; Morin, C.; Rousseau, É. Novel n-3 PUFA monoacylglycerides of pharmacological and medicinal interest: Anti-inflammatory and anti-proliferative effects. Eur. J. Pharmacol. 2016, 792, 70–77. [Google Scholar] [CrossRef]
- Kort, W.J.; Weijma, I.M.; Bijma, A.M.; van Schalkwijk, W.P.; Vergroesen, A.J.; Westbroek, D.L. Omega-3 fatty acids inhibiting the growth of a transplantable rat mammary adenocarcinoma. Clin. Med. 1987, 79, 593–599. [Google Scholar]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Charrière, K.; Ghzaiel, I.; Lizard, G.; Vejux, A. Involvement of Microglia in Neurodegenerative Diseases: Beneficial Effects of Docosahexahenoic Acid (DHA) Supplied by Food or Combined with Nanoparticles. Int. J. Mol. Sci. 2021, 22, 10639. [Google Scholar] [CrossRef]
- Jeromson, S.; Gallagher, I.J.; Galloway, S.D.R.; Hamilton, D.L. Omega-3 Fatty Acids and Skeletal Muscle Health. Mar. Drugs 2015, 13, 6977–7004. [Google Scholar] [CrossRef]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2019, 11, 46. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef]
- Atkin, W.S.; Edwards, R.; Kralj-Hans, I.; Wooldrage, K.; Hart, A.R.; Northover, J.M.; Parkin, D.M.; Wardle, J.; Duffy, S.W.; Cuzick, J. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: A multicentre randomised controlled trial. Lancet 2010, 375, 1624–1633. [Google Scholar] [CrossRef]
- Boursi, B.; Arber, N. Current and future clinical strategies in colon cancer prevention and the emerging role of chemoprevention. Curr. Pharm. Des. 2007, 13, 2274–2282. [Google Scholar] [CrossRef]
- Zauber, A.G.; Winawer, S.J.; O’Brien, M.J.; Lansdorp-Vogelaar, I.; van Ballegooijen, M.; Hankey, B.F.; Shi, W.; Bond, J.H.; Schapiro, M.; Panish, J.F. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012, 366, 687–696. [Google Scholar] [CrossRef]
- Hull, M.A. Nutritional agents with anti-lnflammatory properties in chemoprevention of colorectal neoplasia. Prospect. Chemoprevention Color. Neoplasia 2013, 143–156. [Google Scholar]
- Chan, A.T.; Arber, N.; Burn, J.; Chia, W.K.; Elwood, P.; Hull, M.A.; Logan, R.F.; Rothwell, P.M.; Schrör, K.; Baron, J.A. Aspirin in the Chemoprevention of Colorectal Neoplasia: An OverviewAspirin in Chemoprevention of Colorectal Neoplasia. Cancer Prev. Res. 2012, 5, 164–178. [Google Scholar] [CrossRef]
- Nkondjock, A.; Shatenstein, B.; Maisonneuve, P.; Ghadirian, P. Specific fatty acids and human colorectal cancer: An overview. Cancer Detect. Prev. 2003, 27, 55–66. [Google Scholar] [CrossRef]
- Calviello, G.; Serini, S.; Piccioni, E. n-3 polyunsaturated fatty acids and the prevention of colorectal cancer: Molecular mechanisms involved. Curr. Med. Chem. 2007, 14, 3059–3069. [Google Scholar] [CrossRef]
- Murff, H.J.; Shrubsole, M.J.; Cai, Q.Y.; Smalley, W.E.; Dai, Q.; Milne, G.L.; Ness, R.M.; Zheng, W. Dietary intake of PUFAs and colorectal polyp risk. Am. J. Clin. Nutr. 2012, 95, 703–712. [Google Scholar] [CrossRef]
- Lawson, L.D.; Hughes, B.G. Human absorption of fish oil fatty acids as triacylglycerols, free acids, or ethyl esters. Res. Commun. 1988, 152, 328–335. [Google Scholar] [CrossRef]
- Pietrzyk, L.; Torres, A.; Maciejewski, R.; Torres, K. Obesity and obese-related chronic low-grade inflammation in promotion of colorectal cancer development. Asian Pac. J. Cancer Prev. 2015, 16, 4161–4168. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Ingangi, V.; Minopoli, M.; Ragone, C.; Motti, M.L.; Carriero, M.V. Role of Microenvironment on the Fate of Disseminating Cancer Stem Cells. Front. Oncol. 2019, 9, 82. [Google Scholar] [CrossRef]
- Sellem, L.; Srour, B.; Guéraud, F.; Pierre, F.; Kesse-Guyot, E.; Fiolet, T.; Lavalette, C.; Egnell, M.; Latino-Martel, P.; Fassier, P.; et al. Saturated, mono- and polyunsaturated fatty acid intake and cancer risk: Results from the French prospective cohort NutriNet-Santé. Eur. J. Nutr. 2019, 58, 1515–1527. [Google Scholar] [CrossRef]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The world cancer research fund/American institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: Impact and future directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, A.T.; Coleman, H.G.; Huang, W.-Y.; Kitahara, C.M.; Cantwell, M.M.; Berndt, S.I. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Clin. Nutr. 2015, 102, 881–890. [Google Scholar] [CrossRef]
- He, X.; Wu, K.; Zhang, X.; Nishihara, R.; Cao, Y.; Fuchs, C.S.; Giovannucci, E.L.; Ogino, S.; Chan, A.T.; Song, M. Dietary intake of fiber, whole grains and risk of colorectal cancer: An updated analysis according to food sources, tumor location and molecular subtypes in two large US cohorts. Int. J. Cancer 2019, 145, 3040–3051. [Google Scholar] [CrossRef]
- Bingham, S.A.; Day, N.E.; Luben, R.; Ferrari, P.; Slimani, N.; Norat, T.; Clavel-Chapelon, F.; Kesse, E.; Nieters, A.; Boeing, H.; et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): An observational study. Lancet 2003, 361, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Neil, M.; Teresa, N.; Pietro, F.; Mazda, J.; Bas, B.-d.-M.; Guri, S.; Christina, C.D.; Kim, O.; Anja, O.; Anne, T. Dietary Fibre Intake and Risks of Cancers of the Colon and Rectum in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS ONE 2012, 7, e39361. [Google Scholar]
- Kraja, B.; Muka, T.; Ruiter, R.; de Keyser, C.E.; Hofman, A.; Franco, O.H.; Stricker, B.H.; Kiefte-de Jong, J.C. Dietary Fiber Intake Modifies the Positive Association between n–3 PUFA Intake and Colorectal Cancer Risk in a Caucasian Population. J. Nutr. 2015, 145, 1709–1716. [Google Scholar] [CrossRef]
- Navarro, S.L.; Neuhouser, M.L.; Cheng, T.-Y.D.; Tinker, L.F.; Shikany, J.M.; Snetselaar, L.; Martinez, J.A.; Kato, I.; Beresford, S.A.A.; Chapkin, R.S.; et al. The Interaction between Dietary Fiber and Fat and Risk of Colorectal Cancer in the Women’s Health Initiative. Nutrients 2016, 8, 779. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chan, A.T. Environmental Factors, Gut Microbiota, and Colorectal Cancer Prevention. Clin. Gastroenterol. Hepatol. 2019, 17, 275–289. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T.; Sun, J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology 2020, 158, 322–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Yu, Y.-N.; Wang, J.-L.; Lin, Y.-W.; Kong, X.; Yang, C.-Q.; Yang, L.; Liu, Z.-J.; Yuan, Y.-Z.; Liu, F.; et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am. J. Clin. Nutr. 2013, 97, 1044–1052. [Google Scholar] [CrossRef]
- Li, Z.-H.; Zhong, W.-F.; Liu, S.; Kraus, V.B.; Zhang, Y.-J.; Gao, X.; Lv, Y.-B.; Shen, D.; Zhang, X.-R.; Zhang, P.-D.; et al. Associations of habitual fish oil supplementation with cardiovascular outcomes and all cause mortality: Evidence from a large population based cohort study. BMJ 2020, 368, m456. [Google Scholar] [CrossRef]
- Song, M.; Zhang, X.; Meyerhardt, J.A.; Giovannucci, E.L.; Ogino, S.; Fuchs, C.S.; Chan, A.T. Marine ω-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut 2017, 66, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; Williamson, E.J.; Bassett, J.K.; MacInnis, R.J.; Giles, G.G.; English, D.R. Dietary and biomarker estimates of fatty acids and risk of colorectal cancer. Int. J. Cancer 2015, 137, 1224–1234. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Masset, G.; Brunner, E.J.; Cade, J.E.; Greenwood, D.C.; Stephen, A.M.; Kuh, D.; Bhaniani, A.; Powell, N.; et al. Vitamins, minerals, essential fatty acids and colorectal cancer risk in the United Kingdom Dietary Cohort Consortium. Int. J. Cancer 2012, 131, E320–E325. [Google Scholar] [CrossRef]
- Kantor, E.D.; Lampe, J.W.; Peters, U.; Vaughan, T.L.; White, E. Long-Chain Omega-3 Polyunsaturated Fatty Acid Intake and Risk of Colorectal Cancer. Nutr. Cancer 2014, 66, 716–727. [Google Scholar] [CrossRef]
- Hull, M.A.; Sandell, A.C.; Montgomery, A.A.; Logan, R.F.A.; Clifford, G.M.; Rees, C.J.; Loadman, P.M.; Whitham, D. A randomized controlled trial of eicosapentaenoic acid and/or aspirin for colorectal adenoma prevention during colonoscopic surveillance in the NHS Bowel Cancer Screening Programme (The seAFOod Polyp Prevention Trial): Study protocol for a randomized controlled trial. Trials 2013, 14. [Google Scholar] [CrossRef]
- Smith, W.L. Cyclooxygenases, peroxide tone and the allure of fish oil. Curr. Opin. Cell Biol. 2005, 17, 174–182. [Google Scholar] [CrossRef]
- Greene, E.R.; Huang, S.; Serhan, C.N.; Panigrahy, D. Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediat. 2011, 96, 27–36. [Google Scholar] [CrossRef]
- Larson, M.K.; Ashmore, J.H.; Harris, K.A.; Vogelaar, J.L.; Pottala, J.V.; Sprehe, M.; Harris, W.S. Effects of omega-3 acid ethyl esters and aspirin, alone and in combination, on platelet function in healthy subjects. Thromb. Haemost. 2008, 100, 634–641. [Google Scholar] [CrossRef]
- Block, R.C.; Kakinami, L.; Jonovich, M.; Antonetti, I.; Lawrence, P.; Meednu, N.; CalderonArtero, P.; Mousa, S.A.; Brenna, J.T.; Georas, S. The combination of EPA+ DHA and low-dose aspirin ingestion reduces platelet function acutely whereas each alone may not in healthy humans. Leukot. Essent. Fat. Acids 2012, 87, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Volpato, M.; Hull, M.A. Omega-3 polyunsaturated fatty acids as adjuvant therapy of colorectal cancer. Cancer Metastasis Rev. 2018, 37, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Calviello, G.; Resci, F.; Serini, S.; Piccioni, E.; Toesca, A.; Boninsegna, A.; Monego, G.; Ranelletti, F.O.; Palozza, P. Docosahexaenoic acid induces proteasome-dependent degradation of beta-catenin, down-regulation of survivin and apoptosis in human colorectal cancer cells not expressing COX-2. Carcinog. 2007, 28, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, B.A.; Narayanan, N.K.; Desai, D.; Pittman, B.; Reddy, B.S. Effects of a combination of docosahexaenoic acid and 1,4-phenylene bis(methylene) selenocyanate on cyclooxygenase 2, inducible nitric oxide synthase and beta-catenin pathways in colon cancer cells. Carcinogenesis 2004, 25, 2443–2449. [Google Scholar] [CrossRef]
- Toit-Kohn, J.L.; Louw, L.; Engelbrecht, A.M. Docosahexaenoic acid induces apoptosis in colorectal carcinoma cells by modulating the PI3 kinase and p38 MAPK pathways. J. Nutr. Biochem. 2009, 20, 106–114. [Google Scholar] [CrossRef]
- Engelbrecht, A.M.; Toit-Kohn, J.L.; Ellis, B.; Thomas, M.; Nell, T.; Smith, R. Differential induction of apoptosis and inhibition of the PI3-kinase pathway by saturated, monounsaturated and polyunsaturated fatty acids in a colon cancer cell model. Apoptosis 2008, 13, 1368–1377. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, C.A.; Cai, P.Z.; Xu, F.P.; Zhu, W.J.; Wang, W.W.; Jiang, H.P. Omega-3PUFA Attenuates MNU-Induced Colorectal Cancer in Rats by Blocking PI3K/AKT/Bcl-2 Signaling. OncoTargets Ther. 2020, 13, 1953–1965. [Google Scholar] [CrossRef]
- Schmidt, N.; Møller, G.; Bæksgaard, L.; Østerlind, K.; Stark, K.D.; Lauritzen, L.; Andersen, J.R. Fish oil supplementation in cancer patients. Capsules or nutritional drink supplements? A controlled study of compliance. Clin. Nutr. ESPEN 2020, 35, 63–68. [Google Scholar] [CrossRef]
- Cockbain, A.J.; Volpato, M.; Race, A.D.; Munarini, A.; Fazio, C.; Belluzzi, A.; Loadman, P.M.; Toogood, G.J.; Hull, M.A. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut 2014, 63, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Hossain, T.; Phillips, B.E.; Doleman, B.; Lund, J.N.; Williams, J.P. A double-blind randomized controlled trial of the effects of eicosapentaenoic acid supplementation on muscle inflammation and physical function in patients undergoing colorectal cancer resection. Clin. Nutr. 2020, 39, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Brown, N.M.; McCoy, A.N.; Sandler, R.S.; Keku, T.O. Omega-3 Polyunsaturated Fatty Acids, Gut Microbiota, Microbial Metabolites, and Risk of Colorectal Adenomas. Cancers 2022, 14, 4443. [Google Scholar] [CrossRef]
- Anti, M.; Marra, G.; Armelao, F.; Bartoli, G.M.; Ficarelli, R.; Percesepe, A.; De Vitis, I.; Maria, G.; Sofo, L.; Rapaccini, G.L.; et al. Effect of ω-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer. Gastroenterology 1992, 103, 883–891. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Fuchs, C.S.; Niedzwiecki, D.; Ye, X.; Zhang, S.; Song, M.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; et al. Marine ω-3 Polyunsaturated Fatty Acid and Fish Intake after Colon Cancer Diagnosis and Survival: CALGB 89803 (Alliance). Cancer Epidemiol. Biomark. Prev. 2018, 27, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Aglago, E.K.; Huybrechts, I.; Murphy, N.; Casagrande, C.; Nicolas, G.; Pischon, T.; Fedirko, V.; Severi, G.; Boutron-Ruault, M.C.; Fournier, A.; et al. Consumption of Fish and Long-chain n-3 Polyunsaturated Fatty Acids Is Associated With Reduced Risk of Colorectal Cancer in a Large European Cohort. Clin. Gastroenterol. 2020, 18, 654–666.e656. [Google Scholar] [CrossRef]
- Sasazuki, S.; Inoue, M.; Iwasaki, M.; Sawada, N.; Shimazu, T.; Yamaji, T.; Takachi, R.; Tsugane, S. Intake of n-3 and n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int. J. Cancer 2011, 129, 1718–1729. [Google Scholar] [CrossRef]
- Khankari, N.K.; Banbury, B.L.; Borges, M.C.; Haycock, P.; Albanes, D.; Arndt, V.; Berndt, S.I.; Bézieau, S.; Brenner, H.; Campbell, P.T.; et al. Mendelian Randomization of Circulating Polyunsaturated Fatty Acids and Colorectal Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2020, 29, 860–870. [Google Scholar] [CrossRef]
- Shin, A.; Cho, S.; Sandin, S.; Lof, M.; Oh, M.Y.; Weiderpass, E. Omega-3 and -6 Fatty Acid Intake and Colorectal Cancer Risk in Swedish Women’s Lifestyle and Health Cohort. Cancer Res. Treat. 2020, 52, 848–854. [Google Scholar] [CrossRef]
- Silva Jde, A.; Trindade, E.B.; Fabre, M.E.; Menegotto, V.M.; Gevaerd, S.; Buss Zda, S.; Frode, T.S. Fish oil supplement alters markers of inflammatory and nutritional status in colorectal cancer patients. Nutr. Cancer 2012, 64, 267–273. [Google Scholar] [CrossRef]
- Mocellin, M.C.; Pastore e Silva Jde, A.; Camargo Cde, Q.; Fabre, M.E.; Gevaerd, S.; Naliwaiko, K.; Moreno, Y.M.; Nunes, E.A.; Trindade, E.B. Fish oil decreases C-reactive protein/albumin ratio improving nutritional prognosis and plasma fatty acid profile in colorectal cancer patients. Lipids 2013, 48, 879–888. [Google Scholar] [CrossRef]
- Tutino, V.; De Nunzio, V.; Caruso, M.G.; Veronese, N.; Lorusso, D.; Di Masi, M.; Benedetto, M.L.; Notarnicola, M. Elevated AA/EPA Ratio Represents an Inflammatory Biomarker in Tumor Tissue of Metastatic Colorectal Cancer Patients. Int. J. Mol. Sci. 2019, 20, 2050. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.A.; Sprange, K.; Hepburn, T.; Tan, W.; Shafayat, A.; Rees, C.J.; Clifford, G.; Logan, R.F.; Loadman, P.M.; Williams, E.A.; et al. Eicosapentaenoic acid and aspirin, alone and in combination, for the prevention of colorectal adenomas (seAFOod Polyp Prevention trial): A multicentre, randomised, double-blind, placebo-controlled, 2 × 2 factorial trial. Lancet 2018, 392, 2583–2594. [Google Scholar] [CrossRef]
- Haidari, F.; Abiri, B.; Iravani, M.; Ahmadi-Angali, K.; Vafa, M. Effects of Vitamin D and Omega-3 Fatty Acids Co-Supplementation on Inflammatory Factors and Tumor Marker CEA in Colorectal Cancer Patients Undergoing Chemotherapy: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutr. Cancer 2020, 72, 948–958. [Google Scholar] [CrossRef]
- Han, Y.M.; Jeong, M.; Park, J.M.; Kim, M.Y.; Go, E.J.; Cha, J.Y.; Kim, K.J.; Hahm, K.B. The ω-3 polyunsaturated fatty acids prevented colitis-associated carcinogenesis through blocking dissociation of β-catenin complex, inhibiting COX-2 through repressing NF-κB, and inducing 15-prostaglandin dehydrogenase. Oncotarget 2016, 7, 63583–63595. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chan, A.T.; Fuchs, C.S.; Ogino, S.; Hu, F.B.; Mozaffarian, D.; Ma, J.; Willett, W.C.; Giovannucci, E.L.; Wu, K. Dietary intake of fish, ω-3 and ω-6 fatty acids and risk of colorectal cancer: A prospective study in U.S. men and women. Int. J. Cancer 2014, 135, 2413–2423. [Google Scholar] [CrossRef]
- Sorensen, L.S.; Rasmussen, H.H.; Aardestrup, I.V.; Thorlacius-Ussing, O.; Lindorff-Larsen, K.; Schmidt, E.B.; Calder, P.C. Rapid incorporation of ω-3 fatty acids into colonic tissue after oral supplementation in patients with colorectal cancer: A randomized, placebo-controlled intervention trial. J. Parenter. Enter. Nutr. 2014, 38, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, L.S.; Thorlacius-Ussing, O.; Rasmussen, H.H.; Lundbye-Christensen, S.; Calder, P.C.; Lindorff-Larsen, K.; Schmidt, E.B. Effects of perioperative supplementation with omega-3 fatty acids on leukotriene B4 and leukotriene B5 production by stimulated neutrophils in patients with colorectal cancer: A randomized, placebo-controlled intervention trial. Nutrients 2014, 6, 4043–4057. [Google Scholar] [CrossRef]
- Sorensen, L.S.; Thorlacius-Ussing, O.; Schmidt, E.B.; Rasmussen, H.H.; Lundbye-Christensen, S.; Calder, P.C.; Lindorff-Larsen, K. Randomized clinical trial of perioperative omega-3 fatty acid supplements in elective colorectal cancer surgery. Br. J. Surg. 2014, 101, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Camargo Cde, Q.; Mocellin, M.C.; Pastore Silva Jde, A.; Fabre, M.E.; Nunes, E.A.; Trindade, E.B. Fish oil supplementation during chemotherapy increases posterior time to tumor progression in colorectal cancer. Nutr. Cancer 2016, 68, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chang, Y.-n. Omega-3 polyunsaturated fatty acids in the prevention of postoperative complications in colorectal cancer: A meta-analysis. OncoTargets Ther. 2016, 7435–7443. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; Solomon, M.; Rangan, A.; Ferrie, S.; Carey, S. The Impact of Preoperative Immunonutrition and Standard Polymeric Supplements on Patient Outcomes After Pelvic Exenteration Surgery, Taking Compliance Into Consideration: A Randomized Controlled Trial. J. Parenter. Enter. Nutr. 2020, 44, 806–814. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.; Park, H.-M.; Kim, C.H.; Kim, H.R. Impact of Preoperative Immunonutrition on the Outcomes of Colon Cancer Surgery: Results from a Randomized Controlled Trial. Ann. Surg. 2023, 277, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.W.; Tang, D.N.; Hou, J.; Wei, J.M.; Hua, B.; Sun, J.H.; Cui, H.Y. Impact of fish oil enriched total parenteral nutrition on elderly patients after colorectal cancer surgery. Chin. Med J. 2012, 125, 178–181. [Google Scholar]
- Ma, C.-J.; Wu, J.-M.; Tsai, H.-L.; Huang, C.-W.; Lu, C.-Y.; Sun, L.-C.; Shih, Y.-L.; Chen, C.-W.; Chuang, J.-F.; Wu, M.-H.; et al. Prospective double-blind randomized study on the efficacy and safety of an n-3 fatty acid enriched intravenous fat emulsion in postsurgical gastric and colorectal cancer patients. Nutr. J. 2015, 14, 9. [Google Scholar] [CrossRef]
- Aliyazicioglu, T.; Cantürk, N.Z.; Simsek, T.; Kolayli, F.; Çekmen, M. Effects of standard and/or glutamine dipeptide and/or omega-3 fatty ascid-supplemented parenteral nutrition on neutrophil functions, interleukin-8 level and length of stay—A double blind, controlled, randomised study. East Afr. Med. J. 2013, 90, 59–66. [Google Scholar]
- Trust, R.S.C.H.N.F. PeRioperative Omega Three and the Effect on ImmuNity. 2018. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03598413 (accessed on 24 August 2023).
- Colomer, R.; Moreno-Nogueira, J.M.; García-Luna, P.P.; García-Peris, P.; García-de-Lorenzo, A.; Zarazaga, A.; Quecedo, L.; del Llano, J.; Usán, L.; Casimiro, C. N-3 fatty acids, cancer and cachexia: A systematic review of the literature. Br. J. Nutr. 2007, 97, 823–831. [Google Scholar] [CrossRef]
- Van der Meij, B.; Bauer, J.; Isenring, E.; Brown, T.; Davidson, W.; van Bokhorst, M.; Langius, J.; van Leeuwen, P. The effects of supplementation of n-3 polyunsaturated fatty acids on clinical outcome parameters in patients with cancer: A systematic review. OA Epidemiol. 2013, 1, 2. [Google Scholar]
- Read, J.A.; Beale, P.J.; Volker, D.H.; Smith, N.; Childs, A.; Clarke, S.J. Nutrition intervention using an eicosapentaenoic acid (EPA)-containing supplement in patients with advanced colorectal cancer. Effects on nutritional and inflammatory status: A phase II trial. Support. Care Cancer 2007, 15, 301–307. [Google Scholar] [CrossRef]
- Ryan, A.M.; Reynolds, J.V.; Healy, L.; Byrne, M.; Moore, J.; Brannelly, N.; McHugh, A.; McCormack, D.; Flood, P. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: Results of a double-blinded randomized controlled trial. Ann. Surg. 2009, 249, 355–363. [Google Scholar] [CrossRef]
- Golkhalkhali, B.; Rajandram, R.; Paliany, A.S.; Ho, G.F.; Wan Ishak, W.Z.; Johari, C.S.; Chin, K.F. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: A randomized controlled trial. Asia-Pacific J. Clin. Oncol. 2018, 14, 179–191. [Google Scholar] [CrossRef]
- Silva, J.D.A.P.; Silva, M.M.R.L.; Mello, C.L.; Begnami, M.D.; Silva, M.L.G.; Junior, S.A. Fish oil supplementation and inflammatory response during neoadjuvant chemoradiation for rectal cancer: Results from a prospective, randomized, controlled trial. J. Clin. Oncol. 2018, 36, 3605. [Google Scholar] [CrossRef]
- D’Angelo, L.; Piazzi, G.; Pacilli, A.; Prossomariti, A.; Fazio, C.; Montanaro, L.; Graziani, G.; Fogliano, V.; Munarini, A.; Bianchi, F.; et al. A combination of eicosapentaenoic acid-free fatty acid, epigallocatechin-3-gallate and proanthocyanidins has a strong effect on mTOR signaling in colorectal cancer cells. Carcinog. 2014, 35, 2314–2320. [Google Scholar] [CrossRef]
- Bai, B.; Wu, F.; Ying, K.; Xu, Y.; Shan, L.; Lv, Y.; Gao, X.; Xu, D.; Lu, J.; Xie, B. Therapeutic effects of dihydroartemisinin in multiple stages of colitis-associated colorectal cancer. Theranostics 2021, 11, 6225–6239. [Google Scholar] [CrossRef]
- Mocellin, M.C.; Camargo, C.Q.; Nunes, E.A.; Fiates, G.M.R.; Trindade, E. A systematic review and meta-analysis of the n-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer. Clin. Nutr. 2016, 35, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Fluckiger, A.; Dumont, A.; Derangère, V.; Rébé, C.; de Rosny, C.; Causse, S.; Thomas, C.; Apetoh, L.; Hichami, A.; Ghiringhelli, F.; et al. Inhibition of colon cancer growth by docosahexaenoic acid involves autocrine production of TNFα. Oncogene 2016, 35, 4611–4622. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Ottes Vasconcelos, R.; Fasano, E.; Calviello, G. Epigenetic regulation of gene expression and M2 macrophage polarization as new potential omega-3 polyunsaturated fatty acid targets in colon inflammation and cancer. Expert Opin. Ther. Targets 2016, 20, 843–858. [Google Scholar] [CrossRef]
- Song, M.; Nishihara, R.; Cao, Y.; Chun, E.; Qian, Z.R.; Mima, K.; Inamura, K.; Masugi, Y.; Nowak, J.A.; Nosho, K.; et al. Marine ω-3 Polyunsaturated Fatty Acid Intake and Risk of Colorectal Cancer Characterized by Tumor-Infiltrating T Cells. JAMA Oncol. 2016, 2, 1197–1206. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Shao, W.; Yan, S.; Ding, S. Efficacy and safety of Omega-3 polyunsaturated fatty acids in adjuvant treatments for colorectal cancer: A meta-analysis of randomized controlled trials. Front. Pharmacol. 2023, 14, 1004465. [Google Scholar] [CrossRef]
- Al-Madhagy, S.; Ashmawy, N.S.; Mamdouh, A.; Eldahshan, O.A.; Farag, M.A. A comprehensive review of the health benefits of flaxseed oil in relation to its chemical composition and comparison with other omega-3-rich oils. Eur. J. Med Res. 2023, 28, 1–17. [Google Scholar] [CrossRef] [PubMed]
- El-Agawy, M.S.E.-d.; Badawy, A.M.M.; Rabei, M.R.; Elshaer, M.M.A.; El Nashar, E.M.; Alghamdi, M.A.; Alshehri, M.A.; Elsayed, H.R.H. Methotrexate-induced alteration of renal Aquaporins 1 and 2, oxidative stress and tubular apoptosis can be attenuated by Omega-3 fatty acids supplementation. Int. J. Mol. Sci. 2022, 23, 12794. [Google Scholar] [CrossRef] [PubMed]
- Gibney, M.; Hunter, B. The effects of short-and long-term supplementation with fish oil on the incorporation of n-3 polyunsaturated fatty acids into cells of the immune system in healthy volunteers. Eur. J. Clin. Nutr. 1993, 47, 255–259. [Google Scholar] [PubMed]
- Bays, H. Clinical overview of Omacor: A concentrated formulation of omega-3 polyunsaturated fatty acids. Am. J. Cardiol. 2006, 98, 71–76. [Google Scholar] [CrossRef]
- Krupa, K.; Fritz, K.; Parmar, M. Omega-3 Fatty Acids; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]

| Species | N | Ω3-FA Type | Combination | Chemo | Outcome | Study Reference |
|---|---|---|---|---|---|---|
| Human | 10 | EPA 4.1g/day + DHA 3.6 g/day (FO) | - | - | A remarkable decrease in the average percentage of replicative “S”-phase cells (a parameter in assessing the risk of colon cancer). | [67] |
| Human | 476,160 | EPA + DPA + DHA | - | NO | Ω3FA: ↓↓ risk of CRC | [69] |
| Human | 68,109 | EPA + DHA (FO) | - | NO | No association with CRC risk overall. | [51] |
| Human | 1268 | ω-3 PUFAs (EPA + DHA + DPA) | - | NO | ω-3 PUFAs: inversely related to the risk of cancer in the proximal site of the large bowel. | [70] |
| Human | 18,682 | EPA, DPA, DHA, LA, ALA | - | NO |
| [71] |
| Human | 48,233 | DHA | - | NO |
| [72] |
| Human | 23 | EPA + DHA (FO) | - | 5-FU + irinotecan + folinic acid |
| [73] |
| Human | 11 | EPA + DHA (FO) | - | Capecitabine + Oxaliplatin + 5-FU + leucovorin | Improved CRP values, CRP/albumin status, plasma fatty acid profile, and potentially prevented weight loss during treatment. | [74] |
| Human | 38 | EPA | - | - | Elevated AA (Omega-6)/EPA ratio represents an inflammatory biomarker in tumor tissue of metastatic CRC | [75] |
| Human | 709 | EPA | + Aspirin | - | No reduction CRC risk | [76] |
| Human | 81 | ω-3 capsule | + Vitamin D | YES | IN CRC PATIENTS: co-supplementation ↓↓ inflammatory markers and CEA. | [77] |
| Fat-1 TG mice | - | DHA | - | - | DHA: ↑ apoptosis and ↓ tumor growth. | [78] |
| Human | 123,529 | Total omega-3 PUFA (ALA + EPA + DHA + DPA) Marine omega-3 PUFAs (EPA + DHA + DPA) Total omega-6 PUFAs (LA + AA) | - | - | Fish and marine ω-3 PUFA intake: ↓ risk of distal colon cancer and rectal cancer (men only). | [79] |
| Human | 148 | ω-3 FA–enriched ONS: 2.0 g EPA + 1.0 g DHA | - | - | Potential beneficial effect on local immune function | [80] |
| Human | 148 | ω-3 FA–enriched ONS: 2.0 g EPA + 1.0 g DHA | - | - | ↓↓ production of LTB5 and 5-HEPE. ↓↓ production of LTB4 ↑↑anti-inflammatory effects in the surgical patient. | [81] |
| Human | 148 | ω-3 FA–enriched ONS: 2.0 g EPA + 1.0 g DHA | - | - | Infectious or non-infectious postoperative complications: no differences. | [82] |
| Human | 30 | EPA + DHA (FO) | - | YES | ↑↑ tumor progression time. | [83] |
| Human | 88 | EPA-FFA (FFA) | - | - | Safe + well tolerated Preoperative FFA: ↑↑ postoperative OS and DFS. | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tojjari, A.; Choucair, K.; Sadeghipour, A.; Saeed, A.; Saeed, A. Anti-Inflammatory and Immune Properties of Polyunsaturated Fatty Acids (PUFAs) and Their Impact on Colorectal Cancer (CRC) Prevention and Treatment. Cancers 2023, 15, 4294. https://doi.org/10.3390/cancers15174294
Tojjari A, Choucair K, Sadeghipour A, Saeed A, Saeed A. Anti-Inflammatory and Immune Properties of Polyunsaturated Fatty Acids (PUFAs) and Their Impact on Colorectal Cancer (CRC) Prevention and Treatment. Cancers. 2023; 15(17):4294. https://doi.org/10.3390/cancers15174294
Chicago/Turabian StyleTojjari, Alireza, Khalil Choucair, Arezoo Sadeghipour, Azhar Saeed, and Anwaar Saeed. 2023. "Anti-Inflammatory and Immune Properties of Polyunsaturated Fatty Acids (PUFAs) and Their Impact on Colorectal Cancer (CRC) Prevention and Treatment" Cancers 15, no. 17: 4294. https://doi.org/10.3390/cancers15174294





