The Impact of Sidedness on the Efficacy of Anti-EGFR-Based First-Line Chemotherapy in Advanced Colorectal Cancer Patients in Real-Life Setting—A Nation-Wide Retrospective Analysis (RACER)
Abstract
:Simple Summary
Abstract
1. Introduction
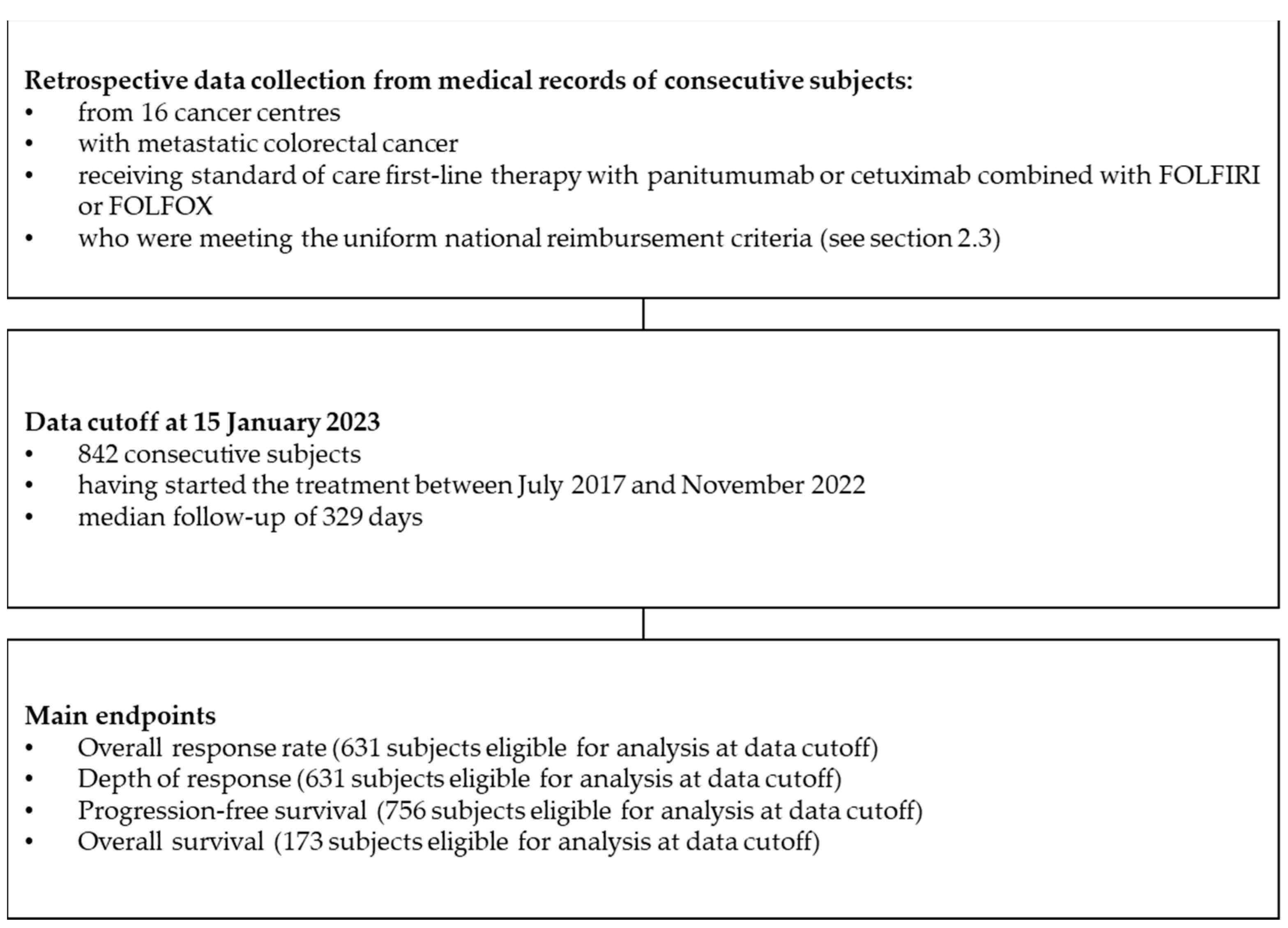
2. Materials and Methods
2.1. Study Design
2.2. National Therapeutic Programme B.4
2.3. Patients
- histologically confirmed colorectal cancer;
- metastatic disease (stage IV);
- disqualification from radical surgery;
- lack of prior systemic treatment due to metastatic disease;
- absence of mutations in the KRAS and NRAS genes (minimal requirements—evaluation of exons 2, 3 and 4 in both genes) and absence of the BRAF V600E mutation;
- disease assessable for response according to the RECIST 1.1 criteria;
- performance status 0–1 according to the Zubrod-WHO classification;
- over 18 years of age;
- results of the complete differentiated blood count:
- platelet count ≥ 1.5 × 105/mm3,
- an absolute neutrophil count ≥ 1500/mm3,
- haemoglobin ≥ 10.0 g/dL;
- adequate organ function:
- total bilirubin concentration not exceeding 2 times the upper limit of normal (except for patients with Gilbert syndrome),
- serum transaminases (alanine and aspartic acid) activity not exceeding 5 times the upper limit of normal,
- creatinine concentration not exceeding 1.5 times the upper limit of normal;
- no contraindications to the FOLFIRI or FOLFOX chemotherapy regimen;
- exclusion of pregnancy;
- absence of brain metastases (in the case of clinical manifestations, exclusion based on imaging examination);
- no contraindications to cetuximab:
- pulmonary fibrosis or interstitial pneumonia,
- hypersensitivity to any excipients.
- hypersensitivity to panitumumab, cetuximab, or any component of chemotherapy;
- disease progression;
- prolonged and clinically significant adverse events ≥ G3;
- pulmonary fibrosis or interstitial pneumonia;
- persistent deterioration of the performance status ECOG ≥ 3.
2.4. Evaluated Data
Characteristics of Patients
2.5. Endpoints
2.6. Statistical Analysis
3. Results
3.1. Characteristics of mCRC Patients Receiving Chemotherapy and Anti-EGFR Combination
3.2. Characteristics of the Subgroups of Patients with Respect to Primary Tumour Location
3.3. The Impact of Sidedness on the Efficacy of Systemic Treatment
3.3.1. Tumour Response
3.3.2. Depth of Response
3.3.3. Progression-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- GLOBOCAN. The Global Cancer Observatory—Cancer Today. 2020. Available online: https://gco.iarc.fr (accessed on 30 January 2021).
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Arnold, M.; Rutherford, M.J.; Guren, M.G.; Cabasag, C.J.; Bardot, A.; Ferlay, J.; Tervonen, H.; Shack, L.; Woods, R.R.; et al. Colon and Rectal Cancer Survival in Seven High-Income Countries 2010–2014: Variation by Age and Stage at Diagnosis (the ICBP SURVMARK-2 Project). Gut 2021, 70, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Guo, F.; Heisser, T.; De Schutter, H.; Van Damme, N.; Nilbert, M.C.; Christensen, J.; Bouvier, A.M.; Bouvier, V.; Launoy, G.; et al. Overall and Stage-Specific Survival of Patients with Screen-Detected Colorectal Cancer in European Countries: A Population-Based Study in 9 Countries. Lancet Reg. Heal.—Eur. 2022, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic Colorectal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network Colon Cancer. Available online: https://pubmed.ncbi.nlm.nih.gov/29262132/ (accessed on 10 June 2023).
- Rossini, D.; Boccaccino, A.; Carullo, M.; Antoniotti, C.; Dima, G.; Ciracì, P.; Marmorino, F.; Moretto, R.; Masi, G.; Cremolini, C. Primary Tumour Side as a Driver for Treatment Choice in RAS Wild-Type Metastatic Colorectal Cancer Patients: A Systematic Review and Pooled Analysis of Randomised Trials. Eur. J. Cancer 2023, 184, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Missiaglia, E.; Jacobs, B.; D’Ario, G.; Di Narzo, A.F.; Soneson, C.; Budinska, E.; Popovici, V.; Vecchione, L.; Gerster, S.; Yan, P.; et al. Distal and Proximal Colon Cancers Differ in Terms of Molecular, Pathological, and Clinical Features. Ann. Oncol. 2014, 25, 1995–2001. [Google Scholar] [CrossRef]
- Gervaz, P.; Bucher, P.; Morel, P. Two Colons-Two Cancers: Paradigm Shift and Clinical Implications. J. Surg. Oncol. 2004, 88, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Meguid, R.A.; Slidell, M.B.; Wolfgang, C.L.; Chang, D.C.; Ahuja, N. Is There a Difference in Survival between Right-versus Left-Sided Colon Cancers? Ann. Surg. Oncol. 2008, 15, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Malakorn, S.; Ouchi, A.; Hu, C.Y.; Sandhu, L.; Dasari, A.; You, Y.Q.N.; Kopetz, E.S.; Ellis, L.M.; Chang, G.J. Tumor Sidedness, Recurrence, and Survival after Curative Resection of Localized Colon Cancer. Clin. Colorectal Cancer 2021, 20, e53–e60. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.M.; Pfau, P.R.; O’Connor, E.S.; King, J.; LoConte, N.; Kennedy, G.; Smith, M.A. Mortality by Stage for Right-versus Left-Sided Colon Cancer: Analysis of Surveillance, Epidemiology, and End Results-Medicare Data. J. Clin. Oncol. 2011, 29, 4401–4409. [Google Scholar] [CrossRef] [PubMed]
- Warschkow, R.; Sulz, M.C.; Marti, L.; Tarantino, I.; Schmied, B.M.; Cerny, T.; Güller, U. Better Survival in Right-Sided versus Left-Sided Stage I–III Colon Cancer Patients. BMC Cancer 2016, 16, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Brennan, K.; Nanji, S.; Berry, S.R.; Booth, C.M. Association between Prognosis and Tumor Laterality in Early-Stage Colon Cancer. JAMA Oncol. 2017, 3, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.; Lueza, B.; Douillard, J.Y.; Peeters, M.; Lenz, H.J.; Venook, A.; Heinemann, V.; Van Cutsem, E.; Pignon, J.P.; Tabernero, J.; et al. Prognostic and Predictive Value of Primary Tumour Side in Patients with RAS Wild-Type Metastatic Colorectal Cancer Treated with Chemotherapy and EGFR Directed Antibodies in Six Randomized Trials. Ann. Oncol. 2017, 28, 1713–1729. [Google Scholar] [CrossRef] [PubMed]
- Obwieszczenie Ministra Zdrowia z Dnia 27 Czerwca 2017 r. w Sprawie Wykazu Refundowanych Leków, Środków Spożywczych Specjalnego Przeznaczenia Żywieniowego Oraz Wyrobów Medycznych; Ministerstwo Zdrowia. 2017. Available online: https://www.gov.pl/web/zdrowie/obwieszczenie-ministra-zdrowia-z-dnia-21-grudnia-2017-r-w-sprawie-wykazu-refundowanych-lekow-srodkow-spozywczych-specjalnego-przeznaczenia-zywieniowego-oraz-wyrobow-medycznych-na-1-stycznia-2018-r (accessed on 15 June 2023).
- Zdrowia, M. Programy Lekowe w Polsce. Available online: https://analizy.mz.gov.pl/app_direct/mpz_2020_prog_lekowe/ (accessed on 15 June 2023).
- R Core Team. R: A Language and Environment for Statistical Computing. 2017. Available online: https://www.r-project.org/ (accessed on 15 June 2023).
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.-J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined with Cetuximab or Bevacizumab on Overall Survival in Patients with KRAS Wild-Type Advanced or Metastatic Colorectal Cancer. JAMA 2017, 317, 2392. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Kaiser, F.; Al-Batran, S.E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus Cetuximab or Bevacizumab for Advanced Colorectal Cancer: Final Survival and per-Protocol Analysis of FIRE-3, a Randomised Clinical Trial. Br. J. Cancer 2021, 124, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Potocki, P.M.; Wójcik, P.; Chmura, Ł.; Goc, B.; Fedewicz, M.; Bielańska, Z.; Swadźba, J.; Konopka, K.; Kwinta, Ł.; Wysocki, P.J. Clinical Characterization of Targetable Mutations (BRAF V600E and KRAS G12C) in Advanced Colorectal Cancer—A Nation-Wide Study. Int. J. Mol. Sci. 2023, 24, 9073. [Google Scholar] [CrossRef] [PubMed]
- Bożyk, A.; Krawczyk, P.; Reszka, K.; Krukowska, K.; Kolak, A.; Mańdziuk, S.; Wojas-Krawczyk, K.; Ramlau, R.; Milanowski, J. Correlation between KRAS, NRAS and BRAF Mutations and Tumor Localizations in Patients with Primary and Metastatic Colorectal Cancer. Arch. Med. Sci. 2021, 18, 1221. [Google Scholar] [CrossRef] [PubMed]
- Boleij, A.; Tack, V.; Taylor, A.; Kafatos, G.; Jenkins-Anderson, S.; Tembuyser, L.; Dequeker, E.; van Krieken, J.H. RAS Testing Practices and RAS Mutation Prevalence among Patients with Metastatic Colorectal Cancer: Results from a Europe-Wide Survey of Pathology Centres. BMC Cancer 2016, 16, 825. [Google Scholar] [CrossRef] [PubMed]
- Kafatos, G.; Niepel, D.; Lowe, K.; Jenkins-Anderson, S.; Westhead, H.; Garawin, T.; Traugottová, Z.; Bilalis, A.; Molnar, E.; Timar, J.; et al. RAS Mutation Prevalence among Patients with Metastatic Colorectal Cancer: A Meta-Analysis of Real-World Data. Biomark. Med. 2017, 11, 751–760. [Google Scholar] [CrossRef] [PubMed]



| Parameter Category | Overall, N = 842 | Right Colon N = 126 | Left Colon, N = 711 | Right Colon vs. Left Colon |
|---|---|---|---|---|
| Sex, N (%) | 0.287 # | |||
| Male | 559 (66.4%) | 78 (61.9%) | 478 (67.2%) | |
| Female | 283 (33.6%) | 48 (38.1%) | 233 (32.8%) | |
| Age [years] | 0.128 ^ | |||
| N | 842 | 126 | 711 | |
| Mean (SD) | 62.3 (9.8) | 63.5 (9.4) | 62.1 (9.9) | |
| Median (Q1; Q3) | 64.0 (57.0; 70.0) | 65.0 (58.0; 70.0) | 63.0 (57.0; 69.0) | |
| Min; max | 25.0; 83.0 | 35.0; 80.0 | 25.0; 83.0 | |
| Weight [kg] | 0.058 ^ | |||
| N | 826 | 126 | 698 | |
| Mean (SD) | 76.3 (16.7) | 74.0 (17.3) | 76.8 (16.6) | |
| Median (Q1; Q3) | 75.0 (64.0; 87.0) | 72.0 (60.0; 85.0) | 76.0 (64.0; 87.0) | |
| Min; max | 39.5; 140.0 | 40.0; 128.0 | 39.5; 140.0 | |
| Height [cm] | 0.869 ^ | |||
| N | 827 | 126 | 699 | |
| Mean (SD) | 168.8 (9.4) | 169.1 (10.0) | 168.8 (9.3) | |
| Median (Q1; Q3) | 169.0 (163.0; 176.0) | 170.0 (162.2; 176.0) | 169.0 (163.0; 175.0) | |
| Min; max | 125.0; 198.0 | 144.0; 197.0 | 125.0; 198.0 | |
| BMI [kg/m2] | 0.007 ^ | |||
| N | 796 | 124 | 670 | |
| Mean (SD) | 26.8 (5.0) | 25.7 (4.9) | 27.0 (5.1) | |
| Median (Q1; Q3) | 26.3 (23.3; 29.8) | 24.8 (22.3; 28.4) | 26.6 (23.4; 30.0) | |
| Min; max | 15.6; 52.3 | 15.6; 43.8 | 15.6; 52.3 | |
| BMI—category, N (%) | 0.022 & | |||
| Underweight | 19 (2.4%) | 5 (4.0%) | 14 (2.1%) | |
| Normal | 303 (38.1%) | 60 (48.4%) | 242 (36.1%) | |
| Overweight | 280 (35.2%) | 35 (28.2%) | 244 (36.4%) | |
| Obese | 194 (24.4%) | 24 (19.4%) | 170 (25.4%) | |
| BSA [m2] | 0.207 ^ | |||
| N | 796 | 124 | 670 | |
| Mean (SD) | 1.9 (0.2) | 1.8 (0.2) | 1.9 (0.2) | |
| Median (Q1; Q3) | 1.9 (1.7; 2.0) | 1.8 (1.6; 2.0) | 1.9 (1.7; 2.0) | |
| Min; max | 1.3; 2.5 | 1.4; 2.5 | 1.3; 2.5 |
| Parameter Category | Overall, N = 842 | Right Colon N = 126 | Left Colon, N = 711 | Right Colon vs. Left Colon |
|---|---|---|---|---|
| Tumour grade, N (%) | 0.115 # | |||
| 1 | 92 (12.8%) | 12 (10.5%) | 80 (13.2%) | |
| 2 | 522 (72.4%) | 78 (68.4%) | 443 (73.1%) | |
| 3 | 107 (14.8%) | 24 (21.1%) | 83 (13.7%) | |
| Mucinous component, N (%) | <0.001 # | |||
| Present | 76 (9.7%) | 30 (24.2%) | 46 (7.0%) | |
| Absent | 709 (90.3%) | 94 (75.8%) | 615 (93.0%) | |
| Initial T score, N (%) (all patients) | 0.177 & | |||
| Tx | 94 (12.0%) | 12 (9.8%) | 82 (12.4%) | |
| T1 | 4 (0.5%) | 1 (0.8%) | 3 (0.5%) | |
| T2 | 49 (6.3%) | 6 (4.9%) | 43 (6.5%) | |
| T3 | 474 (60.5%) | 70 (57.4%) | 404 (61.1%) | |
| T4 | 72 (9.2%) | 10 (8.2%) | 62 (9.4%) | |
| T4a | 38 (4.9%) | 11 (9.0%) | 27 (4.1%) | |
| T4b | 52 (6.6%) | 12 (9.8%) | 40 (6.1%) | |
| Initial N score, N (%) (all patients) | 0.824 & | |||
| Nx | 125 (16.0%) | 17 (14.2%) | 108 (16.3%) | |
| N0 | 128 (16.4%) | 23 (19.2%) | 105 (15.9%) | |
| N1 | 74 (9.5%) | 7 (5.8%) | 67 (10.1%) | |
| N1a | 49 (6.3%) | 7 (5.8%) | 42 (6.4%) | |
| N1b | 89 (11.4%) | 16 (13.3%) | 73 (11.0%) | |
| N1c | 25 (3.2%) | 3 (2.5%) | 22 (3.3%) | |
| N2 | 83 (10.6%) | 13 (10.8%) | 70 (10.6%) | |
| N2a | 92 (11.8%) | 17 (14.2%) | 75 (11.3%) | |
| N2b | 116 (14.9%) | 17 (14.2%) | 99 (15.0%) | |
| Metastases occurrence, N (%) | <0.001 # | |||
| Synchronous | 560 (67.5%) | 93 (73.8%) | 466 (66.4%) | |
| Metachronous | 270 (32.5%) | 33 (26.2%) | 236 (33.6%) | |
| Initial stage for metachronous patients, N (%) | 0.071 # | |||
| I | 14 (5.5%) | 0 (0%) | 14 (6.4%) | |
| II | 57 (22.4%) | 12 (35.3%) | 45 (20.5%) | |
| III | 183 (72%) | 22 (64.7%) | 160 (73.1%) | |
| Prior resection of the primary tumour, N (%) | <0.001 # | |||
| Yes | 558 (70.1%) | 100 (84.0%) | 457 (67.7%) | |
| No | 238 (29.9%) | 19 (16.0%) | 218 (32.3%) | |
| Prior radiotherapy to the primary tumour, N (%) | <0.001 # | |||
| Yes | 178 (21.2%) | 5 (4.0%) | 173 (24.4%) | |
| No | 660 (78.8%) | 121 (96.0%) | 537 (75.6%) | |
| Prior adjuvant/neoadjuvant chemotherapy, N (%) | 0.012 # | |||
| Fluoropirymidine | 173 (23.0%) | 18 (15.9%) | 155 (24.3%) | |
| Fluoropirymidine + oxaliplatin | 86 (11.5%) | 21 (18.6%) | 65 (10.2%) | |
| No | 492 (65.5%) | 74 (65.5%) | 417 (65.5%) | |
| Prior localized therapy for oligometastatic disease, N (%) | 0.290 # | |||
| Yes | 93 (11.2%) | 18 (14.4%) | 75 (10.7%) | |
| No | 735 (88.8%) | 107 (85.6%) | 627 (89.3%) | |
| Sample origin for RAS/BRAF testing, N (%) | 0.079 & | |||
| Primary tumour | 782 (94.0%) | 114 (90.5%) | 667 (94.6%) | |
| Liver metastasis | 28 (3.4%) | 5 (4.0%) | 23 (3.3%) | |
| Non-liver metastasis | 22 (2.6%) | 7 (5.6%) | 15 (2.1%) | |
| Dissemination, N (%) | 0.125 ^ | |||
| Synchronous | 560 (67.5%) | 93 (73.8%) | 466 (66.4%) | |
| Metachronous | 270 (32.5%) | 33 (26.2%) | 236 (33.6%) |
| Parameter Category | Overall, N = 842 | Right Colon, N = 126 | Left Colon, N = 711 | Right Colon vs. Left Colon |
|---|---|---|---|---|
| Metastasis to the liver, N (%) | 0.053 # | |||
| Yes | 615 (73.4%) | 83 (65.9%) | 529 (74.6%) | |
| No | 223 (26.6%) | 43 (34.1%) | 180 (25.4%) | |
| Metastasis to the lungs, N (%) | 0.081 # | |||
| Yes | 237 (28.4%) | 27 (21.6%) | 210 (29.7%) | |
| No | 598 (71.6%) | 98 (78.4%) | 497 (70.3%) | |
| Metastasis to extra-regional lymph nodes, N (%) | 0.095 # | |||
| Yes | 328 (39.1%) | 58 (46.4%) | 270 (38.0%) | |
| No | 510 (60.9%) | 67 (53.6%) | 440 (62.0%) | |
| Metastasis to bones, N (%) | 0.862 # | |||
| Yes | 39 (4.6%) | 5 (4.0%) | 34 (4.8%) | |
| No | 800 (95.4%) | 121 (96.0%) | 676 (95.2%) | |
| Metastases in the peritoneal cavity, N (%) | <0.001 # | |||
| Yes | 189 (22.6%) | 55 (43.7%) | 134 (18.9%) | |
| No | 647 (77.4%) | 71 (56.3%) | 574 (81.1%) | |
| Primary tumour/local recurrence, N (%) | 0.004 # | |||
| Yes | 267 (34.1%) | 26 (22.0%) | 239 (36.0%) | |
| No | 517 (65.9%) | 92 (78.0%) | 425 (64.0%) | |
| Metastasis to other locations, N (%) | 0.226 # | |||
| Yes | 90 (10.8%) | 18 (14.3%) | 72 (10.2%) | |
| No | 746 (89.2%) | 108 (85.7%) | 635 (89.8%) | |
| Baseline SOD (mm) | 0.319 ^ | |||
| N | 759 | 112 | 643 | |
| mean (SD) | 105.5 (77.9) | 95.3 (60.4) | 107.5 (80.6) | |
| median (Q1; Q3) | 87.0 (51.0; 139.0) | 87.5 (41.8; 130.8) | 87.0 (51.0; 140.0) | |
| min; max | 10.0; 840.0 | 10.0; 306.0 | 12.0; 840.0 | |
| Largest dimension of the largest of the liver lesions (mm) | 0.212 ^ | |||
| N | 598 | 79 | 515 | |
| mean (SD) | 52.8 (35.3) | 48.5 (32.2) | 53.6 (35.7) | |
| median (Q1; Q3) | 44.0 (26.2; 70.0) | 40.0 (24.5; 68.5) | 45.0 (27.0; 70.0) | |
| min; max | 1.9; 235.0 | 8.0; 144.0 | 1.9; 235.0 |
| Parameter Category | Overall, N = 631 | Proximal (Right) Colon, N = 101 | Distal (Left) Colon, N = 528 | Proximal (Right) Colon vs. Distal (Left) Colon |
|---|---|---|---|---|
| Depth of response [%] | 0.038 ^ | |||
| N | 631 | 101 | 528 | |
| Mean (SD) | −41.9 (45.1) | −36.7 (41.6) | −43.0 (45.7) | |
| Median (Q1; Q3) | −47.9 (−68.9; −20.2) | −36.7 (−68.5; −8.1) | −50.0 (−69.6; −23.8) | |
| Min; max | −100.0; 366.7 | −100.0; 117.4 | −100.0; 366.7 | |
| Overall response rate, N (%) | 0.010 # | |||
| Complete response | 55 (8.7%) | 12 (11.9%) | 43 (8.1%) | |
| Partial response | 376 (59.6%) | 45 (44.6%) | 330 (62.5%) | |
| Stable disease | 168 (26.6%) | 37 (36.6%) | 130 (24.6%) | |
| Progressive disease | 32 (5.1%) | 7 (6.9%) | 25 (4.7%) | |
| Complete or partial response | 431 (68.3%) | 57 (56.4%) | 373 (70.6%) | −14.2 (−25.2; −3.2) |
| 95% confidence interval | 64.5; 71.9 | 46.2; 66.2 | 66.5; 74.5 | |
| Overall response rate by investigator, N (%) | 0.010 # | |||
| Complete response | 61 (9.7%) | 12 (11.9%) | 49 (9.3%) | |
| Partial response | 392 (62.1%) | 48 (47.5%) | 343 (65.0%) | |
| Stable disease | 134 (21.2%) | 31 (30.7%) | 102 (19.3%) | |
| Progressive disease | 44 (7.0%) | 10 (9.9%) | 34 (6.4%) | |
| Complete or partial response | 453 (71.8%) | 60 (59.4%) | 392 (74.2%) | −14.8 (−25.7; −4.0) |
| 95% confidence interval | 68.1; 75.2 | 49.2; 68.9 | 70.2; 77.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potocki, P.M.; Wiśniowski, R.; Haus, D.; Chowaniec, Z.; Kozaczka, M.; Kustra, M.; Samborska-Plewicka, M.; Szweda, M.; Starzyczny-Słota, D.; Michalik, M.; et al. The Impact of Sidedness on the Efficacy of Anti-EGFR-Based First-Line Chemotherapy in Advanced Colorectal Cancer Patients in Real-Life Setting—A Nation-Wide Retrospective Analysis (RACER). Cancers 2023, 15, 4361. https://doi.org/10.3390/cancers15174361
Potocki PM, Wiśniowski R, Haus D, Chowaniec Z, Kozaczka M, Kustra M, Samborska-Plewicka M, Szweda M, Starzyczny-Słota D, Michalik M, et al. The Impact of Sidedness on the Efficacy of Anti-EGFR-Based First-Line Chemotherapy in Advanced Colorectal Cancer Patients in Real-Life Setting—A Nation-Wide Retrospective Analysis (RACER). Cancers. 2023; 15(17):4361. https://doi.org/10.3390/cancers15174361
Chicago/Turabian StylePotocki, Paweł Michał, Rafał Wiśniowski, Dominik Haus, Zbyszko Chowaniec, Maciej Kozaczka, Magdalena Kustra, Marzenna Samborska-Plewicka, Marcin Szweda, Danuta Starzyczny-Słota, Magdalena Michalik, and et al. 2023. "The Impact of Sidedness on the Efficacy of Anti-EGFR-Based First-Line Chemotherapy in Advanced Colorectal Cancer Patients in Real-Life Setting—A Nation-Wide Retrospective Analysis (RACER)" Cancers 15, no. 17: 4361. https://doi.org/10.3390/cancers15174361






