Prognostic Role of Dynamic Changes in Serological Markers in Metastatic Hormone Naïve Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
3. Statistical Analyses
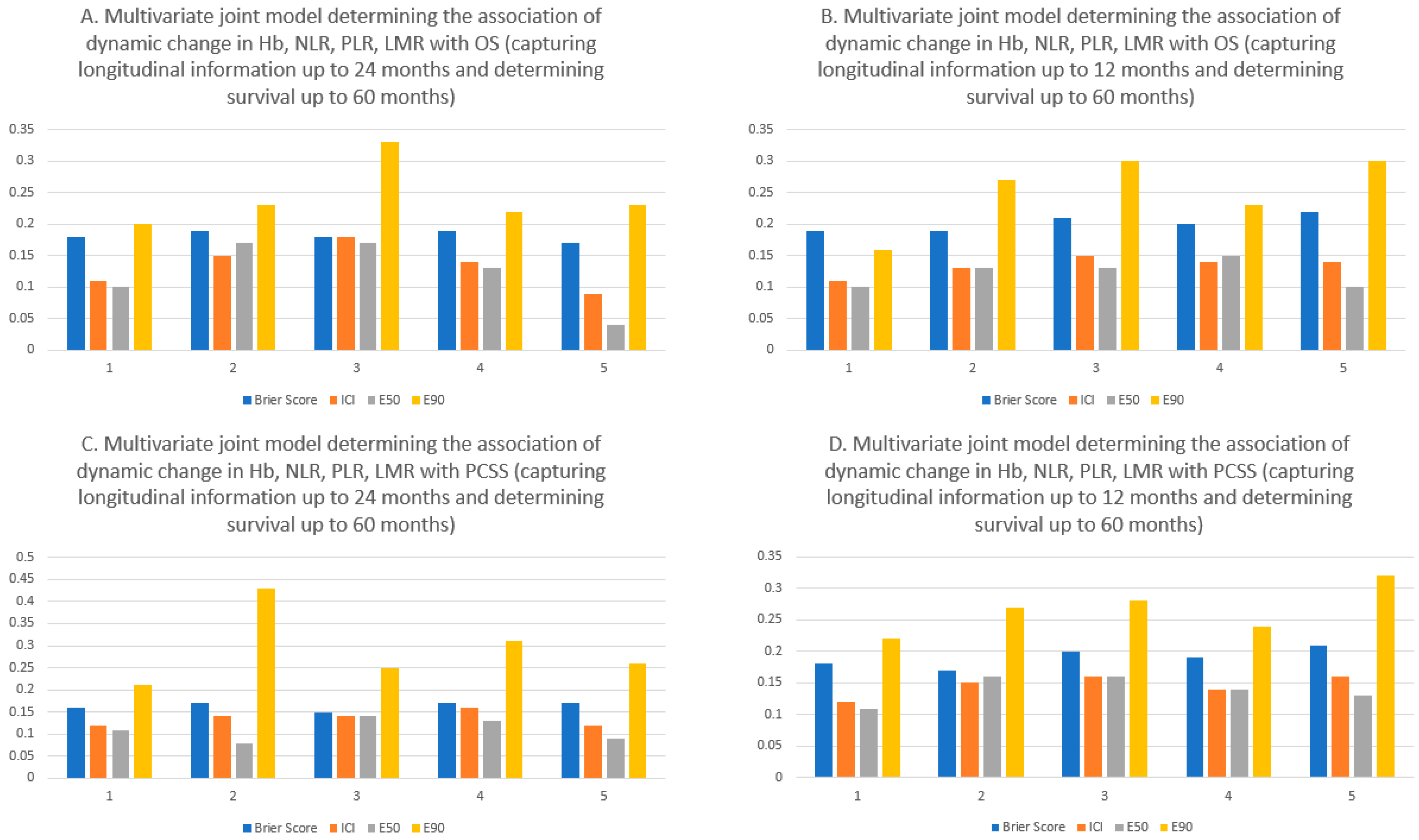
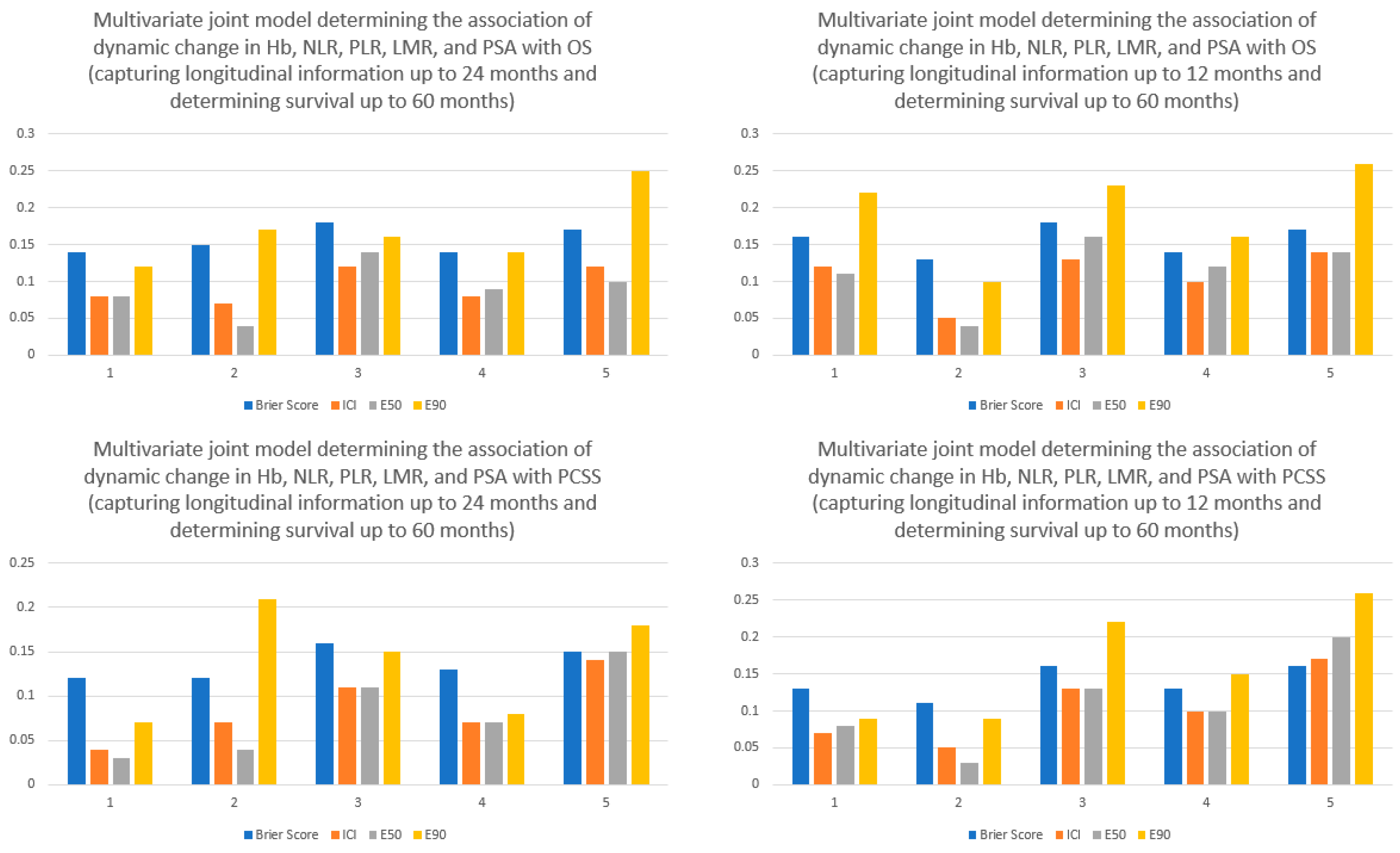
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, S.; Morgan, S.C. Who Dies From Prostate Cancer? An Analysis of the Surveillance, Epidemiology and End Results Database. Clin. Oncol. 2019, 31, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Sandin, R.; Sah, J.; Emir, B.; Mu, Q.; Ratiu, A.; Hong, A.; Serfass, L.; Tagawa, S.T. Treatment Patterns and Survival in Metastatic Castration-Sensitive Prostate Cancer in the US Veterans Health Administration. Cancer Med. 2021, 10, 8570–8580. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus Prednisone Added to Androgen Deprivation Therapy and Docetaxel in de Novo Metastatic Castration-Sensitive Prostate Cancer (PEACE-1): A Multicentre, Open-Label, Randomised, Phase 3 Study with a 2 × 2 Factorial Design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez, A.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin. Oncol. 2021, 39, 2294–2303. [Google Scholar] [CrossRef]
- Roy, S.; Sayyid, R.; Saad, F.; Sun, Y.; Lajkosz, K.; Ong, M.; Klaassen, Z.; Malone, S.; Spratt, D.E.; Wallis, C.J.D.; et al. Addition of Docetaxel to Androgen Receptor Axis–Targeted Therapy and Androgen Deprivation Therapy in Metastatic Hormone-Sensitive Prostate Cancer: A Network Meta-Analysis. Eur. Urol. Oncol. 2022, 5, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the Primary Tumour for Newly Diagnosed, Metastatic Prostate Cancer (STAMPEDE): A Randomised Controlled Phase 3 Trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef]
- Dai, B.; Zhang, S.; Wan, F.-N.; Wang, H.-K.; Zhang, J.-Y.; Wang, Q.-F.; Kong, Y.-Y.; Ma, X.-J.; Mo, M.; Zhu, Y.; et al. Combination of Androgen Deprivation Therapy with Radical Local Therapy Versus Androgen Deprivation Therapy Alone for Newly Diagnosed Oligometastatic Prostate Cancer: A Phase II Randomized Controlled Trial. Eur. Urol. Oncol. 2022, 5, 519–525. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Kessel, A.; Kohli, M.; Swami, U. Current Management of Metastatic Castration-Sensitive Prostate Cancer. Cancer Treat. Res. Commun. 2021, 28, 100384. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.W.; Higano, C.S.; Taplin, M.-E.; Ryan, C.J.; Agarwal, N. Metastatic Castration-Sensitive Prostate Cancer: Optimizing Patient Selection and Treatment. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Tangen, C.M.; Higano, C.; Schelhammer, P.F.; Faulkner, J.; Crawford, E.D.; Wilding, G.; Akdas, A.; Small, E.J.; Donnelly, B.; et al. Absolute Prostate-Specific Antigen Value after Androgen Deprivation Is a Strong Independent Predictor of Survival in New Metastatic Prostate Cancer: Data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin. Oncol. 2006, 24, 3984–3990. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Bjartell, A.; Agarwal, N.; Chung, B.H.; Given, R.W.; Pereira de Santana Gomes, A.J.; Merseburger, A.S.; Özgüroğlu, M.; Soto, Á.J.; Uemura, H.; et al. Deep, Rapid, and Durable Prostate-Specific Antigen Decline with Apalutamide plus Androgen Deprivation Therapy Is Associated with Longer Survival and Improved Clinical Outcomes in TITAN Patients with Metastatic Castration-Sensitive Prostate Cancer. Ann. Oncol. 2023, 34, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; de Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, N.; Chi, K.N.; Özgüroğlu, M.; Rodriguez-Antolin, A.; Feyerabend, S.; Fein, L.; Alekseev, B.Y.; Sulur, G.; Protheroe, A.; Li, S.; et al. Correlation of Prostate-Specific Antigen Kinetics with Overall Survival and Radiological Progression-Free Survival in Metastatic Castration-Sensitive Prostate Cancer Treated with Abiraterone Acetate plus Prednisone or Placebos Added to Androgen Deprivation Therapy: Post Hoc Analysis of Phase 3 LATITUDE Study. Eur. Urol. 2020, 77, 494–500. [Google Scholar] [CrossRef] [PubMed]
- de Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Grönberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in Prostate Carcinogenesis. Nat. Rev. Cancer 2007, 7, 256. [Google Scholar] [CrossRef]
- Taichman, R.S.; Loberg, R.D.; Mehra, R.; Pienta, K.J. The Evolving Biology and Treatment of Prostate Cancer. J. Clin. Invest. 2007, 117, 2351–2361. [Google Scholar] [CrossRef]
- Notario, L.; Piulats, J.M.; Sala, N.; Ferrandiz, U.; González, A.; Etxániz, O.; Heras, L.; Buisan, O.; del Carpio, L.; Álvarez, A.; et al. 667P Impact of Pretreatment Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) on Overall Survival (OS) in Patients (p) with Metastatic Castration-Sensitive Prostate Cancer (MCSPC) Treated with Docetaxel (D) plus Androgen-Deprivation Therapy (ADT). Ann. Oncol. 2020, 31, S537. [Google Scholar] [CrossRef]
- Kumano, Y.; Hasegawa, Y.; Kawahara, T.; Yasui, M.; Miyoshi, Y.; Matsubara, N.; Uemura, H. Pretreatment Neutrophil to Lymphocyte Ratio (NLR) Predicts Prognosis for Castration Resistant Prostate Cancer Patients Underwent Enzalutamide. BioMed Res. Int. 2019, 2019, 9450838. [Google Scholar] [CrossRef]
- Guan, Y.; Xiong, H.; Feng, Y.; Liao, G.; Tong, T.; Pang, J. Revealing the Prognostic Landscape of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Abiraterone or Enzalutamide: A Meta-Analysis. Prostate Cancer Prostatic Dis. 2020, 23, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Koyama, K.; Takezawa, Y.; Nishigaki, Y. Baseline Neutrophil-to-Lymphocyte Ratio Predicts the Prognosis of Castration-Resistant Prostate Cancer Treated with Abiraterone Acetate. Mol Clin. Oncol. 2018, 8, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Loubersac, T.; Nguile-Makao, M.; Pouliot, F.; Fradet, V.; Toren, P. Neutrophil-to-Lymphocyte Ratio as a Predictive Marker of Response to Abiraterone Acetate: A Retrospective Analysis of the COU302 Study. Eur. Urol. Oncol. 2020, 3, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.J.D.; Shayegan, B.; Morgan, S.C.; Hamilton, R.J.; Cagiannos, I.; Basappa, N.S.; Ferrario, C.; Gotto, G.T.; Fernandes, R.; Roy, S.; et al. Prognostic Association between Common Laboratory Tests and Overall Survival in Elderly Men with de Novo Metastatic Castration Sensitive Prostate Cancer: A Population-Based Study in Canada. Cancers 2021, 13, 2844. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Sun, Y.; Wallis, C.J.D.; Morgan, S.C.; Grimes, S.; Malone, J.; Kishan, A.U.; Mukherjee, D.; Spratt, D.E.; Saad, F.; et al. Development and Validation of a Multivariable Prognostic Model in de Novo Metastatic Castrate Sensitive Prostate Cancer. Prostate Cancer Prostatic Dis. 2022, 26, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Lee, H.; Yang, W. Association between Systemic Inflammation and Serum Prostate-Specific Antigen in a Healthy Korean Population. Turk. J. Urol. 2017, 43, 284. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, C.; Gramignano, G.; Astara, G.; Demontis, R.; Sanna, E.; Atzeni, V.; Macciò, A. Pathogenesis and Treatment Options of Cancer Related Anemia: Perspective for a Targeted Mechanism-Based Approach. Front. Physiol. 2018, 9, 1294. [Google Scholar] [CrossRef] [PubMed]
- Halabi, S.; Li, C.; Luo, S. Developing and Validating Risk Assessment Models of Clinical Outcomes in Modern Oncology. JCO Precis. Oncol. 2019, 3, PO.19.00068. [Google Scholar] [CrossRef]
- Macciò, A.; Madeddu, C.; Gramignano, G.; Mulas, C.; Tanca, L.; Cherchi, M.C.; Floris, C.; Omoto, I.; Barracca, A.; Ganz, T. The Role of Inflammation, Iron, and Nutritional Status in Cancer-Related Anemia: Results of a Large, Prospective, Observational Study. Haematologica 2015, 100, 124–132. [Google Scholar] [CrossRef]
- Archer, M.; Dogra, N.; Kyprianou, N. Inflammation as a Driver of Prostate Cancer Metastasis and Therapeutic Resistance. Cancers 2020, 12, 2984. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.P.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone Acetate plus Prednisone in Patients with Newly Diagnosed High-Risk Metastatic Castration-Sensitive Prostate Cancer (LATITUDE): Final Overall Survival Analysis of a Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2019, 20, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Rizopoulos, D.; Hatfield, L.A.; Carlin, B.P.; Takkenberg, J.J.M. Combining Dynamic Predictions From Joint Models for Longitudinal and Time-to-Event Data Using Bayesian Model Averaging. J. Am. Stat. Assoc. 2014, 109, 1385–1397. [Google Scholar] [CrossRef]
- Austin, P.C.; Steyerberg, E.W. The Integrated Calibration Index (ICI) and Related Metrics for Quantifying the Calibration of Logistic Regression Models. Stat. Med. 2019, 38, 4051–4065. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Cronin, A.M.; Elkin, E.B.; Gonen, M. Extensions to Decision Curve Analysis, a Novel Method for Evaluating Diagnostic Tests, Prediction Models and Molecular Markers. BMC Med. Inform. Decis. Mak. 2008, 8, 53. [Google Scholar] [CrossRef]
- Vickers, A.J.; Kattan, M.W.; Daniel, S. Method for Evaluating Prediction Models That Apply the Results of Randomized Trials to Individual Patients. Trials 2007, 8, 14. [Google Scholar] [CrossRef]
- Henderson, R.; Keiding, N. Individual Survival Time Prediction Using Statistical Models. J. Med. Ethics 2005, 31, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Virgo, K.S.; Rumble, R.B.; de Wit, R.; Mendelson, D.S.; Smith, T.J.; Taplin, M.-E.; Wade, J.L.; Bennett, C.L.; Scher, H.I.; Nguyen, P.L.; et al. Initial Management of Noncastrate Advanced, Recurrent, or Metastatic Prostate Cancer: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 1274–1305. [Google Scholar] [CrossRef]
- Naqvi, S.A.A.; bin Riaz, Z.; Riaz, A.; Islam, M.; Siddiqi, R.; Ikram, W.; Jafar, M.A.; Singh, P.; Ravi, P.K.; bin Riaz, I.; et al. Triplet Therapy in Metastatic Castration-Sensitive Prostate Cancer: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2022, 40, 136. [Google Scholar] [CrossRef]
- Kostos, L.; Murphy, D.G.; Azad, A.A. Double or Triple Trouble in Metastatic Hormone-Sensitive Prostate Cancer? Eur. Urol. Oncol. 2022, 5, 503–504. [Google Scholar] [CrossRef]
- Hussain, M.; Tombal, B.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Shore, N.; Kopyltsov, E.; Kalebasty, A.R.; Bögemann, M.; et al. Darolutamide Plus Androgen-Deprivation Therapy and Docetaxel in Metastatic Hormone-Sensitive Prostate Cancer by Disease Volume and Risk Subgroups in the Phase III ARASENS Trial. J Clin. Oncol. 2023, 41, 3595–3607. [Google Scholar] [CrossRef]
| Abiraterone Acetate Plus ADT | ADT Plus Placebo | p-Value | |
|---|---|---|---|
| n (Total number) | 563 | 575 | |
| Age (median [IQR]) | 65.0 [60.0, 70.0] | 65.0 [60.0, 70.0] | 0.44 |
| Age group (%) | |||
| <65 | 210 (37.3) | 220 (38.3) | |
| 65–69 | 104 (18.5) | 132 (23.0) | |
| 70–74 | 135 (24.0) | 108 (18.8) | |
| ≥75 | 114 (20.2) | 115 (20.0) | |
| Presence of liver metastasis (%) | 29 (5.2) | 29 (5.0) | 0.99 |
| Presence of lung metastasis (%) | 70 (12.4) | 69 (12.0) | 0.89 |
| Nodal stage | |||
| N0/Nx (%) | 300 (53.3) | 308 (53.6) | 0.97 |
| N1 (%) | 263 (46.7) | 267 (46.4) | |
| Gleason score | |||
| <9 (%) | 263 (46.7) | 284 (49.4) | 0.40 |
| 9–10 (%) | 300 (53.3) | 291 (50.6) | |
| ECOG performance status | |||
| 0 (%) | 313 (55.6) | 314 (54.8) | 0.83 |
| ≥1 (%) | 250 (44.4) | 260 (45.2) | |
| Number of skeletal metastases | |||
| 0–9 | 193 (34.3) | 201 (35.0) | 0.86 |
| ≥10 (%) | 370 (65.7) | 374 (65.0) | |
| Worst pain score (median (IQR)) | 1.0 (0.0, 4.0) | 1.0 (0.0, 4.0) | 0.66 |
| Baseline PSA (median (IQR)) | 18.4 (3.7, 77.0) | 14.8 (2.9, 76.2) | 0.20 |
| Baseline Hb (g/dL) (median (IQR)) | 13.2 (12.0, 14.3) | 13.3 (12.1, 14.4) | 0.42 |
| Baseline NLR (median (IQR)) | 2.2 (1.6, 3.0) | 2.2 (1.7, 3.0) | 0.97 |
| Baseline PLR (median (IQR)) | 138 (108, 183) | 138 (107, 180) | 0.93 |
| Baseline LMR (median (IQR)) | 4.7 (3.6, 6.0) | 4.8 (3.6, 6.3) | 0.43 |
| Models | Parameters | Hazard Ratio | Lower CI | Upper CI | p-Value |
|---|---|---|---|---|---|
| For OS | ADT alone vs. Abiraterone plus ADT | 1.61 | 1.28 | 2.04 | <0.001 |
| Skeletal lesions (10 or more vs. 0–10) | 1.69 | 1.38 | 2.06 | <0.001 | |
| ECOG performance status (1–2 vs. 0) | 1.31 | 1.11 | 1.56 | 0.004 | |
| Nodal stage | 1.08 | 0.92 | 1.28 | 0.35 | |
| Liver metastasis (yes vs. no) | 1.56 | 1.10 | 2.16 | 0.008 | |
| Gleason score (9–10 vs. <9) | 1.22 | 1.03 | 1.45 | 0.02 | |
| Baseline worst pain score | 1.05 | 1.02 | 1.09 | 0.003 | |
| Baseline Hb | 0.99 | 0.99 | 1.00 | 0.01 | |
| Log of baseline PSA | 0.96 | 0.93 | 1.01 | 0.08 | |
| Dynamic change in the current Hb level by 1 g/dL | 0.80 | 0.75 | 0.86 | <0.001 | |
| Dynamic change in the current NLR level by 1 point | 1.19 | 1.06 | 1.33 | 0.003 | |
| Dynamic change in the current PLR level by 100 points | 1.07 | 0.87 | 1.31 | 0.47 | |
| Dynamic change in the current LMR by 5 points | 0.90 | 0.68 | 1.10 | 0.43 | |
| For PCSS | ADT alone vs. Abiraterone plus ADT | 1.71 | 1.32 | 2.25 | <0.001 |
| Skeletal lesions (10 or more vs. 0–10) | 1.88 | 1.49 | 2.41 | <0.001 | |
| ECOG performance status (1–2 vs. 0) | 1.30 | 1.08 | 1.58 | 0.008 | |
| Nodal stage | 1.03 | 0.85 | 1.25 | 0.80 | |
| Liver metastasis (yes vs. no) | 1.69 | 1.14 | 2.43 | 0.008 | |
| Gleason score (9–10 vs. <9) | 1.27 | 1.05 | 1.54 | 0.02 | |
| Baseline worst pain score | 1.05 | 1.01 | 1.09 | 0.02 | |
| Baseline Hb | 0.99 | 0.98 | 1.00 | 0.006 | |
| Log of baseline PSA | 0.97 | 0.93 | 1.01 | 0.15 | |
| Dynamic change in the current Hb level by 1 g/dL | 0.81 | 0.75 | 0.87 | <0.001 | |
| Dynamic change in the current NLR level by 1 point | 1.11 | 0.97 | 1.27 | 0.13 | |
| Dynamic change in the current PLR level by 100 points | 1.19 | 0.93 | 1.51 | 0.17 | |
| Dynamic change in the current LMR by 5 points | 0.86 | 0.61 | 1.10 | 0.37 |
| Models | Parameters | Hazard Ratio | Lower CI | Upper CI | p-Value |
|---|---|---|---|---|---|
| For OS | ADT alone vs. abiraterone plus ADT | 0.87 | 0.66 | 1.13 | 0.29 |
| Skeletal lesions (10 or more vs. 0–10) | 1.34 | 1.07 | 1.68 | 0.01 | |
| ECOG performance status (1–2 vs. 0) | 1.33 | 1.11 | 1.58 | 0.004 | |
| Nodal stage | 1.14 | 0.96 | 1.36 | 0.16 | |
| Liver metastasis (yes vs. no) | 1.78 | 1.23 | 2.49 | 0.001 | |
| Gleason score (9–10 vs. <9) | 1.17 | 0.98 | 1.38 | 0.07 | |
| Baseline worst pain score | 1.04 | 1.01 | 1.09 | 0.02 | |
| Baseline Hb | 0.99 | 0.99 | 1.00 | 0.001 | |
| Log of baseline PSA | 0.92 | 0.88 | 0.96 | <0.001 | |
| Dynamic change in the current Hb level by 1 g/dL | 0.88 | 0.81 | 0.94 | <0.001 | |
| Dynamic change in the current NLR level by 1 point | 1.26 | 1.11 | 1.44 | <0.001 | |
| Dynamic change in the current PLR level by 100 points | 0.97 | 0.76 | 1.22 | 0.79 | |
| Dynamic change in the current LMR by 5 points | 1.02 | 0.78 | 1.22 | 0.81 | |
| Dynamic change in the log of current PSA value | 1.24 | 1.20 | 1.28 | <0.001 | |
| For PCSS | ADT alone vs. abiraterone plus ADT | 0.82 | 0.59 | 1.15 | 0.24 |
| Skeletal lesions (10 or more vs. 0–10) | 1.39 | 1.07 | 1.82 | 0.02 | |
| ECOG Performance status (1–2 vs. 0) | 1.29 | 1.04 | 1.58 | 0.02 | |
| Nodal stage | 1.11 | 0.91 | 1.35 | 0.30 | |
| Liver metastasis (yes vs. no) | 1.99 | 1.33 | 2.89 | <0.001 | |
| Gleason score (9–10 vs. <9) | 1.21 | 1.00 | 1.51 | 0.05 | |
| Baseline worst pain score | 1.04 | 1.00 | 1.09 | 0.05 | |
| Baseline Hb | 0.99 | 0.98 | 1.00 | 0.001 | |
| Log of baseline PSA | 0.92 | 0.88 | 0.97 | 0.001 | |
| Dynamic change in the current Hb level by 1 g/dL | 0.90 | 0.83 | 0.98 | 0.009 | |
| Dynamic change in the current NLR level by 1 point | 1.22 | 1.04 | 1.44 | 0.007 | |
| Dynamic change in the current PLR level by 100 points | 1.01 | 0.76 | 1.32 | 0.95 | |
| Dynamic change in the current LMR by 5 points | 0.98 | 0.68 | 1.23 | 0.99 | |
| Dynamic change in the log of current PSA value | 1.28 | 1.23 | 1.34 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, S.; Sun, Y.; Wallis, C.J.D.; Kishan, A.U.; Morgan, S.C.; Spratt, D.E.; Malone, S.; Saad, F. Prognostic Role of Dynamic Changes in Serological Markers in Metastatic Hormone Naïve Prostate Cancer. Cancers 2023, 15, 4392. https://doi.org/10.3390/cancers15174392
Roy S, Sun Y, Wallis CJD, Kishan AU, Morgan SC, Spratt DE, Malone S, Saad F. Prognostic Role of Dynamic Changes in Serological Markers in Metastatic Hormone Naïve Prostate Cancer. Cancers. 2023; 15(17):4392. https://doi.org/10.3390/cancers15174392
Chicago/Turabian StyleRoy, Soumyajit, Yilun Sun, Christopher J. D. Wallis, Amar U. Kishan, Scott C. Morgan, Daniel E. Spratt, Shawn Malone, and Fred Saad. 2023. "Prognostic Role of Dynamic Changes in Serological Markers in Metastatic Hormone Naïve Prostate Cancer" Cancers 15, no. 17: 4392. https://doi.org/10.3390/cancers15174392






