Associations of Multiparametric Breast MRI Features, Tumor-Infiltrating Lymphocytes, and Immune Gene Signature Scores Following a Single Dose of Trastuzumab in HER2-Positive Early-Stage Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Criteria and Enrollment
2.2. Breast MRI Acquisition
2.3. Breast MRI Analysis
2.4. DCE-MRI Kinetic Analysis
2.5. DW-MRI Analysis
2.6. Tumor Microenvironment Evaluation
2.6.1. TIL Assessment
2.6.2. RNA Isolation
2.6.3. Immune Signature Scores
2.7. Statistical Analysis
2.7.1. Correlation of MRI Features with TILs
2.7.2. Correlation of MRI Features with Tumor Microenvironment Gene Expression
3. Results
3.1. Patient Demographics
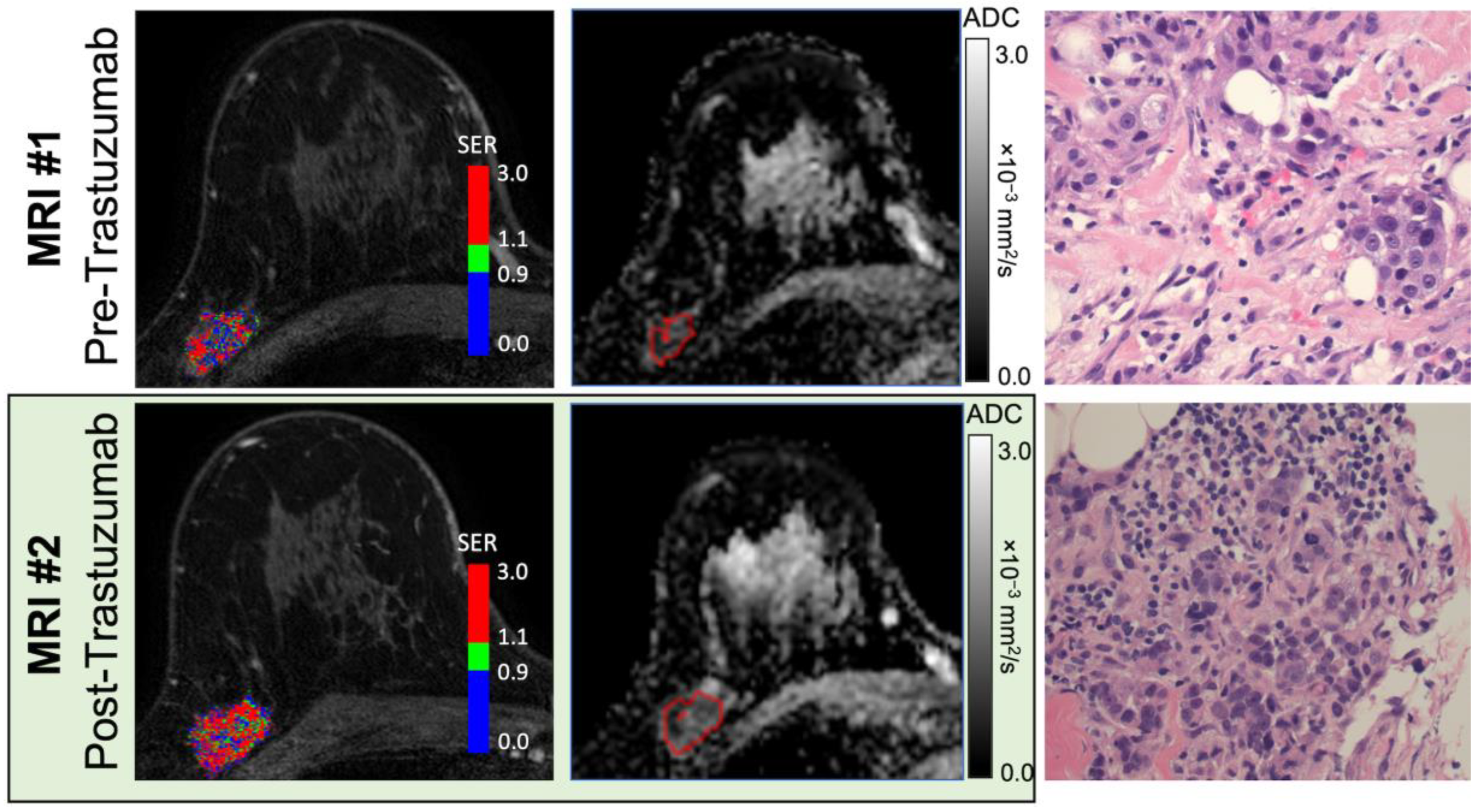
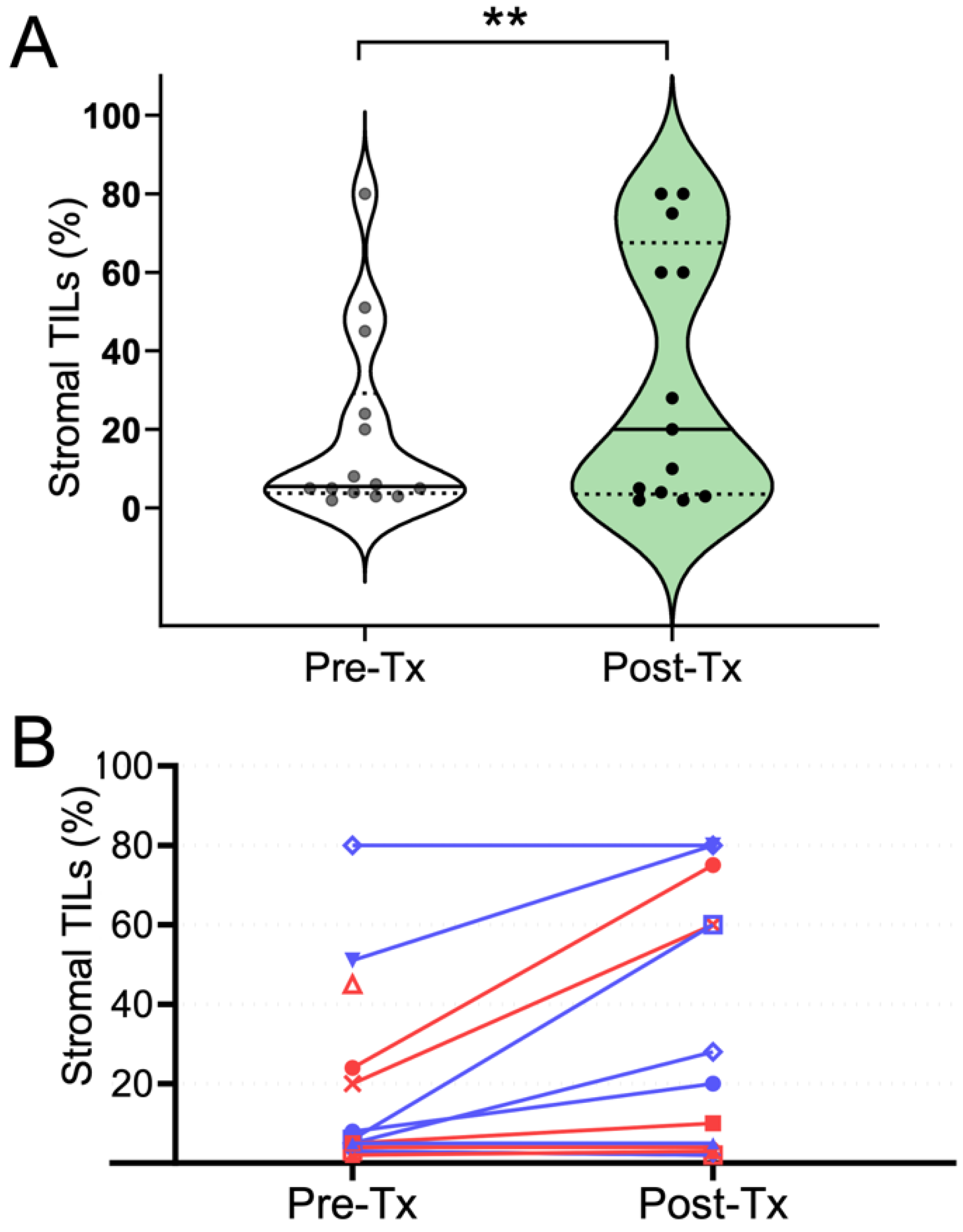
3.2. Peak PE and WF Correlate with an Immune Response Post-Trastuzumab Administration
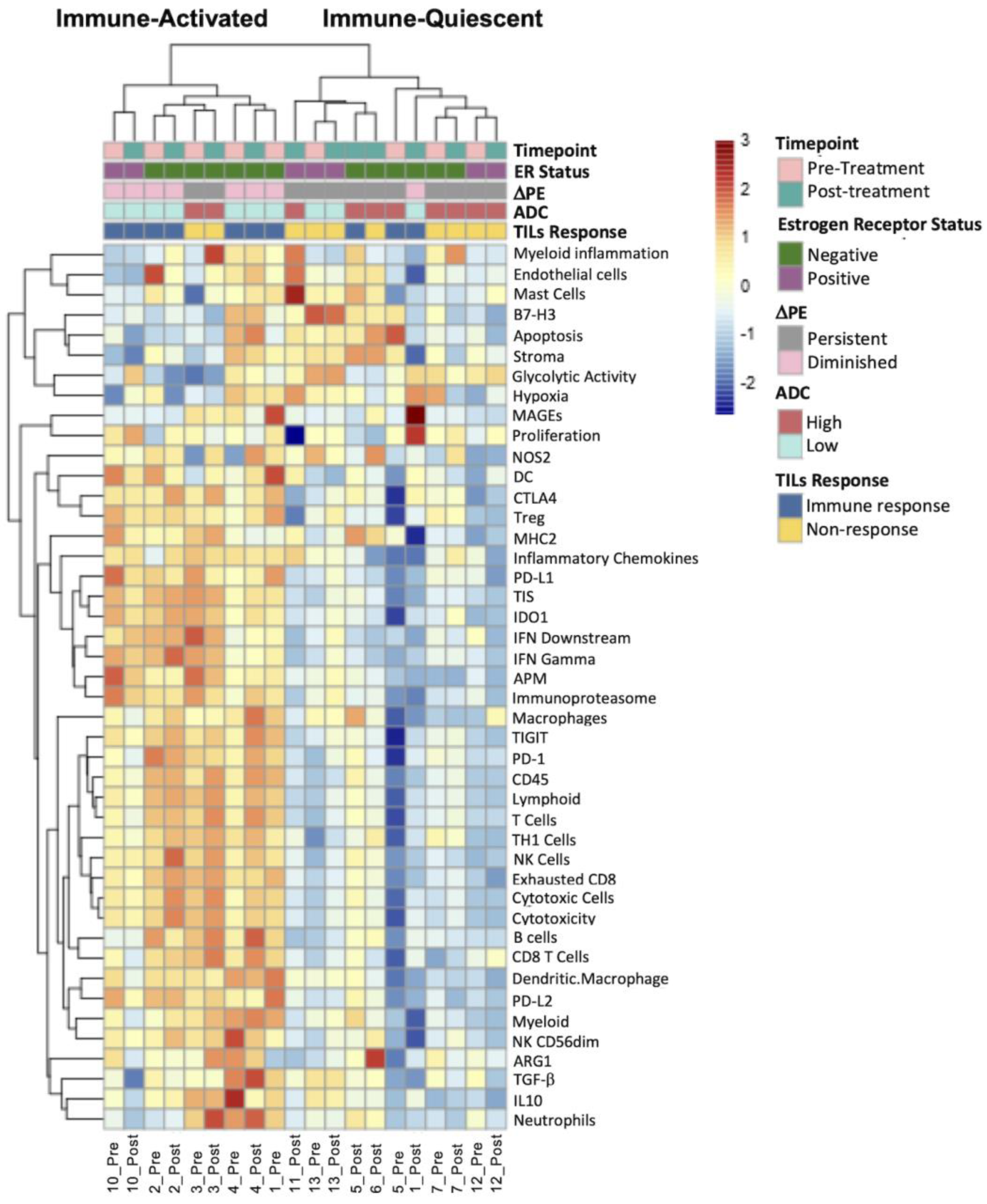
3.3. Diminished Peak PE Was Associated with an Immune-Activated Gene Signature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, F.; van’t Veer, L.J.; Bogaerts, J.; Slaets, L.; Viale, G.; Delaloge, S.; Pierga, J.Y.; Brain, E.; Causeret, S.; DeLorenzi, M.; et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N. Engl. J. Med. 2016, 375, 717–729. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E.; Dees, E.C.; Goetz, M.P.; Olson, J.A.; et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.4.2023. © National Comprehensive Cancer Network, Inc. 2023. All Rights Reserved. Available online: https://www.nccn.org (accessed on 15 August 2023).
- Ignatiadis, M.; Van den Eynden, G.; Roberto, S.; Fornili, M.; Bareche, Y.; Desmedt, C.; Rothé, F.; Maetens, M.; Venet, D.; Holgado, E.; et al. Tumor-Infiltrating Lymphocytes in Patients Receiving Trastuzumab/Pertuzumab-Based Chemotherapy: A TRYPHAENA Substudy. J. Natl. Cancer Inst. 2019, 111, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Gall, V.A.; Philips, A.V.; Qiao, N.; Clise-Dwyer, K.; Perakis, A.A.; Zhang, M.; Clifton, G.T.; Sukhumalchandra, P.; Ma, Q.; Reddy, S.M.; et al. Trastuzumab Increases HER2 Uptake and Cross-Presentation by Dendritic Cells. Cancer Res. 2017, 77, 5374–5383. [Google Scholar] [CrossRef]
- Muntasell, A.; Cabo, M.; Servitja, S.; Tusquets, I.; Martinez-Garcia, M.; Rovira, A.; Rojo, F.; Albanell, J.; Lopez-Botet, M. Interplay between Natural Killer Cells and Anti-HER2 Antibodies: Perspectives for Breast Cancer Immunotherapy. Front. Immunol. 2017, 8, 1544. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Fan, X.; Deng, H.; Brezski, R.J.; Rycyzyn, M.; Jordan, R.E.; Strohl, W.R.; Zou, Q.; Zhang, N.; An, Z. Trastuzumab triggers phagocytic killing of high HER2 cancer cells in vitro and in vivo by interaction with Fcgamma receptors on macrophages. J. Immunol. 2015, 194, 4379–4386. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef]
- Hylton, N.M.; Gatsonis, C.A.; Rosen, M.A.; Lehman, C.D.; Newitt, D.C.; Partridge, S.C.; Bernreuter, W.K.; Pisano, E.D.; Morris, E.A.; Weatherall, P.T.; et al. Neoadjuvant Chemotherapy for Breast Cancer: Functional Tumor Volume by MR Imaging Predicts Recurrence-free Survival-Results from the ACRIN 6657/CALGB 150007 I-SPY 1 TRIAL. Radiology 2016, 279, 44–55. [Google Scholar] [CrossRef]
- Connolly, R.M.; Leal, J.P.; Solnes, L.; Huang, C.Y.; Carpenter, A.; Gaffney, K.; Abramson, V.; Carey, L.A.; Liu, M.C.; Rimawi, M.; et al. Updated Results of TBCRC026: Phase II Trial Correlating Standardized Uptake Value with Pathological Complete Response to Pertuzumab and Trastuzumab in Breast Cancer. J. Clin. Oncol. 2021, 39, 2247–2256. [Google Scholar] [CrossRef]
- Hylton, N.M.; Blume, J.D.; Bernreuter, W.K.; Pisano, E.D.; Rosen, M.A.; Morris, E.A.; Weatherall, P.T.; Lehman, C.D.; Newstead, G.M.; Polin, S.; et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy—Results from ACRIN 6657/I-SPY TRIAL. Radiology 2012, 263, 663–672. [Google Scholar] [CrossRef]
- Partridge, S.C.; Zhang, Z.; Newitt, D.C.; Gibbs, J.E.; Chenevert, T.L.; Rosen, M.A.; Bolan, P.J.; Marques, H.S.; Romanoff, J.; Cimino, L.; et al. Diffusion-weighted MRI Findings Predict Pathologic Response in Neoadjuvant Treatment of Breast Cancer: The ACRIN 6698 Multicenter Trial. Radiology 2018, 289, 618–627. [Google Scholar] [CrossRef]
- Braman, N.; Prasanna, P.; Whitney, J.; Singh, S.; Beig, N.; Etesami, M.; Bates, D.D.B.; Gallagher, K.; Bloch, B.N.; Vulchi, M.; et al. Association of Peritumoral Radiomics with Tumor Biology and Pathologic Response to Preoperative Targeted Therapy for HER2 (ERBB2)-Positive Breast Cancer. JAMA Netw. Open 2019, 2, e192561. [Google Scholar] [CrossRef]
- Jiang, X.; Dudzinski, S.; Beckermann, K.E.; Young, K.; McKinley, E.; McIntyre, O.J.; Rathmell, J.C.; Xu, J.; Gore, J.C. MRI of tumor T cell infiltration in response to checkpoint inhibitor therapy. J. Immunother. Cancer 2020, 8, e000328. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Teng, X.; Rubin, D.L.; Napel, S.; Daniel, B.L.; Li, R. Magnetic resonance imaging and molecular features associated with tumor-infiltrating lymphocytes in breast cancer. Breast Cancer Res. 2018, 20, 101. [Google Scholar] [CrossRef]
- ACR MRI Accreditation. Available online: https://www.acraccreditation.org/modalities/mri (accessed on 16 August 2023).
- Partridge, S.C.; Gibbs, J.E.; Lu, Y.; Esserman, L.J.; Sudilovsky, D.; Hylton, N.M. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am. J. Roentgenol. 2002, 179, 1193–1199. [Google Scholar] [CrossRef]
- Partridge, S.C.; Vanantwerp, R.K.; Doot, R.K.; Chai, X.; Kurland, B.F.; Eby, P.R.; Specht, J.M.; Dunnwald, L.K.; Schubert, E.K.; Lehman, C.D.; et al. Association between serial dynamic contrast-enhanced MRI and dynamic 18F-FDG PET measures in patients undergoing neoadjuvant chemotherapy for locally advanced breast cancer. J. Magn. Reson. Imaging 2010, 32, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Rahbar, H.; Hippe, D.S.; Rendi, M.H.; Parker, E.U.; Shekar, N.; Hirano, M.; Cheung, K.J.; Partridge, S.C. Dynamic contrast-enhanced breast MRI features correlate with invasive breast cancer angiogenesis. NPJ Breast Cancer 2021, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Johnston, B.S.; Kitsch, A.E.; Hippe, D.S.; Korde, L.A.; Javid, S.; Lee, J.M.; Peacock, S.; Lehman, C.D.; Partridge, S.C.; et al. Ductal Carcinoma in Situ: Quantitative Preoperative Breast MR Imaging Features Associated with Recurrence after Treatment. Radiology 2017, 285, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, H.; Zhang, Z.; Chenevert, T.L.; Romanoff, J.; Kitsch, A.E.; Hanna, L.G.; Harvey, S.M.; Moy, L.; DeMartini, W.B.; Dogan, B.; et al. Utility of Diffusion-weighted Imaging to Decrease Unnecessary Biopsies Prompted by Breast MRI: A Trial of the ECOG-ACRIN Cancer Research Group (A6702). Clin. Cancer Res. 2019, 25, 1756–1765. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Tanjak, P.; Chaiboonchoe, A.; Suwatthanarak, T.; Acharayothin, O.; Thanormjit, K.; Chanthercrob, J.; Suwatthanarak, T.; Wannasuphaphol, B.; Chumchuen, K.; Suktitipat, B.; et al. The KRAS-Mutant Consensus Molecular Subtype 3 Reveals an Immunosuppressive Tumor Microenvironment in Colorectal Cancer. Cancers 2023, 15, 1098. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188, 1124. [Google Scholar] [CrossRef]
- Bradley, R.; Braybrooke, J.; Gray, R.G.; Taylor, H.; Denkert, C.; Badve, S.S.; Kim, R.S.; Lacroix-Triki, M.; Regan, M.M.; Hayes, D.F.; et al. Do tumor infiltrating lymphocytes (TILs) predict benefits from trastuzumab therapy for HER2 positive breast cancer? Meta-analysis of individual patient data from 4097 women in 5 trials. J. Clin. Oncol. 2023, 41 (Suppl. 16), 508. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Mathieu, M.C.; Guarneri, V.; Conte, P.; Delaloge, S.; Andre, F.; Goubar, A. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann. Oncol. 2015, 26, 1698–1704. [Google Scholar] [CrossRef]
- Asano, Y.; Kashiwagi, S.; Goto, W.; Takada, K.; Takahashi, K.; Hatano, T.; Takashima, T.; Tomita, S.; Motomura, H.; Ohsawa, M.; et al. Prediction of Treatment Response to Neoadjuvant Chemotherapy in Breast Cancer by Subtype Using Tumor-infiltrating Lymphocytes. Anticancer Res. 2018, 38, 2311–2321. [Google Scholar] [CrossRef] [PubMed]
- Varadan, V.; Gilmore, H.; Miskimen, K.L.; Tuck, D.; Parsai, S.; Awadallah, A.; Krop, I.E.; Winer, E.P.; Bossuyt, V.; Somlo, G.; et al. Immune Signatures Following Single Dose Trastuzumab Predict Pathologic Response to Preoperative Trastuzumab and Chemotherapy in HER2-Positive Early Breast Cancer. Clin. Cancer Res. 2016, 22, 3249–3259. [Google Scholar] [CrossRef]
- Virostko, J.; Hainline, A.; Kang, H.; Arlinghaus, L.R.; Abramson, R.G.; Barnes, S.L.; Blume, J.D.; Avery, S.; Patt, D.; Goodgame, B.; et al. Dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted magnetic resonance imaging for predicting the response of locally advanced breast cancer to neoadjuvant therapy: A meta-analysis. J. Med. Imaging 2018, 5, 011011. [Google Scholar] [CrossRef]
- De Lisa, M.; Pistelli, M.; Giampieri, R.; Macchini, M.; Ponzani, M.; Giuseppetti, G.M.; Santinelli, A.; Bastianelli, L.; Ballatore, Z.; Battelli, N.; et al. Evaluation of stromal tumour-infiltrating lymphocytes (TILs) in breast cancer by Dynamic contrast–enhanced magnetic resonance (DCE-MR) imaging. Ann. Oncol. 2017, 28 (Suppl. 6), VI26–VI27. [Google Scholar] [CrossRef]
- Ku, Y.J.; Kim, H.H.; Cha, J.H.; Shin, H.J.; Baek, S.H.; Lee, H.J.; Gong, G. Correlation Between MRI and the Level of Tumor-Infiltrating Lymphocytes in Patients with Triple-Negative Breast Cancer. AJR Am. J. Roentgenol. 2016, 207, 1146–1151. [Google Scholar] [CrossRef]
- Chenevert, T.L.; McKeever, P.E.; Ross, B.D. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin. Cancer Res. 1997, 3, 1457–1466. [Google Scholar]
- Rozel, S.; Galban, C.J.; Nicolay, K.; Lee, K.C.; Sud, S.; Neeley, C.; Snyder, L.A.; Chenevert, T.L.; Rehemtulla, A.; Ross, B.D.; et al. Synergy between anti-CCL2 and docetaxel as determined by DW-MRI in a metastatic bone cancer model. J. Cell. Biochem. 2009, 107, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Pipe, J.G.; Bonnett, J.; Evelhoch, J.L. Early detection of treatment response by diffusion-weighted 1H-NMR spectroscopy in a murine tumour in vivo. Br. J. Cancer 1996, 73, 61–64. [Google Scholar] [CrossRef][Green Version]
- Guo, Y.; Cai, Y.Q.; Cai, Z.L.; Gao, Y.G.; An, N.Y.; Ma, L.; Mahankali, S.; Gao, J.H. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J. Magn. Reson. Imaging 2002, 16, 172–178. [Google Scholar] [CrossRef]
- Sharma, U.; Danishad, K.K.; Seenu, V.; Jagannathan, N.R. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009, 22, 104–113. [Google Scholar] [CrossRef]
- Brix, G.; Kiessling, F.; Lucht, R.; Darai, S.; Wasser, K.; Delorme, S.; Griebel, J. Microcirculation and microvasculature in breast tumors: Pharmacokinetic analysis of dynamic MR image series. Magn. Reson. Med. 2004, 52, 420–429. [Google Scholar] [CrossRef]
- He, G.; Howard, F.; Pandey, T.; Abe, H.; Nanda, R. Independent validation of simbiosys tumorscope to predict response to neoadjuvant chemotherapy (NACT) in early breast cancer (EBC). J. Clin. Oncol. 2021, 39, 582. [Google Scholar] [CrossRef]
- Cole, J.A.; Peterson, J.R.; Earnest, T.M.; Hallock, M.J.; Pfeiffer, J.R.; Pandey, T.; Braun, E. SimBioSys TumorScope: Spatio-temporal modeling of the tumor microenvironment to predict chemotherapeutic response. J. Clin. Oncol. 2020, 38 (Suppl. 15), e12650. [Google Scholar] [CrossRef]
- Braman, N.M.; Etesami, M.; Prasanna, P.; Dubchuk, C.; Gilmore, H.; Tiwari, P.; Plecha, D.; Madabhushi, A. Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res. 2017, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Locy, H.; Verhulst, S.; Cools, W.; Waelput, W.; Brock, S.; Cras, L.; Schiettecatte, A.; Jonckheere, J.; van Grunsven, L.A.; Vanhoeij, M.; et al. Assessing Tumor-Infiltrating Lymphocytes in Breast Cancer: A Proposal for Combining Immunohistochemistry and Gene Expression Analysis to Refine Scoring. Front. Immunol. 2022, 13, 794175. [Google Scholar] [CrossRef] [PubMed]

| Patient ID | Age at Diagnosis (Years) | ER Status (Allred Score) | Combined Histologic Grade | Days to Post-Trastuzumab MRI |
|---|---|---|---|---|
| 1 | 41 | Negative | 2 | 16 |
| 2 | 39 | Negative | 3 | 11 |
| 3 | 37 | Negative | 3 | 14 |
| 4 | 57 | Negative | 2 | 14 |
| 5 | 40 | Negative | 3 | 18 |
| 6 * | 60 | Negative | 1 | 14 |
| 7 | 61 | Negative | 3 | 11 |
| 8 * | 53 | Positive (3/8) | 2 | 21 |
| 9 | 47 | Positive (4/8) | 3 | 15 |
| 10 | 37 | Positive (7/8) | 3 | 28 |
| 11 | 64 | Positive (8/8) | 2 | 14 |
| 12 | 69 | Positive (8/8) | 3 | 36 |
| 13 *,+ | 51 | Positive (8/8) | 3 | 14 |
| 14 | 52 | Positive (8/8) | 2 | 14 |
| Pre-Trastuzumab | Post-Trastuzumab | ΔTILs (ρ) | |
|---|---|---|---|
| TILs (ρ) | TILs (ρ) | ||
| Functional Tumor Volume (FTV) | |||
| FTV MRI #1 | 0.14 | 0.03 | 0.03 |
| FTV MRI #2 | −0.32 | −0.19 | −0.05 |
| ΔFTV | −0.37 | −0.18 | 0.19 |
| Wash-Out Fraction (WF) | |||
| WF MRI #1 | 0.34 | 0.22 | 0.51 |
| WF MRI #2 | 0.08 | 0.18 | 0.26 |
| ΔWF | −0.11 | 0.07 | −0.11 |
| Signal Enhancement Ratio (SER) | |||
| SER MRI #1 | 0.28 | 0.22 | 0.33 |
| SER MRI #2 | 0.23 | 0.36 | 0.38 |
| ΔSER | 0.25 | 0.35 | 0.32 |
| Peak Percent Enhancement (PE) | |||
| PE MRI #1 | 0.24 | 0.25 | 0.48 |
| PE MRI #2 | −0.1 | -0.1 | 0.11 |
| ΔPE | −0.45 | -0.51 | −0.69 |
| Apparent Diffusion Coefficient (ADC) | |||
| ADC MRI #1 | −0.28 | -0.51 | −0.67 |
| ADC MRI #2 | −0.06 | -0.27 | −0.49 |
| ΔADC | 0.06 | 0.11 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennedy, L.C.; Kazerouni, A.S.; Chau, B.; Biswas, D.; Alvarez, R.; Durenberger, G.; Dintzis, S.M.; Stanton, S.E.; Partridge, S.C.; Gadi, V. Associations of Multiparametric Breast MRI Features, Tumor-Infiltrating Lymphocytes, and Immune Gene Signature Scores Following a Single Dose of Trastuzumab in HER2-Positive Early-Stage Breast Cancer. Cancers 2023, 15, 4337. https://doi.org/10.3390/cancers15174337
Kennedy LC, Kazerouni AS, Chau B, Biswas D, Alvarez R, Durenberger G, Dintzis SM, Stanton SE, Partridge SC, Gadi V. Associations of Multiparametric Breast MRI Features, Tumor-Infiltrating Lymphocytes, and Immune Gene Signature Scores Following a Single Dose of Trastuzumab in HER2-Positive Early-Stage Breast Cancer. Cancers. 2023; 15(17):4337. https://doi.org/10.3390/cancers15174337
Chicago/Turabian StyleKennedy, Laura C., Anum S. Kazerouni, Bonny Chau, Debosmita Biswas, Rebeca Alvarez, Grace Durenberger, Suzanne M. Dintzis, Sasha E. Stanton, Savannah C. Partridge, and Vijayakrishna Gadi. 2023. "Associations of Multiparametric Breast MRI Features, Tumor-Infiltrating Lymphocytes, and Immune Gene Signature Scores Following a Single Dose of Trastuzumab in HER2-Positive Early-Stage Breast Cancer" Cancers 15, no. 17: 4337. https://doi.org/10.3390/cancers15174337
APA StyleKennedy, L. C., Kazerouni, A. S., Chau, B., Biswas, D., Alvarez, R., Durenberger, G., Dintzis, S. M., Stanton, S. E., Partridge, S. C., & Gadi, V. (2023). Associations of Multiparametric Breast MRI Features, Tumor-Infiltrating Lymphocytes, and Immune Gene Signature Scores Following a Single Dose of Trastuzumab in HER2-Positive Early-Stage Breast Cancer. Cancers, 15(17), 4337. https://doi.org/10.3390/cancers15174337






