Patient Preferences in Metastatic Breast Cancer Care: A Scoping Review
Abstract
:Simple Summary
Abstract
1. Introduction
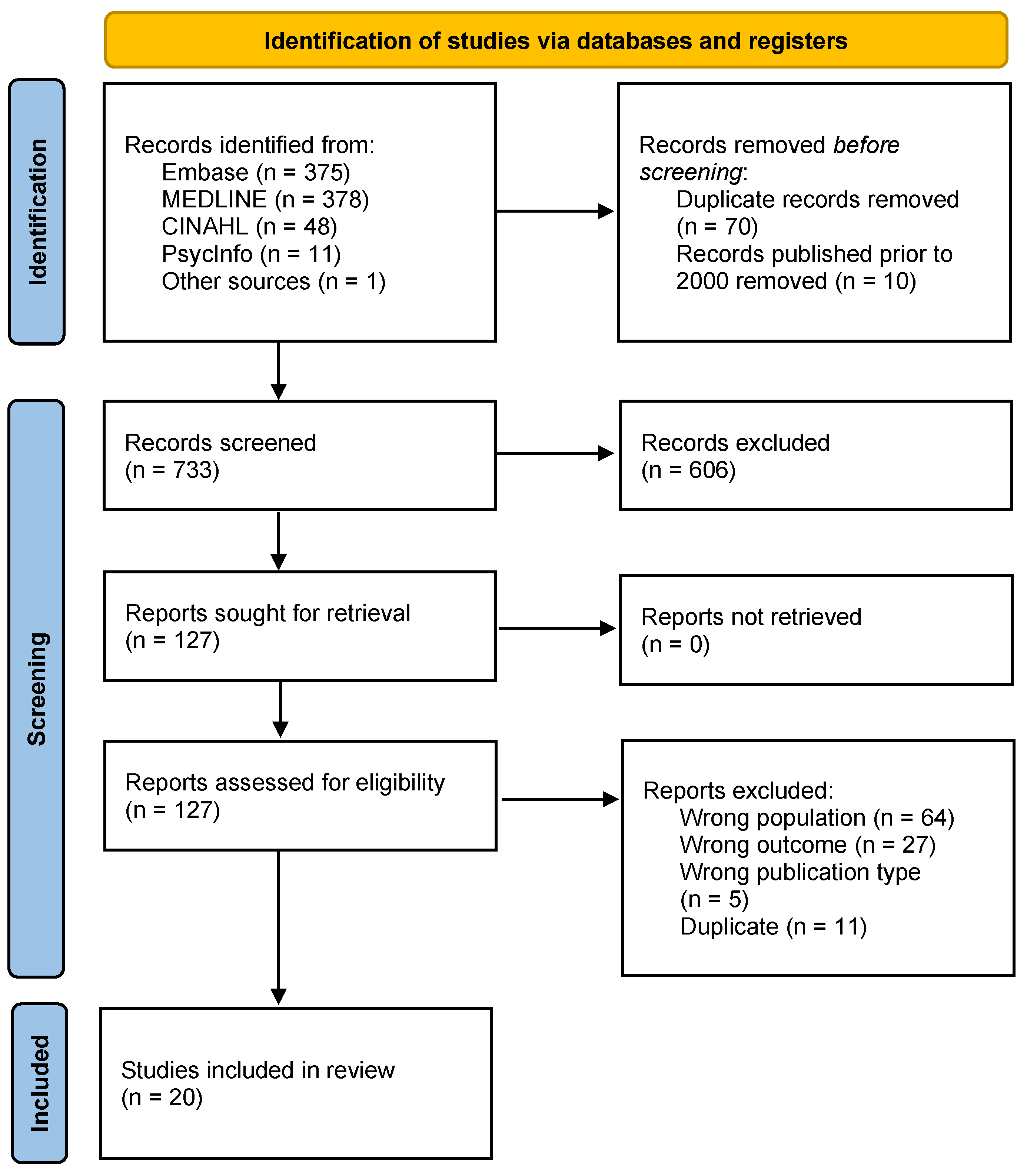
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Analysis
3. Results
3.1. Treatment Preferences
3.2. Communication and Decision-Making Preferences
3.3. Supportive Care Preferences
4. Discussion
4.1. Study Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Harbeck, N.; Mertz, S.; Fenech, D. Evolving Psychosocial, Emotional, Functional, and Support Needs of Women with Advanced Breast Cancer: Results from the Count Us, Know Us, Join Us and Here & Now Surveys. Breast 2016, 28, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288. [Google Scholar] [CrossRef]
- Budhwani, S.; Moineddin, R.; Wodchis, W.P.; Zimmermann, C.; Howell, D. Longitudinal Symptom Burden Trajectories in a Population-Based Cohort of Women with Metastatic Breast Cancer: A Group-Based Trajectory Modeling Analysis. Curr. Oncol. 2021, 28, 879–897. [Google Scholar] [CrossRef]
- Cleeland, C.; Von Moos, R.; Walker, M.S.; Wang, Y.; Gao, J.; Chavez-MacGregor, M.; Liede, A.; Arellano, J.; Balakumaran, A.; Qian, Y. Burden of Symptoms Associated with Development of Metastatic Bone Disease in Patients with Breast Cancer. Support. Care Cancer 2016, 24, 3557–3565. [Google Scholar] [CrossRef] [PubMed]
- Hamer, J.; McDonald, R.; Zhang, L.; Verma, S.; Leahey, A.; Ecclestone, C.; Bedard, G.; Pulenzas, N.; Bhatia, A.; Chow, R.; et al. Quality of Life (QOL) and Symptom Burden (SB) in Patients with Breast Cancer. Support. Care Cancer 2017, 25, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Kenne Sarenmalm, E.; Öhlén, J.; Jonsson, T.; Gaston-Johansson, F. Coping with Recurrent Breast Cancer: Predictors of Distressing Symptoms and Health-Related Quality of Life. J. Pain Symptom Manag. 2007, 34, 24–39. [Google Scholar] [CrossRef]
- Fraenkel, L. Incorporating Patients’ Preferences Into Medical Decision Making. Med. Care Res. Rev. 2013, 70, 80S–93S. [Google Scholar] [CrossRef]
- Ryan, M. Using Conjoint Analysis to Elicit Preferences for Health Care. BMJ 2000, 320, 1530–1533. [Google Scholar] [CrossRef]
- Ryan, M.; Gerard, K. Using Discrete Choice Experiments to Value Health Care Programmes: Current Practice and Future Research Reflections. Appl. Health Econ. Health Policy 2003, 2, 55–64. [Google Scholar]
- Guerra, R.L.; Castaneda, L.; de Albuquerque, R.d.C.R.; Ferreira, C.B.T.; Corrêa, F.d.M.; Fernandes, R.R.A.; de Almeida, L.M. Patient Preferences for Breast Cancer Treatment Interventions: A Systematic Review of Discrete Choice Experiments. Patient 2019, 12, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Hamelinck, V.C.; Bastiaannet, E.; Pieterse, A.H.; Jannink, I.; van de Velde, C.J.H.; Liefers, G.-J.; Stiggelbout, A.M. Patients’ Preferences for Surgical and Adjuvant Systemic Treatment in Early Breast Cancer: A Systematic Review. Cancer Treat. Rev. 2014, 40, 1005–1018. [Google Scholar] [CrossRef]
- Duric, V.; Stockler, M. Patients’ Preferences for Adjuvant Chemotherapy in Early Breast Cancer: A Review of What Makes It Worthwhile? Lancet Oncol. 2001, 2, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Minami, C.A.; King, T.A.; Mittendorf, E.A. Patient Preferences for Locoregional Therapy in Early-Stage Breast Cancer. Breast Cancer Res. Treat. 2020, 183, 291–309. [Google Scholar] [CrossRef]
- Lafranconi, A.; Pylkkänen, L.; Deandrea, S.; Bramesfeld, A.; Lerda, D.; Neamțiu, L.; Saz-Parkinson, Z.; Posso, M.; Rigau, D.; Sola, I.; et al. Intensive Follow-up for Women with Breast Cancer: Review of Clinical, Economic and Patient’s Preference Domains through Evidence to Decision Framework. Health Qual. Life Outcomes 2017, 15, 206. [Google Scholar] [CrossRef] [PubMed]
- Cherny, N.I.; Paluch-Shimon, S.; Berner-Wygoda, Y. Palliative Care: Needs of Advanced Breast Cancer Patients. BCTT 2018, 10, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Decker, T.; Soling, U.; Hahn, A.; Maintz, C.; Kurbacher, C.M.; Vehling-Kaiser, U.; Sent, D.; Klare, P.; Hagen, V.; Chiabudini, M.; et al. Final Results from IMPROVE: A Randomized, Controlled, Open-Label, Two-Arm, Cross-over Phase IV Study to Determine Patients’ Preference for Everolimus in Combination with Exemestane or Capecitabine in Combination with Bevacizumab in Advanced HR-Positive, HER2-Negative Breast Cancer. BMC Cancer 2020, 20, 286. [Google Scholar] [CrossRef]
- Ciruelos, E.M.; Montano, A.; Rodriguez, C.A.; Gonzalez-Flores, E.; Lluch, A.; Garrigos, L.; Quiroga, V.; Anton, A.; Malon, D.; Chacon, J.I.; et al. Phase III Study to Evaluate Patient’s Preference of Subcutaneous versus Intravenous Trastuzumab in HER2-Positive Metastatic Breast Cancer Patients: Results from the ChangHER Study (GEICAM/2012-07). Eur. J. Cancer Care 2020, 29, e13253. [Google Scholar] [CrossRef]
- Pivot, X.; Spano, J.P.; Espie, M.; Cottu, P.; Jouannaud, C.; Pottier, V.; Moreau, L.; Extra, J.M.; Lortholary, A.; Rivera, P.; et al. Patients’ Preference of Trastuzumab Administration (Subcutaneous versus Intravenous) in HER2-Positive Metastatic Breast Cancer: Results of the Randomised MetaspHer Study. Eur. J. Cancer Care 2017, 82, 230–236. [Google Scholar] [CrossRef]
- Amin, S.; Tolaney, S.M.; Janelle Cambron-Mellott, M.; Beusterien, K.; MacUlaitis, M.C.; Mulvihill, E.; Shinde, R.; McLaurin, K. Benefit-Risk Trade-Offs in Treatment Choice in Advanced HER2 Negative Breast Cancer: Patient and Oncologist Perspectives. Future Oncol. 2022, 18, 1927–1941. [Google Scholar] [CrossRef]
- Maculaitis, M.C.; Liu, X.; Will, O.; Hanson, M.; McRoy, L.; Berk, A.; Crastnopol, M. Oncologist and Patient Preferences for Attributes of CDK4/6 Inhibitor Regimens for the Treatment of Advanced/Metastatic HR Positive/HER2 Negative Breast Cancer: Discrete Choice Experiment and Best-Worst Scaling. Patient Prefer. Adherence 2020, 14, 2201–2214. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, C.; Botha, W.; Vondeling, G.T.; Klein, K.; Wang, K.; Singh, J.; Hackshaw, M.D. Patient Preferences for Features of HER2-Targeted Treatment of Advanced or Metastatic Breast Cancer: A Discrete-Choice Experiment Study. Breast Cancer 2022, 30, 23–25. [Google Scholar] [CrossRef] [PubMed]
- daCosta DiBonaventura, M.; Copher, R.; Basurto, E.; Faria, C.; Lorenzo, R. Patient Preferences and Treatment Adherence among Women Diagnosed with Metastatic Breast Cancer. Am. Health Drug Benefits 2014, 7, 386–396. [Google Scholar]
- Lalla, D.; Carlton, R.; Santos, E.; Bramley, T.; D’Souza, A. Willingness to Pay to Avoid Metastatic Breast Cancer Treatment Side Effects: Results from a Conjoint Analysis. Springerplus 2014, 3, 350. [Google Scholar] [CrossRef]
- Smith, M.L.; White, C.B.; Railey, E.; Sledge Jr, G.W. Examining and Predicting Drug Preferences of Patients with Metastatic Breast Cancer: Using Conjoint Analysis to Examine Attributes of Paclitaxel and Capecitabine. Breast Cancer Res. Treat. 2014, 145, 83–89. [Google Scholar] [CrossRef]
- Spaich, S.; Kinder, J.; Hetjens, S.; Fuxius, S.; Gerhardt, A.; Sutterlin, M. Patient Preferences Regarding Chemotherapy in Metastatic Breast Cancer-a Conjoint Analysis for Common Taxanes. Front. Oncol. 2018, 8, 535. [Google Scholar] [CrossRef]
- Reinisch, M.; Marschner, N.; Otto, T.; Korfel, A.; Stoffregen, C.; Wockel, A. Patient Preferences: Results of a German Adaptive Choice-Based Conjoint Analysis (Market Research Study Sponsored by Eli Lilly and Company) in Patients on Palliative Treatment for Advanced Breast Cancer. Breast Care 2021, 16, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, M.; Szczylik, C. Oral Treatment of Metastatic Breast Cancer with Capecitabine: What Influences the Decision-Making Process? Eur. J. Cancer Care 2010, 19, 131–136. [Google Scholar] [CrossRef]
- Delrieu, L.; Vallance, J.K.; Morelle, M.; Fervers, B.; Pialoux, V.; Friedenreich, C.; Dufresne, A.; Bachelot, T.; Heudel, P.-E.; Tredan, O.; et al. Physical Activity Preferences before and after Participation in a 6-Month Physical Activity Intervention among Women with Metastatic Breast Cancer. Eur. J. Cancer Care 2020, 29, e13169. [Google Scholar] [CrossRef]
- Fallowfield, L.; Stebbing, J.; Braybrooke, J.; Langridge, C.; Jenkins, V. The Preferences and Experiences of Different Bisphosphonate Treatments in Women with Breast Cancer. Psychooncology 2011, 20, 755–761. [Google Scholar] [CrossRef]
- Ejem, D.; Dionne-Odom, J.N.; Turkman, Y.; Knight, S.J.; Willis, D.; Kaufman, P.A.; Bakitas, M. Incongruence between Women’s Survey- and Interview-Determined Decision Control Preferences: A Mixed Methods Study of Decision-Making in Metastatic Breast Cancer. Psychooncology 2018, 27, 1950–1957. [Google Scholar] [CrossRef]
- Rocque, G.B.; Rasool, A.; Williams, B.R.; Wallace, A.S.; Niranjan, S.J.; Halilova, K.I.; Turkman, Y.E.; Ingram, S.A.; Williams, C.P.; Forero-Torres, A.; et al. What Is Important When Making Treatment Decisions in Metastatic Breast Cancer? A Qualitative Analysis of Decision-Making in Patients and Oncologists. Oncologist 2019, 24, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- ten Tusscher, M.R.; Groen, W.G.; Geleijn, E.; Sonke, G.S.; Konings, I.R.; Van der Vorst, M.J.; van Zweeden, A.; Aaronson, N.K.; Stuiver, M.M. Physical Problems, Functional Limitations, and Preferences for Physical Therapist-Guided Exercise Programs among Dutch Patients with Metastatic Breast Cancer: A Mixed Methods Study. Support. Care Cancer 2019, 27, 3061–3070. [Google Scholar] [CrossRef]
- Butow, P.N.; Dowsett, S.; Hagerty, R.; Tattersall, M.H.N. Communicating Prognosis to Patients with Metastatic Disease: What Do They Really Want to Know? Support. Care Cancer 2002, 10, 161–168. [Google Scholar] [CrossRef]
- Niranjan, S.J.; Turkman, Y.; Williams, B.R.; Williams, C.P.; Halilova, K.I.; Smith, T.; Knight, S.J.; Bhatia, S.; Rocque, G.B. “I’d Want to Know, Because a Year’s Not a Long Time to Prepare for a Death”: Role of Prognostic Information in Shared Decision Making among Women with Metastatic Breast Cancer. J. Pallait Med. 2020, 23, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Schulman-Green, D.; Bradley, E.H.; Knobf, M.T.; Prigerson, H.; Digiovanna, M.P.; McCorkle, R.; McCorkle, R. Self-Management and Transitions in Women with Advanced Breast Cancer. J. Pain. Symptom Manag. 2011, 42, 517–525. [Google Scholar] [CrossRef]
- Tometich, D.B.; Hyland, K.A.; Soliman, H.; Jim, H.S.L.; Oswald, L. Living with Metastatic Cancer: A Roadmap for Future Research. Cancers 2020, 12, 3684. [Google Scholar] [CrossRef]
- Omori, Y.; Enatsu, S.; Cai, Z.; Ishiguro, H. Patients’ Preferences for Postmenopausal Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer Treatments in Japan. Breast Cancer 2019, 26, 652–662. [Google Scholar] [CrossRef]
- Shrestha, A.; Martin, C.; Burton, M.; Walters, S.; Collins, K.; Wyld, L. Quality of Life versus Length of Life Considerations in Cancer Patients: A Systematic Literature Review. Psychooncology 2019, 28, 1367–1380. [Google Scholar] [CrossRef]
- Seghers, P.A.L.; Wiersma, A.; Festen, S.; Stegmann, M.E.; Soubeyran, P.; Rostoft, S.; O’Hanlon, S.; Portielje, J.E.A.; Hamaker, M.E. Patient Preferences for Treatment Outcomes in Oncology with a Focus on the Older Patient—A Systematic Review. Cancers 2022, 14, 1147. [Google Scholar] [CrossRef]
- Dhakal, P.; Wichman, C.S.; Pozehl, B.; Weaver, M.; Fisher, A.L.; Vose, J.; Bociek, R.G.; Bhatt, V.R. Preferences of Adults with Cancer for Systemic Cancer Treatment: Do Preferences Differ Based on Age? Future Oncol. 2022, 18, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Fried, T.R.; Bradley, E.H. What Matters to Seriously Ill Older Persons Making End-of-Life Treatment Decisions? A Qualitative Study. J. Pallait. Med. 2003, 6, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Back, A.L. Patient-Clinician Communication Issues in Palliative Care for Patients with Advanced Cancer. J. Clin. Oncol. 2020, 38, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Saracino, R.M.; Polacek, L.C.; Applebaum, A.J.; Rosenfeld, B.; Pessin, H.; Breitbart, W. Health Information Preferences and Curability Beliefs Among Patients with Advanced Cancer. J. Pain Symptom Manag. 2021, 61, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hagerty, R.G.; Butow, P.N.; Ellis, P.M.; Lobb, E.A.; Pendlebury, S.C.; Leighl, N.; Mac Leod, C.; Tattersall, M.H.N. Communicating with Realism and Hope: Incurable Cancer Patients’ Views on the Disclosure of Prognosis. J. Clin. Oncol. 2005, 23, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Mosher, C.E.; Johnson, C.; Dickler, M.; Norton, L.; Massie, M.J.; DuHamel, K. Living with Metastatic Breast Cancer: A Qualitative Analysis of Physical, Psychological, and Social Sequelae. Breast J. 2013, 19, 285–292. [Google Scholar] [CrossRef]
- Keane, D.; Phillips, G.; Mitchell, N.; Connolly, R.M.; Hegarty, J. Improving Quality of Life and Symptom Experience in Patients with Metastatic Breast Cancer: A Systematic Review of Supportive Care Interventions. Psychooncology 2023, 32, 1192–1207. [Google Scholar] [CrossRef]
- Lipsett, A.; Barrett, S.; Haruna, F.; Mustian, K.; O’Donovan, A. The Impact of Exercise during Adjuvant Radiotherapy for Breast Cancer on Fatigue and Quality of Life: A Systematic Review and Meta-Analysis. Breast 2017, 32, 144–155. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Liu, D. Effects of Exercise on the Quality of Life in Breast Cancer Patients: A Systematic Review of Randomized Controlled Trials. Support. Care Cancer 2019, 27, 9–21. [Google Scholar] [CrossRef]
- Gebruers, N.; Camberlin, M.; Theunissen, F.; Tjalma, W.; Verbelen, H.; Van Soom, T.; Van Breda, E. The Effect of Training Interventions on Physical Performance, Quality of Life, and Fatigue in Patients Receiving Breast Cancer Treatment: A Systematic Review. Support. Care Cancer 2019, 27, 109–122. [Google Scholar] [CrossRef]
- Nadler, M.B.; Desnoyers, A.; Langelier, D.M.; Amir, E. The Effect of Exercise on Quality of Life, Fatigue, Physical Function, and Safety in Advanced Solid Tumor Cancers: A Meta-Analysis of Randomized Control Trials. J. Pain Symptom Manag. 2019, 58, 899–908.e7. [Google Scholar] [CrossRef]
- Dittus, K.L.; Gramling, R.E.; Ades, P.A. Exercise Interventions for Individuals with Advanced Cancer: A Systematic Review. Prev. Med. 2017, 104, 124–132. [Google Scholar] [CrossRef]
- Weller, S.; Hart, N.H.; Bolam, K.A.; Mansfield, S.; Santa Mina, D.; Winters-Stone, K.M.; Campbell, A.; Rosenberger, F.; Wiskemann, J.; Quist, M.; et al. Exercise for Individuals with Bone Metastases: A Systematic Review. Crit. Rev. Oncol. Hematol. 2021, 166, 103433. [Google Scholar] [CrossRef]
- Yang, H.-C.; Thornton, L.M.; Shapiro, C.L.; Andersen, B.L. Surviving Recurrence: Psychological and Quality-of-Life Recovery. Cancer 2008, 112, 1178–1187. [Google Scholar] [CrossRef]
- Park, E.M.; Gelber, S.; Rosenberg, S.M.; Seah, D.S.E.; Schapira, L.; Come, S.E.; Partridge, A.H. Anxiety and Depression in Young Women with Metastatic Breast Cancer: A Cross-Sectional Study. Psychosomatics 2018, 59, 251–258. [Google Scholar] [CrossRef]
- McFarland, D.C.; Shaffer, K.M.; Tiersten, A.; Holland, J. Physical Symptom Burden and Its Association with Distress, Anxiety, and Depression in Breast Cancer. Psychosomatics 2018, 59, 464–471. [Google Scholar] [CrossRef]
- Pieterse, A.H.; Stiggelbout, A.M.; Baas-Thijssen, M.C.M.; Van De Velde, C.J.H.; Marijnen, C.A.M. Benefit from Preoperative Radiotherapy in Rectal Cancer Treatment: Disease-Free Patients’ and Oncologists’ Preferences. Br. J. Cancer 2007, 97, 717–724. [Google Scholar] [CrossRef]
- Kunneman, M.; Branda, M.E.; Hargraves, I.; Pieterse, A.H.; Montori, V.M. Fostering Choice Awareness for Shared Decision Making: A Secondary Analysis of Video-Recorded Clinical Encounters. Mayo Clin. Proc. Innov. Qual. Outcomes 2018, 2, 60–68. [Google Scholar] [CrossRef]
- Kunneman, M.; Engelhardt, E.G.; Ten Hove, F.L.; Marijnen, C.A.M.; Portielje, J.E.A.; Smets, E.M.A.; De Haes, H.J.C.J.M.; Stiggelbout, A.M.; Pieterse, A.H. Deciding about (Neo-)Adjuvant Rectal and Breast Cancer Treatment: Missed Opportunities for Shared Decision Making. Acta Oncol. 2016, 55, 134–139. [Google Scholar] [CrossRef]
- Kunneman, M.; Marijnen, C.A.M.; Rozema, T.; Ceha, H.M.; Grootenboers, D.A.R.H.; Neelis, K.J.; Stiggelbout, A.M.; Pieterse, A.H. Decision Consultations on Preoperative Radiotherapy for Rectal Cancer: Large Variation in Benefits and Harms That Are Addressed. Br. J. Cancer 2015, 112, 39–43. [Google Scholar] [CrossRef]
- Engelhardt, E.G.; Pieterse, A.H.; Van Der Hout, A.; De Haes, H.J.C.J.M.; Kroep, J.R.; Quarles Van Ufford-Mannesse, P.; Portielje, J.E.A.; Smets, E.M.A.; Stiggelbout, A.M. Use of Implicit Persuasion in Decision Making about Adjuvant Cancer Treatment: A Potential Barrier to Shared Decision Making. Eur. J. Cancer Care 2016, 66, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Lederer, B. The Importance of Supportive Care in Breast Cancer Patients. Breast Care 2014, 9, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Kida, K.; Olver, I.; Yennu, S.; Tripathy, D.; Ueno, N.T. Optimal Supportive Care for Patients with Metastatic Breast Cancer According to Their Disease Progression Phase. JCO Oncol. Pract. 2021, 17, 177–183. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Study Design | Sample Size | Participants | Mean Age (±SD or Range) 1 | De Novo Status | Preference Type | Outcome Measures * | Follow-Up | Results * |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Preferences | ||||||||||
| Specific Drug Preferences | ||||||||||
| Decker et al., 2020 [17] | Germany | RCT | n = 192 (Patients) n = 13 (HCPs) | Post-menopausal women with HR+/HER2− locally recurrent and inoperable or metastatic breast cancer Physicians | Arm A: 64.4 (47–83.6) Arm B: 65.9 (49.8–86.0) | NR | Preferences for combined antihormonal therapy (everolimus + exemestane) or chemo- and anti-angiogenic therapy (capecitabine + bevacizumab) | PPQ | 3 mo; 24 mo | Patients and healthcare providers tended to prefer capecitabine + bevacizumab. |
| Treatment Administration Preferences | ||||||||||
| Ciruelos et al., 2020 [18] | Spain | RCT | n = 166 (Patients) n = 39 (HCPs) | Women with HER2+ metastatic breast cancer Oncology nurses, medical oncologists, general nurses, others | 60 (35–93) | De novo: n = 61 (36.7%) Recurrence: n = 105 (63.3%) | Preferences for subcutaneous versus intravenous trastuzumab administration | Researcher-developed survey | After 2 cycles; after 4 cycles | Most patients and healthcare providers tended to prefer subcutaneous trastuzumab. |
| Fallowfield et al., 2011 [30] | UK | Mixed methods | n = 79 | Women with breast cancer with bone metastases | Oral Group: 62.3 ± 11.9 IV Group: 62.6 ± 13.2 | NR | Preferences for oral versus intravenous bisphosphonates administration | Semi-structured interviews | 3 mo; 6 mo | Both oral and intravenous bisphosphonates had disadvantages but were acceptable to most patients. |
| Gornas and Szczylik 2010 [28] | Poland | Cross-sectional observational | n = 215 | Women with metastatic breast cancer | 52 (27–77) | NR | Preferences for oral versus intravenous capecitabine administration | Researcher-developed survey | None | Most patients tended to prefer oral chemotherapy due to increased convenience and the possibility of staying at home during treatment. |
| Pivot et al., 2017 [19] | France | RCT | n = 113 | Patients with HER2+ metastatic breast cancer | 59.4 (34.7–84.9) | De novo: n = 58 (51.3%) Recurrence: n = 55 (48.7%) | Preferences for subcutaneous versus intravenous trastuzumab administration | PPQ | After 3 cycles | Most patients tended to prefer subcutaneous trastuzumab. |
| Treatment Characteristic Preferences | ||||||||||
| Amin et al., 2022 [20] | USA | DCE | n = 169 (Patients) n = 117 (HCPs) | Patients with locally advanced or metastatic triple-negative breast cancer or endocrine refractory HR+ breast cancer Oncologists | 54.2 ± 9.2 | NR | HER2- treatment preferences (OS, PFS, neuropathy, neutropenia, nausea, alopecia, immune-related AEs) | Researcher-developed surveys | None | Improving OS was most important to patients and HCPs, followed by improving nausea and neuropathy. |
| DaCosta DiBonaventura et al., 2014 [23] | USA | Conjoint Analysis | n = 181 | Women with metastatic breast cancer | 52.2 ± 9.1 | NR | Treatment preferences (OS, quality of life, treatment side-effects, dosing regime) and trade-offs between treatment side effects and effectiveness/quality of life | Interviews to develop survey (n = 10) Researcher-developed survey | None | Improving OS was most important to patients, followed by improving alopecia, fatigue, neutropenia, neuropathy and nausea/vomiting. |
| Lalla et al., 2014 [24] | USA | Conjoint Analysis | n = 298 | Patients with metastatic breast cancer | <30 to 71+ | NR | Treatment preferences and willingness to pay to avoid treatment side effects | Researcher-developed survey | None | Patients were willing to pay the most to avoid severe diarrhea, followed by being hospitalized due to infection, severe nausea and tingling in hands and feet. |
| Maculaitis et al., 2020 [21] | USA | DCE | n = 513 (Patients) n = 209 (HCPs) | Postmenopausal women with HR+/HER2- metastatic breast cancer Medical oncologists | 47.4 ± 9.9 | NR | CDK4/6 inhibitor treatment preferences (dose reduction, treatment side effects, dose regimen, dose schedule) and trade-offs between treatment benefits and risks | Interviews to develop survey (patients, n = 10; oncologists n = 8) Researcher-developed survey | None | Avoiding diarrhoea and Grade 3–4 neutropenia were of most importance to patients and oncologists. |
| Mansfield et al., 2022 [22] | USA, UK Japan | DCE | n = 302 | Patients with advanced or metastatic breast cancer | 47.6 ± 11.5 | NR | HER2- treatment preferences (PFS, treatment side-effects) and trade-offs between treatment benefits and risk | Researcher-developed survey | None | Improving PFS was most important to patients, followed by reducing the risk of heart failure. |
| Smith et al., 2014 [25] | USA | Conjoint Analysis | n = 641 | Patients with metastatic breast cancer | 40–80+ | NR | Paclitaxel and capecitabine preferences (benefit, treatment side effects) | Researcher-developed survey | None | Treatment benefit was more important than treatment side effects to patients. |
| Spaich et al., 2018 [26] | Germany | Conjoint Analysis | n = 100 | Patients with metastatic breast cancer | 64.4 ± 10.6 | NR | Taxane chemotherapy preferences (PFS, application time, cycle, premedication, treatment side effects) | Researcher-developed survey | None | Avoiding Grade 3–4 neutropenia was most important to patients, followed by alopecia, Grade 2–4 neuropathy and PFS. |
| Reinisch et al., 2021 [27] | Germany | Conjoint Analysis | n = 104 | Postmenopausal women with HR+/HER2- locally advanced or metastatic breast cancer | 50–70+ | Recurrence: n = 72 (69%) De novo: n = 32 (31%) | Palliative treatment preferences and importance of OS/PFS relative to quality of life/treatment side-effects | Interviews to develop survey (n = 12) Researcher-developed survey | None | Improving quality of life (physical agility and mobility) was most important to patients, followed by OS and PFS. |
| Communication and Decision-making Preferences | ||||||||||
| Butow et al., 2002 [34] | Australia | Qualitative | n = 17 (Patients) n = 13 (HCPs) | Women with metastatic breast cancer Oncologists, nurses, psychiatrist, psychologist, social worker, breast cancer advocate | 50 (38–80) | NR | Views towards disclosing prognosis and the ideal manner in which to structure the discussion | Semi-structured interviews | None | Open and repeated negotiations for patient preferences for information. Patients tended to prefer aclear, straight-forward presentation of prognosis. |
| Ejem et al., 2018 [31] | USA | Mixed methods | n = 22 | Women with metastatic breast cancer | 62 (33–87) | NR | Treatment decision-making preferences (“shared” versus “independent” versus “delegated” decision making) | CPS Semi-structured interviews | 3 mo | Patients selected a “shared” treatment decision-making style using the CPS. Interview descriptions reflected a passive process where patients followed oncologists’ treatment suggestions. |
| Niranjan et al., 2020 [35] | USA | Qualitative | n = 44 (Patients) n = 34 (HCPs) | Women with metastatic breast cancer Oncologists, nurses, lay investigators | 50% of patients were 55 and over | NR | Communication preferences regarding prognosis, crisis support, treatment information, and timing of communication | Interviews (Patients) Focus Groups (HCPs) | None | Most patients expressed wanting prognostic information but varied in the timing of when they wanted the information. |
| Rocque et al., 2019 [32] | USA | Mixed methods | n = 20 (Patients) n = 11 (HCPs) | Women with metastatic breast cancer Community oncologists, academic oncologists | 25–65+ | NR | Factors influencing decision-making in treatment selection | CPS Interviews (Patients) Focus Group or Interviews (HCPs) | None | Patients and HCPs consider treatment characteristics when making decisions. Patients tend to have broader considerations than HCPs and incorporate more contextual factors. |
| Supportive Care Preferences | ||||||||||
| Delrieu et al., 2020 [29] | France | Single-arm intervention trial | n = 49 | Women with metastatic breast cancer | 55 ± 10.4 | De novo: n = 14 (28.6%), Recurrence: n = 35 (71.4%) | Physical activity preferences | Researcher-developed survey | 6 mo | Physical activity preferences varied. Most patients tended to prefer receiving counselling from a physical therapist specialist, and preferred exercise during treatment, in the company of others and at home (baseline) or in a fitness centre (6 mo). |
| Schulman-Green et al., 2011 [36] | USA | Qualitative | n = 15 | Women with metastatic breast cancer | 52 (37–91) | NR | Self-management preferences, practices, and experiences | Semi-structured interviews | None | Self-managed preferences vary. HCPs should repeatedly explore patients’ self-management preferences and ability to self-manage. |
| ten Tusscher et al., 2019 [33] | The Netherlands | Mixed methods | n = 114 | Patients with metastatic breast cancer | 63.5 ± 10.2 | NR | Exercise-based physical therapy program preferences | Researcher-developed survey Focus groups (n = 6) | None | Exercise-based physical therapy program preferences vary. Patients tend to prefer high-quality, physical therapist-guided, tailored exercise programs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bland, K.A.; Mustafa, R.; McTaggart-Cowan, H. Patient Preferences in Metastatic Breast Cancer Care: A Scoping Review. Cancers 2023, 15, 4331. https://doi.org/10.3390/cancers15174331
Bland KA, Mustafa R, McTaggart-Cowan H. Patient Preferences in Metastatic Breast Cancer Care: A Scoping Review. Cancers. 2023; 15(17):4331. https://doi.org/10.3390/cancers15174331
Chicago/Turabian StyleBland, Kelcey A., Reem Mustafa, and Helen McTaggart-Cowan. 2023. "Patient Preferences in Metastatic Breast Cancer Care: A Scoping Review" Cancers 15, no. 17: 4331. https://doi.org/10.3390/cancers15174331
APA StyleBland, K. A., Mustafa, R., & McTaggart-Cowan, H. (2023). Patient Preferences in Metastatic Breast Cancer Care: A Scoping Review. Cancers, 15(17), 4331. https://doi.org/10.3390/cancers15174331






