Diagnostic Approaches for Neuroendocrine Neoplasms of Unknown Primary (NEN-UPs) and Their Prognostic Relevance—A Retrospective, Long-Term Single-Center Experience
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Collection of Data
2.3. Data Analysis
3. Results
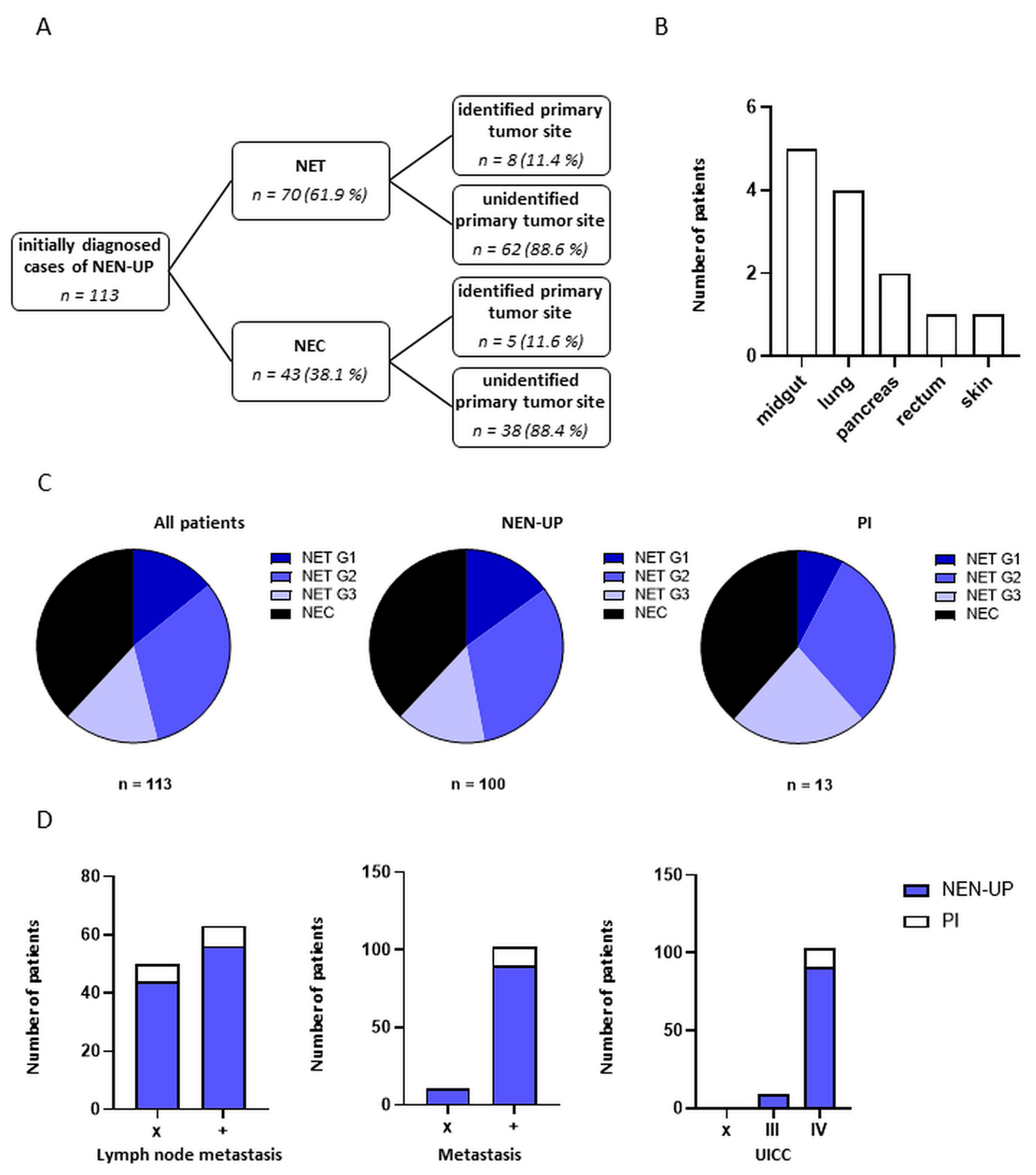
3.1. Demographic and Clinical Characteristics of Patients with NEN-UP
3.2. Assessment and Comparison of the Diagnostic Work-Up Performed in Patients with NEN-UP
3.3. Association and Predictive Value of Diagnostic Instruments and Detection of Primary Tumor Sites in Patients with NEN-UP
3.4. Treatment Strategies for Patients with NEN-UP
3.5. Clinical Outcomes of Patients with NEN-UP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schimmack, S.; Svejda, B.; Lawrence, B.; Kidd, M.; Modlin, I.M. The diversity and commonalities of gastroenteropancreatic neuroendocrine tumors. Langenbecks Arch. Surg. 2011, 396, 273–298. [Google Scholar] [CrossRef] [PubMed]
- Perren, A.; Couvelard, A.; Scoazec, J.Y.; Costa, F.; Borbath, I.; Delle Fave, G.; Gorbounova, V.; Gross, D.; Grossma, A.; Jense, R.T.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology 2017, 105, 196–200. [Google Scholar] [CrossRef]
- Rickman, D.S.; Beltran, H.; Demichelis, F.; Rubin, M.A. Biology and evolution of poorly differentiated neuroendocrine tumors. Nat. Med. 2017, 23, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Kloppel, G. Neuroendocrine Neoplasms: Dichotomy, Origin and Classifications. Visc. Med. 2017, 33, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Ekeblad, S.; Skogseid, B.; Dunder, K.; Oberg, K.; Eriksson, B. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin. Cancer Res. 2008, 14, 7798–7803. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Greco, F.A.; Pavlidis, N.; Daugaard, G.; Oien, K.; Pentheroudakis, G.; Committee, E.G. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v133–v138. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Usman, N.; Jayaraj, J.S.; Janapala, R.N.; Kashif, T. Cancer of Unknown Primary: A Review on Clinical Guidelines in the Development and Targeted Management of Patients with the Unknown Primary Site. Cureus 2019, 11, e5552. [Google Scholar] [CrossRef]
- Massard, C.; Loriot, Y.; Fizazi, K. Carcinomas of an unknown primary origin--diagnosis and treatment. Nat. Rev. Clin. Oncol. 2011, 8, 701–710. [Google Scholar] [CrossRef]
- Pavlidis, N.; Pentheroudakis, G. Cancer of unknown primary site. Lancet 2012, 379, 1428–1435. [Google Scholar] [CrossRef]
- Stella, G.M.; Senetta, R.; Cassenti, A.; Ronco, M.; Cassoni, P. Cancers of unknown primary origin: Current perspectives and future therapeutic strategies. J. Transl. Med. 2012, 10, 12. [Google Scholar] [CrossRef]
- Dyrvig, A.K.; Yderstraede, K.B.; Gerke, O.; Jensen, P.B.; Hess, S.; Hoilund-Carlsen, P.F.; Green, A. Cancer of unknown primary: Registered procedures compared with national integrated cancer pathway for illuminating external validity. Medicine 2017, 96, e6693. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.; Allardice, G.; Scott, I.; Oien, K.; Brewster, D.; Morrison, D.S. Cancers of unknown primary diagnosed during hospitalization: A population-based study. BMC Cancer 2017, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Wagland, R.; Bracher, M.; Drosdowsky, A.; Richardson, A.; Symons, J.; Mileshkin, L.; Schofield, P. Differences in experiences of care between patients diagnosed with metastatic cancer of known and unknown primaries: Mixed-method findings from the 2013 cancer patient experience survey in England. BMJ Open 2017, 7, e017881. [Google Scholar] [CrossRef] [PubMed]
- Mnatsakanyan, E.; Tung, W.C.; Caine, B.; Smith-Gagen, J. Cancer of unknown primary: Time trends in incidence, United States. Cancer Causes Control 2014, 25, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Urban, D.; Rao, A.; Bressel, M.; Lawrence, Y.R.; Mileshkin, L. Cancer of unknown primary: A population-based analysis of temporal change and socioeconomic disparities. Br. J. Cancer 2013, 109, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Rassy, E.; Pavlidis, N. The currently declining incidence of cancer of unknown primary. Cancer Epidemiol. 2019, 61, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Loffler, H.; Puthenparambil, J.; Hielscher, T.; Neben, K.; Kramer, A. Patients with cancer of unknown primary: A retrospective analysis of 223 patients with adenocarcinoma or undifferentiated carcinoma. Dtsch. Arztebl. Int. 2014, 111, 481–487. [Google Scholar] [CrossRef]
- Kang, S.; Jeong, J.H.; Yoon, S.; Yoo, C.; Kim, K.P.; Cho, H.; Ryoo, B.Y.; Jung, J.; Kim, J.E. Real-world data analysis of patients with cancer of unknown primary. Sci. Rep. 2021, 11, 23074. [Google Scholar] [CrossRef]
- Varghese, A.M.; Arora, A.; Capanu, M.; Camacho, N.; Won, H.H.; Zehir, A.; Gao, J.; Chakravarty, D.; Schultz, N.; Klimstra, D.S.; et al. Clinical and molecular characterization of patients with cancer of unknown primary in the modern era. Ann. Oncol. 2017, 28, 3015–3021. [Google Scholar] [CrossRef]
- Rassy, E.; Parent, P.; Lefort, F.; Boussios, S.; Baciarello, G.; Pavlidis, N. New rising entities in cancer of unknown primary: Is there a real therapeutic benefit? Crit. Rev. Oncol. Hematol. 2020, 147, 102882. [Google Scholar] [CrossRef]
- Spigel, D.R.; Hainsworth, J.D.; Greco, F.A. Neuroendocrine carcinoma of unknown primary site. Semin. Oncol. 2009, 36, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Alexandraki, K.; Angelousi, A.; Boutzios, G.; Kyriakopoulos, G.; Rontogianni, D.; Kaltsas, G. Management of neuroendocrine tumors of unknown primary. Rev. Endocr. Metab. Disord. 2017, 18, 423–431. [Google Scholar] [CrossRef]
- Stoyianni, A.; Pentheroudakis, G.; Pavlidis, N. Neuroendocrine carcinoma of unknown primary: A systematic review of the literature and a comparative study with other neuroendocrine tumors. Cancer Treat. Rev. 2011, 37, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Catena, L.; Bichisao, E.; Milione, M.; Valente, M.; Platania, M.; Pusceddu, S.; Ducceschi, M.; Zilembo, N.; Formisano, B.; Bajetta, E. Neuroendocrine tumors of unknown primary site: Gold dust or misdiagnosed neoplasms? Tumori 2011, 97, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Capdevila, J.; Crespo-Herrero, G.; Diaz-Perez, J.A.; Martinez Del Prado, M.P.; Alonso Orduna, V.; Sevilla-Garcia, I.; Villabona-Artero, C.; Beguiristain-Gomez, A.; Llanos-Munoz, M.; et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): Results from the National Cancer Registry of Spain (RGETNE). Ann. Oncol. 2010, 21, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Berner, A.M.; Pipinikas, C.; Ryan, A.; Dibra, H.; Moghul, I.; Webster, A.; Luong, T.V.; Thirlwell, C. Diagnostic Approaches to Neuroendocrine Neoplasms of Unknown Primary Site. Neuroendocrinology 2020, 110, 563–573. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Chauhan, A.; Rau, J.; Diebold, A.E.; Opoku-Boateng, A.; Ramcharan, T.; Boudreaux, J.P.; Woltering, E.A. Neuroendocrine tumors (NETs) of unknown primary: Is early surgical exploration and aggressive debulking justifiable? Chin. Clin. Oncol. 2016, 5, 4. [Google Scholar] [CrossRef]
- Pavel, M.; Valle, J.W.; Eriksson, B.; Rinke, A.; Caplin, M.; Chen, J.; Costa, F.; Falkerby, J.; Fazio, N.; Gorbounova, V.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms: Systemic Therapy—Biotherapy and Novel Targeted Agents. Neuroendocrinology 2017, 105, 266–280. [Google Scholar] [CrossRef]
- Park, J.S.; Yim, J.J.; Kang, W.J.; Chung, J.K.; Yoo, C.G.; Kim, Y.W.; Han, S.K.; Shim, Y.S.; Lee, S.M. Detection of primary sites in unknown primary tumors using FDG-PET or FDG-PET/CT. BMC Res. Notes 2011, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Greco, F.A. Cancer of Unknown Primary Site: New Treatment Paradigms in the Era of Precision Medicine. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Zaun, G.; Schuler, M.; Herrmann, K.; Tannapfel, A. CUP Syndrome-Metastatic Malignancy with Unknown Primary Tumor. Dtsch. Arztebl. Int. 2018, 115, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Conway, A.M.; Mitchell, C.; Kilgour, E.; Brady, G.; Dive, C.; Cook, N. Molecular characterisation and liquid biomarkers in Carcinoma of Unknown Primary (CUP): Taking the ‘U’ out of ‘CUP’. Br. J. Cancer 2019, 120, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Le, N.S.; Grasl, S.; Janik, S.; Simmel, H.; Schratter-Sehn, A.U.; Hamzavi, J.S.; Franz, P.; Erovic, B.M. Carcinoma of Unknown Primary (CUP) versus CUP Turned to Primary Carcinoma of the Head and Neck-An Analysis of Diagnostic Methods and the Impact of Primary Tumor on Clinical Outcome. Diagnostics 2022, 12, 894. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.F.; Assenov, Y.; Martin-Subero, J.I.; Balint, B.; Siebert, R.; Taniguchi, H.; Yamamoto, H.; Hidalgo, M.; Tan, A.C.; Galm, O.; et al. A DNA methylation fingerprint of 1628 human samples. Genome Res. 2012, 22, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Moran, S.; Martinez-Cardus, A.; Sayols, S.; Musulen, E.; Balana, C.; Estival-Gonzalez, A.; Moutinho, C.; Heyn, H.; Diaz-Lagares, A.; de Moura, M.C.; et al. Epigenetic profiling to classify cancer of unknown primary: A multicentre, retrospective analysis. Lancet Oncol. 2016, 17, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Moran, S.; Martinez-Cardus, A.; Boussios, S.; Esteller, M. Precision medicine based on epigenomics: The paradigm of carcinoma of unknown primary. Nat. Rev. Clin. Oncol. 2017, 14, 682–694. [Google Scholar] [CrossRef]
- Massimino, K.P.; Han, E.; Pommier, S.J.; Pommier, R.F. Laparoscopic surgical exploration is an effective strategy for locating occult primary neuroendocrine tumors. Am. J. Surg. 2012, 203, 628–631. [Google Scholar] [CrossRef]
- Rinke, A.; Wiedenmann, B.; Auernhammer, C.; Bartenstein, P.; Bartsch, D.K.; Begum, N.; Faiss, S.; Fottner, C.; Gebauer, B.; Goretzki, P.; et al. S2k-Leitlinie Neuroendokrine Tumore. Z. Gastroenterol. 2018, 56, 583–681. [Google Scholar]
- Wong, B.; Vickers, M.M.; Wheatley-Price, P. The Diminishing Importance of Primary Site Identification in Cancer of Unknown Primary: A Canadian Single-Center Experience. Front. Oncol. 2021, 11, 634563. [Google Scholar] [CrossRef] [PubMed]
- Juhlin, C.C.; Zedenius, J.; Hoog, A. Metastatic Neuroendocrine Neoplasms of Unknown Primary: Clues from Pathology Workup. Cancers 2022, 14, 2210. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Erlenbach-Wunsch, K.; Konukiewitz, B.; Schmitt, A.M.; Rieker, R.J.; Vieth, M.; Kiesewetter, F.; Hartmann, A.; Zamboni, G.; Perren, A.; et al. ISL1 expression is not restricted to pancreatic well-differentiated neuroendocrine neoplasms, but is also commonly found in well and poorly differentiated neuroendocrine neoplasms of extrapancreatic origin. Mod. Pathol. 2013, 26, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Juhlin, C.C.; Zedenius, J.; Hoog, A. Clinical Routine Application of the Second-generation Neuroendocrine Markers ISL1, INSM1, and Secretagogin in Neuroendocrine Neoplasia: Staining Outcomes and Potential Clues for Determining Tumor Origin. Endocr. Pathol. 2020, 31, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Bocchini, M.; Nicolini, F.; Severi, S.; Bongiovanni, A.; Ibrahim, T.; Simonetti, G.; Grassi, I.; Mazza, M. Biomarkers for Pancreatic Neuroendocrine Neoplasms (PanNENs) Management—An Updated Review. Front. Oncol. 2020, 10, 831. [Google Scholar] [CrossRef] [PubMed]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.B.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.J.; Kjaer, A.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar] [CrossRef] [PubMed]
- Velayoudom-Cephise, F.L.; Duvillard, P.; Foucan, L.; Hadoux, J.; Chougnet, C.N.; Leboulleux, S.; Malka, D.; Guigay, J.; Goere, D.; Debaere, T.; et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr. Relat. Cancer 2013, 20, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Heetfeld, M.; Chougnet, C.N.; Olsen, I.H.; Rinke, A.; Borbath, I.; Crespo, G.; Barriuso, J.; Pavel, M.; O’Toole, D.; Walter, T.; et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2015, 22, 657–664. [Google Scholar] [CrossRef]
- Raj, N.; Valentino, E.; Capanu, M.; Tang, L.H.; Basturk, O.; Untch, B.R.; Allen, P.J.; Klimstra, D.S.; Reidy-Lagunes, D. Treatment Response and Outcomes of Grade 3 Pancreatic Neuroendocrine Neoplasms Based on Morphology: Well Differentiated Versus Poorly Differentiated. Pancreas 2017, 46, 296–301. [Google Scholar] [CrossRef]
- Becx, M.N.; Minczeles, N.S.; Brabander, T.; de Herder, W.W.; Nonnekens, J.; Hofland, J. A Clinical Guide to Peptide Receptor Radionuclide Therapy with (177)Lu-DOTATATE in Neuroendocrine Tumor Patients. Cancers 2022, 14, 5792. [Google Scholar] [CrossRef]
- Thang, S.P.; Lung, M.S.; Kong, G.; Hofman, M.S.; Callahan, J.; Michael, M.; Hicks, R.J. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN)—A single-institution retrospective analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.A.; Fazio, N.; Granberg, D.; Grozinsky-Glasberg, S.; Ahmadzadehfar, H.; Grana, C.M.; Zandee, W.T.; Cwikla, J.; Walter, M.A.; Oturai, P.S.; et al. Peptide receptor radionuclide therapy in gastroenteropancreatic NEN G3: A multicenter cohort study. Endocr. Relat. Cancer 2019, 26, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.J.; Ueberroth, B.E.; McGarrah, P.W.; Buckner Petty, S.A.; Kendi, A.T.; Starr, J.; Hobday, T.J.; Halfdanarson, T.R.; Sonbol, M.B. Treatment Outcomes of Well-Differentiated High-Grade Neuroendocrine Tumors. Oncologist 2021, 26, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Johnbeck, C.B.; Knigge, U.; Kjaer, A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: Current status and review of the literature. Future Oncol. 2014, 10, 2259–2277. [Google Scholar] [CrossRef] [PubMed]
- Binderup, T.; Knigge, U.; Loft, A.; Mortensen, J.; Pfeifer, A.; Federspiel, B.; Hansen, C.P.; Hojgaard, L.; Kjaer, A. Functional imaging of neuroendocrine tumors: A head-to-head comparison of somatostatin receptor scintigraphy, 123I-MIBG scintigraphy, and 18F-FDG PET. J. Nucl. Med. 2010, 51, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Has Simsek, D.; Kuyumcu, S.; Turkmen, C.; Sanli, Y.; Aykan, F.; Unal, S.; Adalet, I. Can complementary 68Ga-DOTATATE and 18F-FDG PET/CT establish the missing link between histopathology and therapeutic approach in gastroenteropancreatic neuroendocrine tumors? J. Nucl. Med. 2014, 55, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Bahri, H.; Laurence, L.; Edeline, J.; Leghzali, H.; Devillers, A.; Raoul, J.L.; Cuggia, M.; Mesbah, H.; Clement, B.; Boucher, E.; et al. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: A long-term evaluation. J. Nucl. Med. 2014, 55, 1786–1790. [Google Scholar] [CrossRef]
- Kayani, I.; Conry, B.G.; Groves, A.M.; Win, T.; Dickson, J.; Caplin, M.; Bomanji, J.B. A comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in pulmonary neuroendocrine tumors. J. Nucl. Med. 2009, 50, 1927–1932. [Google Scholar] [CrossRef]
- Squires, M.H., III; Volkan Adsay, N.; Schuster, D.M.; Russell, M.C.; Cardona, K.; Delman, K.A.; Winer, J.H.; Altinel, D.; Sarmiento, J.M.; El-Rayes, B.; et al. Octreoscan Versus FDG-PET for Neuroendocrine Tumor Staging: A Biological Approach. Ann. Surg. Oncol. 2015, 22, 2295–2301. [Google Scholar] [CrossRef]
- Bartlett, E.K.; Roses, R.E.; Gupta, M.; Shah, P.K.; Shah, K.K.; Zaheer, S.; Wachtel, H.; Kelz, R.R.; Karakousis, G.C.; Fraker, D.L. Surgery for metastatic neuroendocrine tumors with occult primaries. J. Surg. Res. 2013, 184, 221–227. [Google Scholar] [CrossRef]
- Wang, S.C.; Parekh, J.R.; Zuraek, M.B.; Venook, A.P.; Bergsland, E.K.; Warren, R.S.; Nakakura, E.K. Identification of unknown primary tumors in patients with neuroendocrine liver metastases. Arch. Surg. 2010, 145, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Manguso, N.; Gangi, A.; Johnson, J.; Harit, A.; Nissen, N.; Jamil, L.; Lo, S.; Wachsman, A.; Hendifar, A.; Amersi, F. The role of pre-operative imaging and double balloon enteroscopy in the surgical management of small bowel neuroendocrine tumors: Is it necessary? J. Surg. Oncol. 2018, 117, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Bellutti, M.; Fry, L.C.; Schmitt, J.; Seemann, M.; Klose, S.; Malfertheiner, P.; Monkemuller, K. Detection of neuroendocrine tumors of the small bowel by double balloon enteroscopy. Dig. Dis. Sci. 2009, 54, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Van Tuyl, S.A.; van Noorden, J.T.; Timmer, R.; Stolk, M.F.; Kuipers, E.J.; Taal, B.G. Detection of small-bowel neuroendocrine tumors by video capsule endoscopy. Gastrointest. Endosc. 2006, 64, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Smith, G.; Clift, A.K.; Martin, J. Capsule endoscopy to detect primary tumour site in metastatic neuroendocrine tumours. Dig. Liver Dis. 2014, 46, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Oberg, K.; Couvelard, A.; Delle Fave, G.; Gross, D.; Grossman, A.; Jensen, R.T.; Pape, U.F.; Perren, A.; Rindi, G.; Ruszniewski, P.; et al. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Biochemical Markers. Neuroendocrinology 2017, 105, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Bajetta, E.; Ferrari, L.; Martinetti, A.; Celio, L.; Procopio, G.; Artale, S.; Zilembo, N.; Di Bartolomeo, M.; Seregni, E.; Bombardieri, E. Chromogranin A, neuron specific enolase, carcinoembryonic antigen, and hydroxyindole acetic acid evaluation in patients with neuroendocrine tumors. Cancer 1999, 86, 858–865. [Google Scholar] [CrossRef]
- Carling, R.S.; Degg, T.J.; Allen, K.R.; Bax, N.D.; Barth, J.H. Evaluation of whole blood serotonin and plasma and urine 5-hydroxyindole acetic acid in diagnosis of carcinoid disease. Ann. Clin. Biochem. 2002, 39, 577–582. [Google Scholar] [CrossRef]
- Lindholm, D.P.; Oberg, K. Biomarkers and molecular imaging in gastroenteropancreatic neuroendocrine tumors. Horm. Metab. Res. 2011, 43, 832–837. [Google Scholar] [CrossRef]
- Korse, C.M.; Taal, B.G.; Vincent, A.; van Velthuysen, M.L.; Baas, P.; Buning-Kager, J.C.; Linders, T.C.; Bonfrer, J.M. Choice of tumour markers in patients with neuroendocrine tumours is dependent on the histological grade. A marker study of Chromogranin A, Neuron specific enolase, Progastrin-releasing peptide and cytokeratin fragments. Eur. J. Cancer 2012, 48, 662–671. [Google Scholar] [CrossRef]
- Sherman, S.K.; Maxwell, J.E.; O’Dorisio, M.S.; O’Dorisio, T.M.; Howe, J.R. Pancreastatin predicts survival in neuroendocrine tumors. Ann. Surg. Oncol. 2014, 21, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Mamikunian, P.; Ardill, J.E.; O’Dorisio, T.M.; Krutzik, S.R.; Vinik, A.I.; Go, V.L.; Armstrong, L.; Mamikunian, G.; Woltering, E.A. Validation of neurokinin a assays in the United States and Europe. Pancreas 2011, 40, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Ardill, J.E.; O’Dorisio, T.M. Circulating biomarkers in neuroendocrine tumors of the enteropancreatic tract: Application to diagnosis, monitoring disease, and as prognostic indicators. Endocrinol. Metab. Clin. North Am. 2010, 39, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Hinterleitner, M.; Sipos, B.; Wagner, V.; Grottenthaler, J.M.; Lauer, U.M.; Zender, L.; Hinterleitner, C. Platelet-Expressed Synaptophysin (pSyn) as Novel Biomarker in Neuroendocrine Malignancies. Cancers 2021, 13, 2286. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Frilling, A.; Salem, R.R.; Alaimo, D.; Drymousis, P.; Wasan, H.S.; Callahan, S.; Faiz, O.; Weng, L.; Teixeira, N.; et al. Blood measurement of neuroendocrine gene transcripts defines the effectiveness of operative resection and ablation strategies. Surgery 2016, 159, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Kirkwood, A.A.; Tsigani, T.; Lowe, H.; Goldstein, R.; Hartley, J.A.; Caplin, M.E.; Meyer, T. Early Changes in Circulating Tumor Cells Are Associated with Response and Survival Following Treatment of Metastatic Neuroendocrine Neoplasms. Clin. Cancer Res. 2016, 22, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Rassy, E.; Labaki, C.; Chebel, R.; Boussios, S.; Smith-Gagen, J.; Greco, F.A.; Pavlidis, N. Systematic review of the CUP trials characteristics and perspectives for next-generation studies. Cancer Treat. Rev. 2022, 107, 102407. [Google Scholar] [CrossRef]
- Begum, N.; Hubold, C.; Buchmann, I.; Thorns, C.; Bouchard, R.; Lubienski, A.; Schloricke, E.; Zimmermann, M.; Lehnert, H.; Bruch, H.P.; et al. Diagnostics and therapy for neuroendocrine neoplasia of an unknown primary—A plea for open exploration. Zentralbl. Chir. 2014, 139, 284–291. [Google Scholar] [CrossRef]
- Begum, N.; Wellner, U.; Thorns, C.; Brabant, G.; Hoffmann, M.; Burk, C.G.; Lehnert, H.; Keck, T. CUP Syndrome in Neuroendocrine Neoplasia: Analysis of Risk Factors and Impact of Surgical Intervention. World J. Surg. 2015, 39, 1443–1451. [Google Scholar] [CrossRef]
- Thapa, P.; Ranade, R.; Ostwal, V.; Shrikhande, S.V.; Goel, M.; Basu, S. Performance of 177Lu-DOTATATE-based peptide receptor radionuclide therapy in metastatic gastroenteropancreatic neuroendocrine tumor: A multiparametric response evaluation correlating with primary tumor site, tumor proliferation index, and dual tracer imaging characteristics. Nucl. Med. Commun. 2016, 37, 1030–1037. [Google Scholar] [CrossRef]
- Citterio, D.; Pusceddu, S.; Facciorusso, A.; Coppa, J.; Milione, M.; Buzzoni, R.; Bongini, M.; de Braud, F.; Mazzaferro, V. Primary tumour resection may improve survival in functional well-differentiated neuroendocrine tumours metastatic to the liver. Eur. J. Surg. Oncol. 2017, 43, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Bertani, E.; Fazio, N.; Botteri, E.; Chiappa, A.; Falconi, M.; Grana, C.; Bodei, L.; Papis, D.; Spada, F.; Bazolli, B.; et al. Resection of the primary pancreatic neuroendocrine tumor in patients with unresectable liver metastases: Possible indications for a multimodal approach. Surgery 2014, 155, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Capurso, G.; Bettini, R.; Rinzivillo, M.; Boninsegna, L.; Delle Fave, G.; Falconi, M. Role of resection of the primary pancreatic neuroendocrine tumour only in patients with unresectable metastatic liver disease: A systematic review. Neuroendocrinology 2011, 93, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Bertani, E.; Fazio, N.; Radice, D.; Zardini, C.; Grana, C.; Bodei, L.; Funicelli, L.; Ferrari, C.; Spada, F.; Partelli, S.; et al. Resection of the Primary Tumor Followed by Peptide Receptor Radionuclide Therapy as Upfront Strategy for the Treatment of G1-G2 Pancreatic Neuroendocrine Tumors with Unresectable Liver Metastases. Ann. Surg. Oncol. 2016, 23, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Kaemmerer, D.; Twrznik, M.; Kulkarni, H.R.; Horsch, D.; Sehner, S.; Baum, R.P.; Hommann, M.; Center for Neuroendocrine Tumors, Bad Berka—ENETS Center of Excellence. Prior Resection of the Primary Tumor Prolongs Survival After Peptide Receptor Radionuclide Therapy of Advanced Neuroendocrine Neoplasms. Ann. Surg. 2021, 274, e45–e53. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]



| All Patients (%) | NEN-UP (%) | PI (%) | p-Value | |
|---|---|---|---|---|
| number | 113 | 100 (88.5) | 13 (11.5) | |
| Sex | 0.075 | |||
| female | 50 (44.2) | 41 (41) | 9 (69.2) | |
| male | 63 (55.8) | 59 (59) | 4 (30.8) | |
| Age (in years) | 0.2969 | |||
| average | 63.4 | 63.4 | 63.4 | |
| <30 | 2 (1.8) | 2 (2) | 0 (0) | |
| 30–39 | 5 (4.4) | 3 (3) | 2 (15.4) | |
| 40–49 | 8 (7.1) | 7 (7) | 1 (7.7) | |
| 50–59 | 23 (20.4) | 20 (20) | 3 (23.1) | |
| 60–69 | 32 (28.3) | 28 (28) | 4 (30.8) | |
| 70–79 | 35 (31.0) | 33 (33) | 2 (15.4) | |
| ≥80 | 8 (7.1) | 7 (7) | 1 (7.7) | |
| Body-mass-index (kg/m2) | 0.2725 | |||
| average | 25 | 25.3 | 23.4 | |
| Histological Subtype | >0.9999 | |||
| NET G1 | 16 (14.2) | 15 (15) | 1 (7.7) | |
| NET G2 | 36 (31.9) | 32 (32) | 4 (30.8) | |
| NET G3 | 18 (15.9) | 15 (15) | 3 (23.1) | |
| NEC | 43 (38.1) | 38 (38) | 5 (38.5) | |
| Proliferation index | 0.4614 | |||
| Average (range) | 36% (1–100%) | 35% (1–100%) | 36% (1–90%) | |
| Metastatic sites | 0.7292 | |||
| liver | 77 (68.1) | 65 (65) | 12 (92.3) | |
| lung | 26 (23) | 24 (24) | 2 (15.4) | |
| bones | 33 (29.2) | 30 (30) | 3 (23.1) | |
| lymph nodes | 61 (54) | 54 (54) | 7 (53.8) | |
| brain | 8 (7.1) | 8 (8) | 0 (0) | |
| others | 39 (34.5) | 34 (34) | 5 (38.5) | |
| Number of metastatic sites | ||||
| 1 | 38 (33.6) | 36 (36) | 2 (15.4) | 0.4225 |
| 2 | 34 (30.1) | 29 (29) | 5 (38.5) | |
| 3 | 20 (17.7) | 17 (17) | 3 (23.1) | |
| 4 | 15 (13.3) | 12 (12) | 3 (23.1) | |
| ≥5 | 6 (5.3) | 6 (6) | 0 (0) | |
| Origin of initial histology | 0.0466 | |||
| liver | 65 (57.5) | 57 (57) | 8 (61.5) | |
| lung | 1 (0.8) | 0 (0) | 1 (7.7) | |
| genitourinary | 6 (5.3) | 4 (4) | 2 (15.4) | |
| gastrointestinal | 9 (8) | 8 (8) | 1 (7.7) | |
| skin | 3 (2.7) | 2 (2) | 1 (7.7) | |
| lymph node | 18 (15.9) | 18 (18) | 0 (0) | |
| central nervous system | 4 (3.5) | 4 (4) | 0 (0) | |
| musculoskeletal | 4 (3.5) | 4 (4) | 0 (0) | |
| others | 3 (2.7) | 3 (3) | 0 (0) | |
| Date of initial diagnosis | 0.1898 | |||
| 2010–2014 | 17 (15) | 15 (15) | 2 (15.4) | |
| 2015–2019 | 51 (45.1) | 48 (48) | 3 (23.1) | |
| 2020–2023 | 45 (39.8) | 37 (37) | 8 (61.5) |
| All Patients (%) | NEN-UP (%) | PI (%) | p-Value | |
|---|---|---|---|---|
| Number | 113 | 100 (88.5) | 13 (11.5) | |
| Bloodwork | ||||
| CgA | 75 (66.4) | 65 (65) | 10 (76.9) | 0.538 |
| NSE | 60 (53.1) | 48 (48) | 12 (92.3) | 0.0025 |
| CEA | 28 (24.8) | 26 (26) | 2 (15.4) | 0.5134 |
| CA19-9 | 29 (25.7) | 26 (26) | 3 (23.1) | >0.9999 |
| LDH | 87 (77) | 74 (74) | 13 (100) | 0.037 |
| WBC | 89 (78.8) | 76 (76) | 13 (100) | 0.0669 |
| ANC | 73 (64.6) | 61 (61) | 12 (92.3) | 0.0307 |
| ALC | 73 (64.6) | 61 (61) | 12 (92.3) | 0.0307 |
| Hb | 89 (78.8) | 76 (76) | 13 (100) | 0.0669 |
| PLT | 88 (77.9) | 75 (75) | 13 (100) | 0.0686 |
| Urinalysis | ||||
| 24 h 5-hydroxyindoleacetic acid | 6 (5.3) | 6 (6) | 0 (0) | >0.9999 |
| Immunohistochemistry | ||||
| Synaptophysin | 92 (81.4) | 79 (79) | 13 (100) | 0.1223 |
| CgA | 74 (65.5) | 62 (62) | 12 (92.3) | 0.0326 |
| CD56 | 41 (36.3) | 37 (37) | 4 (30.8) | 0.7664 |
| CDX2 | 66 (58.4) | 56 (56) | 10 (76.9) | 0.2319 |
| TTF-1 | 76 (67.3) | 68 (68) | 8 (61.5) | 0.7549 |
| ISLET-1 | 40 (35.4) | 34 (34) | 6 (46.2) | 0.5385 |
| Serotonin | 18 (15.9) | 16 (16) | 2 (15.4) | >0.9999 |
| Gastrin | 3 (2.7) | 3 (3) | 0 (0) | >0.9999 |
| CK20 | 29 (25.7) | 29 (29) | 0 (0) | 0.0203 |
| Adherence to IHC guidelines | 0.4942 | |||
| Full adherence (5 out of 5 markers) | 32 (28.3) | 27 (27) | 5 (38.5) | |
| Partial adherence (3 or 4 markers) | 40 (35.4) | 35 (35) | 5 (38.5) | |
| Minimal adherence (1 or 2 markers) | 27 (23.9) | 24 (24) | 3 (23.1) | |
| No adherence (no marker) | 14 (12.4) | 14 (14) | 0 (0) | |
| Imaging | ||||
| FDG-PET | 24 (21.2) | 23 (23) | 1 (7.7) | 0.2935 |
| SSTR-PET | 71 (62.8) | 62 (62) | 9 (69.2) | 0.7643 |
| Endoscopy | 0.6438 | |||
| Gastroscopy | 11 (9.7) | 9 (9) | 2 (15.4) | |
| Colonoscopy | 7 (6.2) | 6 (6) | 1 (7.7) | |
| Gastroscopy + Colonoscopy | 42 (37.2) | 36 (36) | 6 (46.2) | |
| no endoscopy | 53 (46.9) | 49 (49) | 4 (30.8) | |
| NGS | ||||
| 31 (27.4) | 26 (26) | 5 (38.5) | 0.3398 | |
| CIN | ||||
| 30 (26.5) | 25 (25) | 5 (38.5) | 0.3253 | |
| Diagnostic lines § | 0.5673 | |||
| 0 | 3 (2.7) | 3 (3) | 0 (0) | |
| 1 | 11 (9.7) | 11 (11) | 0 (0) | |
| 2 | 25 (22.1) | 23 (23) | 2 (15.4) | |
| 3 | 34 (30.1) | 29 (29) | 5 (38.5) | |
| 4 | 40 (35.4) | 34 (34) | 6 (46.2) |
| All Patients (%) | NET | NEC | p-Value | |
|---|---|---|---|---|
| Number | 113 | 70 (61.9) | 43 (38.1) | |
| Bloodwork | ||||
| CgA | 75 (66.4) | 53 (75.7) | 22 (51.2) | 0.0132 |
| NSE | 60 (53.1) | 35 (50) | 25 (58.1) | 0.4416 |
| CEA | 28 (24.8) | 15 (21.4) | 13 (30.2) | 0.3701 |
| CA19-9 | 29 (25.7) | 17 (24.3) | 12 (27.9) | 0.6649 |
| LDH | 87 (77) | 55 (78.6) | 32 (74.4) | 0.6496 |
| WBC | 89 (78.8) | 56 (80) | 33 (76.7) | 0.8132 |
| ANC | 73 (64.6) | 42 (60) | 31 (72.1) | 0.2273 |
| ALC | 73 (64.6) | 42 (60) | 31 (72.1) | 0.2273 |
| Hb | 89 (78.8) | 56 (80) | 33 (76.7) | 0.8132 |
| PLT | 88 (77.9) | 55 (78.6) | 33 (76.7) | 0.8197 |
| Urinalysis | ||||
| 24 h urinary 5-hydroxyindoleacetic acid | 6 (5.3) | 6 (8.6) | 0 (0) | >0.9999 |
| Immunohistochemistry | ||||
| Synaptophysin | 92 (81.4) | 55 (78.6) | 37 (86) | 0.4557 |
| CgA | 74 (65.5) | 49 (62) | 25 (58.1) | 0.2251 |
| CD56 | 41 (36.3) | 18 (25.7) | 23 (53.5) | 0.0052 |
| CDX2 | 66 (58.4) | 44 (62.9) | 22 (51.2) | 0.2432 |
| TTF-1 | 76 (67.3) | 45 (64.3) | 31 (72.1) | 0.4172 |
| ISLET-1 | 40 (35.4) | 30 (42.9) | 10 (23.3) | 0.0432 |
| Serotonin | 18 (15.9) | 16 (22.9) | 2 (4.7) | 0.0152 |
| Gastrin | 3 (2.7) | 3 (4.9) | 0 (0) | 0.2865 |
| CK20 | 29 (25.7) | 14 (20) | 15 (34.9) | 0.1195 |
| Adherence to IHC guidelines | 0.0797 | |||
| Full adherence (5 out of 5 markers) | 32 (28.3) | 25 (35.7) | 7 (16.3) | |
| Partial adherence (3 or 4 markers) | 40 (35.4) | 21 (30) | 19 (44.2) | |
| Minimal adherence (1 or 2 markers) | 27 (23.9) | 14 (20) | 13 (30.2) | |
| No adherence (no marker) | 14 (12.4) | 10 (14.3) | 4 (9.3) | |
| Imaging | ||||
| FDG-PET | 24 (21.2) | 8 (11.4) | 16 (37.2) | 0.0011 |
| SSTR-PET | 71 (62.8) | 60 (85.7) | 11 (25.6) | <0.0001 |
| Endoscopy | 0.6482 | |||
| Gastroscopy | 11 (9.7) | 8 (11.4) | 3 (7.0) | |
| Colonoscopy | 7 (6.2) | 4 (5.7) | 3 (7.0) | |
| Gastroscopy + Colonoscopy | 42 (37.2) | 28 (40) | 14 (32.6) | |
| no endoscopy | 53 (46.9) | 30 (42.9) | 23 (53.5) | |
| NGS | ||||
| 31 (27.4) | 15 (21.4) | 16 (37.2) | 0.0679 | |
| CIN | ||||
| 30 (26.5) | 15 (21.4) | 15 (34.9) | 0.1296 | |
| Diagnostic lines § | 0.0334 | |||
| 0 | 3 (2.7) | 0 (0) | 3 (7) | |
| 1 | 11 (9.7) | 6 (8.6) | 5 (11.6) | |
| 2 | 25 (22.1) | 15 (21.4) | 10 (23.3) | |
| 3 | 34 (30.1) | 18 (25.7) | 16 (37.2) | |
| 4 | 40 (35.4) | 31 (44.3) | 9 (20.9) |
| Diagnostic Test | Odds Ratio (95% CI) | p-Value |
|---|---|---|
| Blood work | ||
| CgA | 1.80 (0.51–8.39) | 0.397 |
| NSE | 13 (2.42–241.2) | 0.016 |
| LDH | x | x |
| Endoscopy | ||
| Any form of endoscopy | 2.16 (0.66–8.40) | 0.224 |
| Gastroscopy | 1.84 (0.26–8.37) | 0.471 |
| Colonoscopy | 1.31 (0.07–8.60) | 0.812 |
| Gastroscopy + Colonoscopy | 1.52 (0.46–4.93) | 0.478 |
| Imaging | ||
| PET-based imaging | 0.83 (0.25–3.27) | 0.775 |
| SSTR-PET | 1.38 (0.42–5.37) | 0.613 |
| FDG-PET | x | x |
| SSTR- and FDG-PET | 0.61 (0.03–3.56) | 0.65 |
| Diagnostic Lines | 1.67 (0.92–3.47) | 0.122 |
| Immunohistochemistry | ||
| Adherence to IHC guidelines | 1.91 (1.21–3.48) | 0.015 |
| Synaptophysin | x | x |
| CgA | 7.36 (1.37–136.6) | 0.06 |
| CD56 | 0.757 (0.19–2.50) | 0.661 |
| CDX2 | 2.62 (0.75–12.2) | 0.162 |
| TTF-1 | 0.753 (0.23–2.66) | 0.641 |
| ISLET-1 | 1.664 (0.50–5.39) | 0.392 |
| Serotonin | 0.955 (0.14–4.01) | 0.955 |
| Gastrin | x | x |
| CK20 | x | x |
| NGS | 1.78 (0.50–5.83) | 0.348 |
| All Patients (%) | NEN-UP (%) | PI (%) | p-Value | |
|---|---|---|---|---|
| Treatment regimen | ||||
| Systemic Therapy | ||||
| Chemotherapy | 58 (51.3) | 49 (49) | 9 (69.2) | 0.24 |
| SSA | 42 (37.2) | 35 (35) | 7 (53.8) | 0.2274 |
| Radiation Therapy | ||||
| Local radiotherapy | 26 (23.0) | 24 (24) | 2 (15.4) | 0.7291 |
| PRRT | 25 (22.1) | 18 (18) | 7 (53.8) | 0.008 |
| SIRT | 4 (3.5) | 3 (3) | 1 (7.7) | 0.391 |
| Local therapy | ||||
| Surgery | 39 (34.5) | 32 (32) | 7 (53.8) | 0.1326 |
| RFA | 1 (0.9) | 1 (1) | 0 (0) | >0.9999 |
| BSC | 8 (7.1) | 7 (7) | 1 (7.7) | >0.9999 |
| Lines of systemic therapy | 0.0127 | |||
| 1 | 55 (48.7) | 51 (51) | 4 (30.8) | |
| 2 | 17 (15) | 11 (11) | 6 (46.2) | |
| 3 | 14 (12.4) | 13 (13) | 1 (7.7) | |
| 4+ | 8 (4.4) | 5 (5) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, M.; Hinterleitner, C.; Singer, S.; Lauer, U.M.; Zender, L.; Hinterleitner, M. Diagnostic Approaches for Neuroendocrine Neoplasms of Unknown Primary (NEN-UPs) and Their Prognostic Relevance—A Retrospective, Long-Term Single-Center Experience. Cancers 2023, 15, 4316. https://doi.org/10.3390/cancers15174316
Schmidt M, Hinterleitner C, Singer S, Lauer UM, Zender L, Hinterleitner M. Diagnostic Approaches for Neuroendocrine Neoplasms of Unknown Primary (NEN-UPs) and Their Prognostic Relevance—A Retrospective, Long-Term Single-Center Experience. Cancers. 2023; 15(17):4316. https://doi.org/10.3390/cancers15174316
Chicago/Turabian StyleSchmidt, Moritz, Clemens Hinterleitner, Stephan Singer, Ulrich M. Lauer, Lars Zender, and Martina Hinterleitner. 2023. "Diagnostic Approaches for Neuroendocrine Neoplasms of Unknown Primary (NEN-UPs) and Their Prognostic Relevance—A Retrospective, Long-Term Single-Center Experience" Cancers 15, no. 17: 4316. https://doi.org/10.3390/cancers15174316
APA StyleSchmidt, M., Hinterleitner, C., Singer, S., Lauer, U. M., Zender, L., & Hinterleitner, M. (2023). Diagnostic Approaches for Neuroendocrine Neoplasms of Unknown Primary (NEN-UPs) and Their Prognostic Relevance—A Retrospective, Long-Term Single-Center Experience. Cancers, 15(17), 4316. https://doi.org/10.3390/cancers15174316





