Expanding the Clinical Utility of Targeted RNA Sequencing Panels beyond Gene Fusions to Complex, Intragenic Structural Rearrangements
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Library Preparation and Sequencing
2.3. Sequencing
2.4. Sequence Data Analysis
2.5. Orthogonal Confirmation
2.6. Statistical Analysis
3. Results
3.1. Overall Clinical Cohort Characteristics
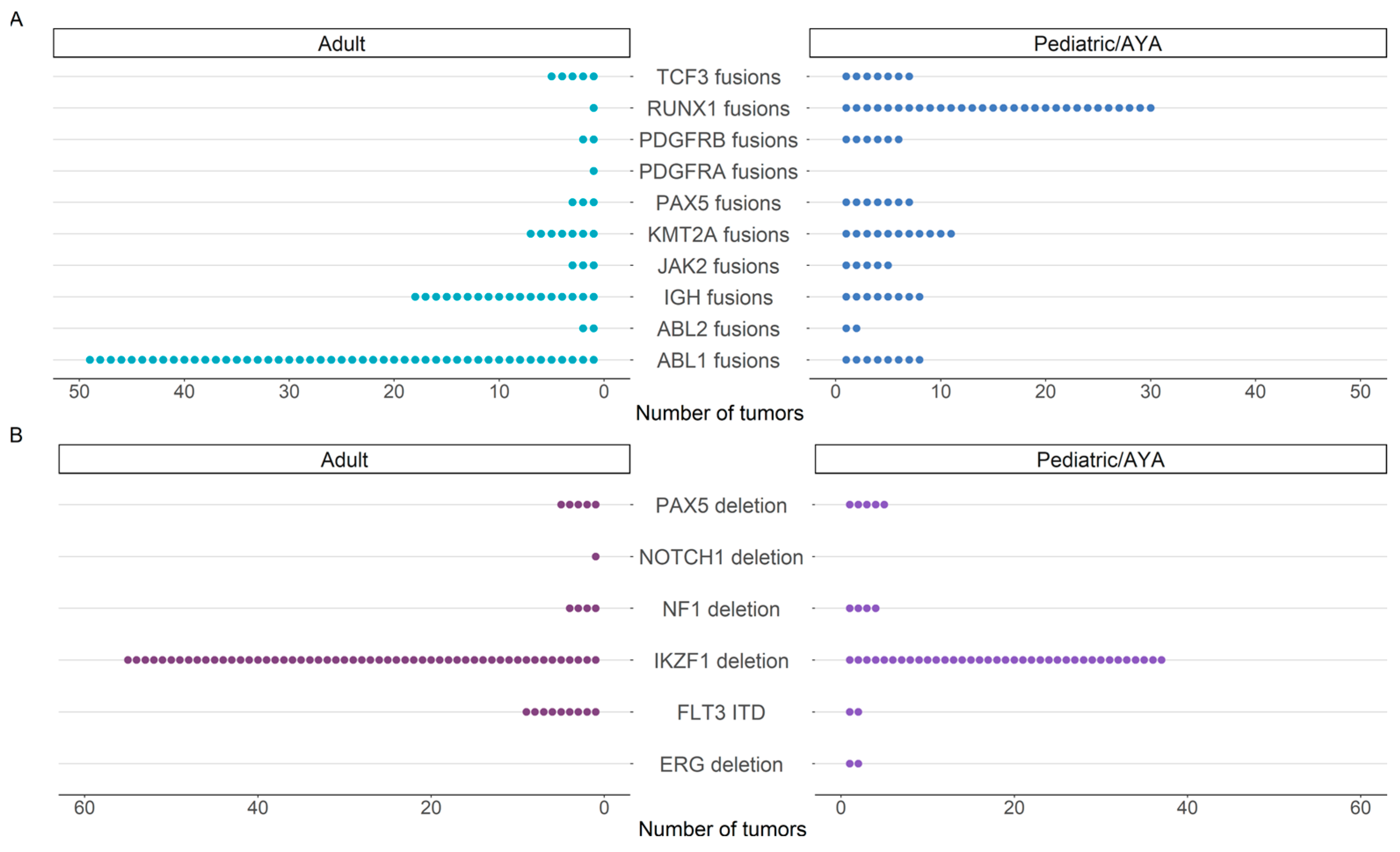
3.2. Three-Year Diagnostic Yield for Hematologic Malignancies
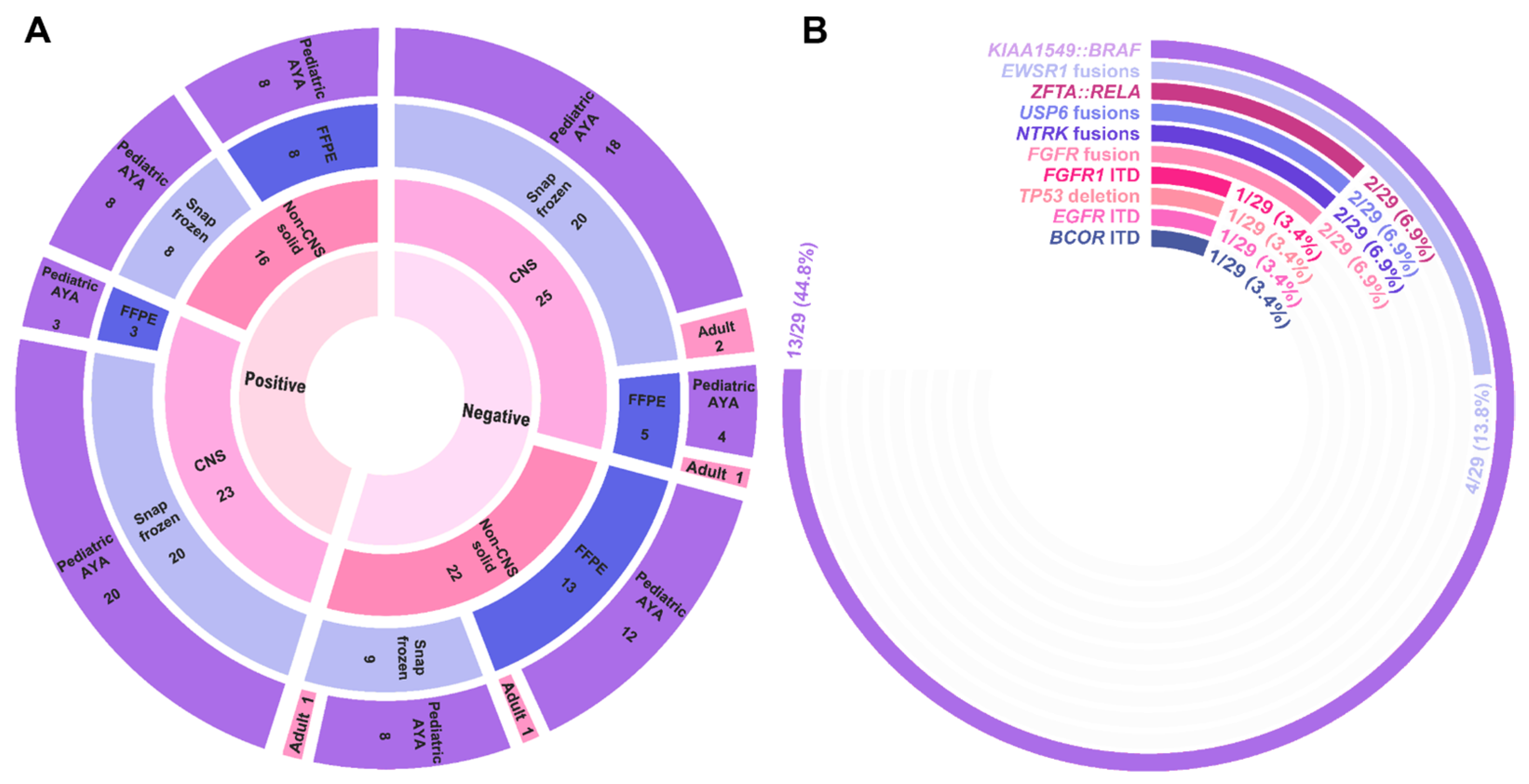
3.3. Three-Year Diagnostic Yield for CNS and Non-CNS Solid Tumors
3.4. Clinical Utility of Identified Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitelman, F.; Johansson, B.; Mertens, F. The impact of translocations and gene fusions on cancer causation. Nat. Rev. Cancer 2007, 7, 233–245. [Google Scholar] [CrossRef]
- Baik, C.S.; Wu, D.; Smith, C.; Martins, R.G.; Pritchard, C.C. Durable response to tyrosine kinase inhibitor therapy in a lung cancer patient harboring epidermal growth factor receptor tandem kinase domain duplication. J. Thorac. Oncol. 2015, 10, e97–e99. [Google Scholar] [CrossRef]
- Chiang, S.; Lee, C.H.; Stewart, C.J.R.; Oliva, E.; Hoang, L.N.; Ali, R.H.; Hensley, M.L.; Arias-Stella, J.A.; Frosina, D.; Jungbluth, A.A.; et al. BCOR is a robust diagnostic immunohistochemical marker of genetically diverse high-grade endometrial stromal sarcoma, including tumors exhibiting variant morphology. Mod. Pathol. 2017, 30, 1251–1261. [Google Scholar] [CrossRef]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef]
- Fina, F.; Barets, D.; Colin, C.; Bouvier, C.; Padovani, L.; Nanni-Metellus, I.; Ouafik, L.H.; Scavarda, D.; Korshunov, A.; Jones, D.T.W.; et al. Droplet digital PCR is a powerful technique to demonstrate frequent FGFR1 duplication in dysembryoplastic neuroepithelial tumors. Oncotarget 2017, 8, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Gallant, J.N.; Sheehan, J.H.; Shaver, T.M.; Bailey, M.; Lipson, D.; Chandramohan, R.; Brewer, M.R.; York, S.J.; Kris, M.G.; Pietenpol, J.A.; et al. EGFR kinase domain duplication (EGFR -KDD) is a novel oncogenic driver in lung cancer that is clinically responsive to afatinib. Cancer Discov. 2015, 5, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Butler, E.; Wilson, R.; Roy, A.; Zheng, Y.; Liem, P.; Rakheja, D.; Pavlick, D.; Young, L.L.; Rosenzweig, M. Novel PDGFRB rearrangement in multifocal infantile myofibromatosis is tumorigenic and sensitive to imatinib. Mol. Case Stud. 2019, 5, a004440. [Google Scholar] [CrossRef]
- Jones, D.T.W.; Hutter, B.; Jäger, N.; Korshunov, A.; Kool, M.; Warnatz, H.J.; Zichner, T.; Lambert, S.R.; Ryzhova, M.; Quang, D.A.K.; et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat. Genet. 2013, 45, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Mohankumar, K.M.; Punchihewa, C.; Weinlich, R.; Dalton, J.D.; Li, Y.; Lee, R.; Tatevossian, R.G.; Phoenix, T.N.; Thiruvenkatam, R.; et al. C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature 2014, 506, 451–455. [Google Scholar] [CrossRef]
- Ryall, S.; Zapotocky, M.; Fukuoka, K.; Nobre, L.; Stucklin, A.G.; Bennett, J.; Siddaway, R.; Li, C.; Pajovic, S.; Arnoldo, A. Integrated molecular and clinical analysis of 1000 pediatric low-grade gliomas. Cancer Cell 2020, 37, 569–583.e5. [Google Scholar] [CrossRef] [PubMed]
- Stanulla, M.; Cavé, H.; Moorman, A.V. IKZF1 deletions in pediatric acute lymphoblastic leukemia: Still a poor prognostic marker? Blood 2020, 135, 252–260. [Google Scholar] [CrossRef]
- Tallman, M.S.; Wang, E.S.; Altman, J.K.; Appelbaum, F.R.; Bhatt, V.R.; Bixby, D.; Coutre, S.E.; De Lima, M.; Fathi, A.T.; Fiorella, M.; et al. Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 721–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Xue, X.; Ou, Q.; Wu, X.; Liang, Y.; Wang, X.; You, M.; Shao, Y.W.; Zhang, Z. Clinical outcomes of EGFR kinase domain duplication to targeted therapies in NSCLC. Int. J. Cancer 2019, 144, 2677–2682. [Google Scholar] [CrossRef] [PubMed]
- Wegert, J.; Vokuhl, C.; Collord, G.; Del Castillo Velasco-Herrera, M.; Farndon, S.J.; Guzzo, C.; Jorgensen, M.; Anderson, J.; Slater, O.; Duncan, C.; et al. Recurrent intragenic rearrangements of EGFR and BRAF in soft tissue tumors of infants. Nat. Commun. 2018, 9, 2378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, G.; Miller, C.P.; Tatevossian, R.G.; Dalton, J.D.; Tang, B.; Orisme, W.; Punchihewa, C.; Parker, M.; Qaddoumi, I.; et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat. Genet. 2013, 45, 602–612. [Google Scholar] [CrossRef]
- Mertens, F.; Johansson, B.; Fioretos, T.; Mitelman, F. The emerging complexity of gene fusions in cancer. Nat. Rev. Cancer 2015, 15, 371–381. [Google Scholar] [CrossRef]
- Kiyoi, H.; Towatari, M.; Yokota, S.; Hamaguchi, M.; Ohno, R.; Saito, H.; Naoe, T. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia 1998, 12, 1333–1337. [Google Scholar] [CrossRef]
- Griffith, J.; Black, J.; Faerman, C.; Swenson, L.; Wynn, M.; Lu, F.; Lippke, J.; Saxena, K. The Structural Basis for Autoinhibition of FLT3 by the Juxtamembrane Domain. Mol. Cell 2004, 13, 169–178. [Google Scholar] [CrossRef]
- Lei, L.; Stohr, B.A.; Berry, S.; Lockwood, C.M.; Davis, J.L.; Rudzinski, E.R.; Kunder, C.A. Recurrent EGFR alterations in NTRK3 fusion negative congenital mesoblastic nephroma. Pract. Lab. Med. 2020, 21, e00164. [Google Scholar] [CrossRef]
- Zhao, M.; Yin, M.; Kuick, C.H.; Chen, H.; Aw, S.J.; Merchant, K.; Ng, E.H.Q.; Gunaratne, S.; Loh, A.H.P.; Gu, W.; et al. Congenital mesoblastic nephroma is characterised by kinase mutations including EGFR internal tandem duplications, the ETV6–NTRK3 fusion, and the rare KLHL7–BRAF fusion. Histopathology 2020, 77, 611–621. [Google Scholar] [CrossRef]
- Brown, P.; Inaba, H.; Annesley, C.; Beck, J.; Colace, S.; Dallas, M.; DeSantes, K.; Kelly, K.; Kitko, C.; Lacayo, N.; et al. Pediatric acute lymphoblastic leukemia, version 2.2020. JNCCN J. Natl. Compr. Cancer Netw. 2020, 18, 81–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.M.; Harvey, R.C.; Mullighan, C.G.; Gastier-Foster, J.; Wharton, W.; Kang, H.; Borowitz, M.J.; Camitta, B.M.; Carroll, A.J.; Devidas, M.; et al. Outcome modeling with CRLF2, IKZF1, JAK, and minimal residual disease in pediatric acute lymphoblastic leukemia: A Children’s Oncology Group study. Blood 2012, 119, 3512–3522. [Google Scholar] [CrossRef] [PubMed]
- Bridge, J.A. The role of cytogenetics and molecular diagnostics in the diagnosis of soft-tissue tumors. Mod. Pathol. 2014, 27, 580–597. [Google Scholar] [CrossRef] [PubMed]
- Paratala, B.S.; Dolfi, S.C.; Khiabanian, H.; Rodriguez-Rodriguez, L.; Ganesan, S.; Hirshfield, K.M. Emerging Role of Genomic Rearrangements in Breast Cancer: Applying Knowledge from Other Cancers. Biomark. Cancer 2016, 8s1, BIC.S34417. [Google Scholar] [CrossRef]
- Akkari, Y.M.N.; Baughn, L.B.; Dubuc, A.M.; Smith, A.C.; Mallo, M.; Dal Cin, P.; Diez Campelo, M.; Gallego, M.S.; Granada Font, I.; Haase, D.T.; et al. Guiding the global evolution of cytogenetic testing for hematologic malignancies. Blood 2022, 139, 2273–2284. [Google Scholar] [CrossRef]
- Tian, L.; Li, Y.; Edmonson, M.N.; Zhou, X.; Newman, S.; McLeod, C.; Thrasher, A.; Liu, Y.; Tang, B.; Rusch, M.C.; et al. CICERO: A versatile method for detecting complex and diverse driver fusions using cancer RNA sequencing data. Genome Biol. 2020, 21, 126. [Google Scholar] [CrossRef]
- Uhrig, S.; Ellermann, J.; Walther, T.; Burkhardt, P.; Fröhlich, M.; Hutter, B.; Toprak, U.H.; Neumann, O.; Stenzinger, A.; Scholl, C.; et al. Accurate and efficient detection of gene fusions from RNA sequencing data. Genome Res. 2021, 31, 448–460. [Google Scholar] [CrossRef]
- Ye, K.; Schulz, M.H.; Long, Q.; Apweiler, R.; Ning, Z. Pindel: A pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics 2009, 25, 2865–2871. [Google Scholar] [CrossRef]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef]
- Bennett, J.T.; Tan, T.Y.; Alcantara, D.; Tétrault, M.; Timms, A.E.; Jensen, D.; Collins, S.; Nowaczyk, M.J.; Lindhurst, M.J.; Christensen, K.M. Mosaic activating mutations in FGFR1 cause encephalocraniocutaneous lipomatosis. Am. J. Hum. Genet. 2016, 98, 579–587. [Google Scholar] [CrossRef]
- Gupta, A.; Liu, H.; Schieffer, K.M.; Koo, S.C.; Cottrell, C.E.; Mardis, E.R.; Roberts, R.D.; Yeager, N.D. Targeted Therapy in a Young Adult with a Novel Epithelioid Tumor Driven by a PRRC2B-ALK Fusion. J. Natl. Compr. Cancer Netw. 2021, 19, 1116–1121. [Google Scholar] [CrossRef]
- Logan, S.J.; Schieffer, K.M.; Conces, M.R.; Stonerock, E.; Miller, A.R.; Fitch, J.; LaHaye, S.; Voytovich, K.; McGrath, S.; Magrini, V. Novel morphologic findings in PLAG1-rearranged soft tissue tumors. Genes Chromosom. Cancer 2021, 60, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Brčić, I.; Igrec, J.; Halbwedl, I.; Viertler, C.; Liegl-Atzwanger, B. Expanding the spectrum of PLAG1-rearranged lipoblastomas arising in patients over 45, with identification of novel fusion partners. Mod. Pathol. 2022, 35, 283–285. [Google Scholar] [CrossRef]
- Chung, C.T.; Antonescu, C.R.; Dickson, B.C.; Chami, R.; Marrano, P.; Fan, R.; Shago, M.; Hameed, M.; Thorner, P.S. Pediatric fibromyxoid soft tissue tumor with PLAG1 fusion: A novel entity? Genes Chromosomes Cancer 2021, 60, 263–271. [Google Scholar] [CrossRef]
- Fritchie, K.; Wang, L.; Yin, Z.; Nakitandwe, J.; Hedges, D.; Horvai, A.; Mora, J.T.; Folpe, A.L.; Bahrami, A. Lipoblastomas presenting in older children and adults: Analysis of 22 cases with identification of novel PLAG1 fusion partners. Mod. Pathol. 2021, 34, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Kautto, E.A.; Schieffer, K.M.; McGrath, S.; Miller, A.R.; Hernandez-Gonzalez, M.E.; Choi, S.; Conces, M.R.; Fernandez-Faith, E.; Ho, M.L.; Lee, K.; et al. Expanding the clinical phenotype of FGFR1 internal tandem duplication. Cold Spring Harb. Mol. Case Stud. 2022, 8, a006174. [Google Scholar] [CrossRef]
- Koo, S.C.; Schieffer, K.M.; Lee, K.; Gupta, A.; Pfau, R.B.; Avenarius, M.R.; Stonerock, E.; LaHaye, S.; Fitch, J.; Setty, B.A.; et al. EGFR internal tandem duplications in fusion-negative congenital and neonatal spindle cell tumors. Genes Chromosomes Cancer 2023, 62, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Biederman, L.E.; Lee, K.; Yeager, N.D.; Sribnick, E.A.; Shenoy, A. CIC::NUTM1 sarcoma mimicking primitive myxoid mesenchymal tumour of infancy: Report of a case. Histopathology 2022, 81, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Avenarius, M.R.; Miller, C.R.; Arnold, M.A.; Koo, S.; Roberts, R.; Hobby, M.; Grossman, T.; Moyer, Y.; Wilson, R.K.; Mardis, E.R.; et al. Genetic Characterization of Pediatric Sarcomas by Targeted RNA Sequencing. J. Mol. Diagn. 2020, 22, 1238–1245. [Google Scholar] [CrossRef]
- Chang, F.; Lin, F.; Cao, K.; Surrey, L.F.; Aplenc, R.; Bagatell, R.; Resnick, A.C.; Santi, M.; Storm, P.B.; Tasian, S.K.; et al. Development and Clinical Validation of a Large Fusion Gene Panel for Pediatric Cancers. J. Mol. Diagn. 2019, 21, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Hindi, I.; Shen, G.; Tan, Q.; Cotzia, P.; Snuderl, M.; Feng, X.; Jour, G. Feasibility and clinical utility of a pan-solid tumor targeted RNA fusion panel: A single center experience. Exp. Mol. Pathol. 2020, 114, 104403. [Google Scholar] [CrossRef]
- Selvam, P.; Kelly, K.; Hesse, A.N.; Spitzer, D.; Reddi, H.V. Evaluating gene fusions in solid tumors—Clinical experience using an RNA based 53 gene next-generation sequencing panel. Cancer Genet. 2019, 233, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Su, X.; Zhang, J.; Radtke, I.; Phillips, L.A.; Miller, C.B.; Ma, J.; Liu, W.; Cheng, C.; Schulman, B.A.; et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N. Engl. J. Med. 2009, 360, 470–480. [Google Scholar] [CrossRef]
- Marke, R.; Leeuwen, F.N.v.; Scheijen, B. The many faces of IKZF1 in B-cell precursor acute lymphoblastic leukemia. Haematologica 2018, 103, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Medvedovic, J.; Ebert, A.; Tagoh, H.; Busslinger, M. Pax5: A master regulator of B cell development and leukemogenesis. Adv. Immunol. 2011, 111, 179–206. [Google Scholar] [CrossRef]
- Gu, Z.; Churchman, M.L.; Roberts, K.G.; Moore, I.; Zhou, X.; Nakitandwe, J.; Hagiwara, K.; Pelletier, S.; Gingras, S.; Berns, H.; et al. PAX5 -driven subtypes of B-progenitor acute lymphoblastic leukemia. Nat. Genet. 2019, 51, 296–307. [Google Scholar] [CrossRef]
- Mullighan, C.G.; Goorha, S.; Radtke, I.; Miller, C.B.; Coustan-Smith, E.; Dalton, J.D.; Girtman, K.; Mathew, S.; Ma, J.; Pounds, S.B. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 2007, 446, 758–764. [Google Scholar] [CrossRef]
- Schwab, C.; Nebral, K.; Chilton, L.; Leschi, C.; Waanders, E.; Boer, J.M.; Žaliová, M.; Sutton, R.; Öfverholm, I.I.; Ohki, K.; et al. Intragenic amplification of PAX5: A novel subgroup in B-cell precursor acute lymphoblastic leukemia? Blood Adv. 2017, 1, 1473. [Google Scholar] [CrossRef]
- Gilliland, D.G.; Griffin, J.D. The roles of FLT3 in hematopoiesis and leukemia. Blood 2002, 100, 1532–1542. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Sturm, D.; Orr, B.A.; Toprak, U.H.; Hovestadt, V.; Jones, D.T.W.; Capper, D.; Sill, M.; Buchhalter, I.; Northcott, P.A.; Leis, I.; et al. New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs. Cell 2016, 164, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Appay, R.; Macagno, N.; Padovani, L.; Korshunov, A.; Kool, M.; André, N.; Scavarda, D.; Pietsch, T.; Figarella-Branger, D. HGNET-BCOR Tumors of the Cerebellum: Clinicopathologic and Molecular Characterization of 3 Cases. Am. J. Surg. Pathol. 2017, 41, 1254–1260. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nobusawa, S.; Nakata, S.; Nakada, M.; Arakawa, Y.; Mineharu, Y.; Sugita, Y.; Yoshioka, T.; Araki, A.; Sato, Y.; et al. CNS high-grade neuroepithelial tumor with BCOR internal tandem duplication: A comparison with its counterparts in the kidney and soft tissue. Brain Pathol. 2018, 28, 710–720. [Google Scholar] [CrossRef]
- Ferris, S.P.; Velazquez Vega, J.; Aboian, M.; Lee, J.C.; Van Ziffle, J.; Onodera, C.; Grenert, J.P.; Saunders, T.; Chen, Y.Y.; Banerjee, A.; et al. High-grade neuroepithelial tumor with BCOR exon 15 internal tandem duplication—A comprehensive clinical, radiographic, pathologic, and genomic analysis. Brain Pathol. 2020, 30, 46–62. [Google Scholar] [CrossRef]
- De Lima, L.; Sürme, M.B.; Gessi, M.; Mastronuzzi, A.; Miele, E.; Tamburrini, G.; Massimi, L. Central nervous system high-grade neuroepithelial tumor with BCOR alteration (CNS HGNET-BCOR)-case-based reviews. Childs Nerv. Syst. 2020, 36, 1589–1599. [Google Scholar] [CrossRef]
- Al-Ibraheemi, A.; Putra, J.; Tsai, H.K.; Cano, S.; Lip, V.; Pinches, R.S.; Restrepo, T.; Alexandrescu, S.; Janeway, K.A.; Duraisamy, S.; et al. Assessment of BCOR Internal Tandem Duplications in Pediatric Cancers by Targeted RNA Sequencing. J. Mol. Diagn. JMD 2021, 23, 1269–1278. [Google Scholar] [CrossRef]
- Ueno-Yokohata, H.; Okita, H.; Nakasato, K.; Akimoto, S.; Hata, J.; Koshinaga, T.; Fukuzawa, M.; Kiyokawa, N. Consistent in-frame internal tandem duplications of BCOR characterize clear cell sarcoma of the kidney. Nat. Genet. 2015, 47, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kumar, V.; Zorman, B.; Fang, E.; Haines, K.M.; Doddapaneni, H.; Hampton, O.A.; White, S.; Bavle, A.A.; Patel, N.R.; et al. Recurrent internal tandem duplications of BCOR in clear cell sarcoma of the kidney. Nat. Commun. 2015, 6, 8891. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv. Anat. Pathol. 2021, 28, 44–58. [Google Scholar] [CrossRef]
- Kao, E.; Pinto, N.; Trobaugh-Lotrario, A.; Deutsch, G.H.; Wu, Y.; Wang, W.; Rudzinski, E.R.; Liu, Y.J. Tyrosine kinase-altered spindle cell neoplasms with EGFR internal tandem duplications. Genes Chromosom. Cancer 2022, 61, 616–621. [Google Scholar] [CrossRef]
- De Unamuno Bustos, B.; Estal, R.M.; Simó, G.P.; De Juan Jimenez, I.; Muñoz, B.E.; Serna, M.R.; De Miquel, V.A.; Ros, M.L.; Sánchez, R.B.; Enguídanos, E.N.; et al. Towards Personalized Medicine in Melanoma: Implementation of a Clinical Next-Generation Sequencing Panel. Sci. Rep. 2017, 7, 495. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, A.; Wordsworth, S.; Fermont, J.M.; Page, S.; Kaur, K.; Camps, C.; Kaisaki, P.; Gupta, A.; Talbot, D.; Middleton, M.; et al. Clinical applicability and cost of a 46-gene panel for genomic analysis of solid tumours: Retrospective validation and prospective audit in the UK National Health Service. PLoS Med. 2017, 14, e1002230. [Google Scholar] [CrossRef] [PubMed]
- Pennell, N.A.; Mutebi, A.; Zhou, Z.-Y.; Ricculli, M.L.; Tang, W.; Wang, H.; Guerin, A.; Arnhart, T.; Dalal, A.; Sasane, M.; et al. Economic Impact of Next-Generation Sequencing Versus Single-Gene Testing to Detect Genomic Alterations in Metastatic Non–Small-Cell Lung Cancer Using a Decision Analytic Model. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liebers, M.; Zhelyazkova, B.; Cao, Y.; Panditi, D.; Lynch, K.D.; Chen, J.; Robinson, H.E.; Shim, H.S.; Chmielecki, J.; et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat. Med. 2014, 20, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Krystel-Whittemore, M.; Taylor, M.S.; Rivera, M.; Lennerz, J.K.; Le, L.P.; Dias-Santagata, D.; Iafrate, A.J.; Deshpande, V.; Chebib, I.; Nielsen, G.P.; et al. Novel and established EWSR1 gene fusions and associations identified by next-generation sequencing and fluorescence in-situ hybridization. Hum. Pathol. 2019, 93, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Straube, J.; Ling, V.Y.; Hill, G.R.; Lane, S.W. The impact of age, NPM1(mut), and FLT3(ITD) allelic ratio in patients with acute myeloid leukemia. Blood 2018, 131, 1148–1153. [Google Scholar] [CrossRef]
- Mullighan, C.G.; Miller, C.B.; Radtke, I.; Phillips, L.A.; Dalton, J.; Ma, J.; White, D.; Hughes, T.P.; Le Beau, M.M.; Pui, C.H.; et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature 2008, 453, 110–114. [Google Scholar] [CrossRef]
- Dupain, C.; Harttrampf, A.C.; Urbinati, G.; Geoerger, B.; Massaad-Massade, L. Relevance of Fusion Genes in Pediatric Cancers: Toward Precision Medicine. Mol. Ther. Nucleic Acids 2017, 6, 315–326. [Google Scholar] [CrossRef]
- Gröbner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef]

| Conventional Karyotype | Fluorescence In Situ Hybridization | Conventional Microarray | RT-PCR | Genome Sequencing | AMP-Based RNA-Seq (e.g., Archer) | RNA-Seq | |
|---|---|---|---|---|---|---|---|
| Analyte | Metaphase Chromosomes | Interphase nuclei or Metaphase Chromosomes | DNA | RNA | DNA | RNA | RNA |
| Estimated turnaround time ¥ | 3–7 days | 1–2 days | 3–7 days | 1–5 days | 7–14 days | 7–14 days | 7–14 days |
| Targeted | No | Yes | No | Yes | No | Yes | No |
| Gene fusion resolution | Cytogenetic Resolution (Mb); Breakpoints hone and refine likely involved genes | Gene locus resolution (Mb- kb); Probe signal patterns hone and refine involved genes | Molecular Resolution (kb- bp); Breakpoints hone and refine involved genes | Molecular Resolution (bp); Confirms both gene partners | Molecular Resolution (bp); Confirms both gene partners | Molecular Resolution (bp); Confirms both gene partners | Molecular Resolution (bp); Confirms both gene partners |
| Intragenic deletion * | No | No | Yes | Yes | Yes | Yes | Yes |
| ITD resolution * | No | No | Yes | Yes | Yes | Yes | Yes |
| Individuals (n = 522) | |
|---|---|
| Sex | |
| Male | 306 (59%) |
| Female | 216 (41%) |
| Age at the time of testing | |
| 0–18 years | 249 (48%) |
| >18 years | 273 (52%) |
| Specimen source | |
| Bone marrow | 382 (73%) |
| Peripheral blood | 137 (26%) |
| Other | 3 (1%) |
| Indication for testing | |
| Acute lymphoid leukemia | 222 (43%) |
| B-cell acute lymphoid leukemia | 226 (43%) |
| Acute leukemia, NOS | 33 (6%) |
| Acute myeloid leukemia | 5 (1%) |
| MPO negative blasts | 4 (1%) |
| Acute megakaryoblastic leukemia | 3 (1%) |
| Abnormal B cell population | 3 (1%) |
| Chronic myeloid leukemia | 2 (<1%) |
| Diffuse large B cell lymphoma | 2 (<1%) |
| Mixed lineage leukemia | 2 (<1%) |
| Myeloid neoplasm | 2 (<1%) |
| Eosinophilia | 1 (<1%) |
| Myelodysplastic syndrome | 1 (<1%) |
| Other | 11 (2%) |
| Indication not provided | 5 (1%) |
| Myeloid neoplasm | 2 (<1%) |
| Individuals (n = 37) | |
|---|---|
| Sex | |
| Male | 40 (47%) |
| Female | 26 (53%) |
| Age at the time of testing | |
| 0–18 years | 81 (94%) |
| >18 years | 5 (6%) |
| Specimen source | |
| Snap frozen | 57 (66%) |
| Formalin-fixed paraffin-embedded | 29 (34%) |
| Tumor type | |
| Non-CNS solid tumor | 38 (44%) |
| CNS tumor | 48 (56%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schieffer, K.M.; Moccia, A.; Bucknor, B.A.; Stonerock, E.; Jayaraman, V.; Jenkins, H.; McKinney, A.; Koo, S.C.; Mathew, M.T.; Mardis, E.R.; et al. Expanding the Clinical Utility of Targeted RNA Sequencing Panels beyond Gene Fusions to Complex, Intragenic Structural Rearrangements. Cancers 2023, 15, 4394. https://doi.org/10.3390/cancers15174394
Schieffer KM, Moccia A, Bucknor BA, Stonerock E, Jayaraman V, Jenkins H, McKinney A, Koo SC, Mathew MT, Mardis ER, et al. Expanding the Clinical Utility of Targeted RNA Sequencing Panels beyond Gene Fusions to Complex, Intragenic Structural Rearrangements. Cancers. 2023; 15(17):4394. https://doi.org/10.3390/cancers15174394
Chicago/Turabian StyleSchieffer, Kathleen M., Amanda Moccia, Brianna A. Bucknor, Eileen Stonerock, Vijayakumar Jayaraman, Heather Jenkins, Aimee McKinney, Selene C. Koo, Mariam T. Mathew, Elaine R. Mardis, and et al. 2023. "Expanding the Clinical Utility of Targeted RNA Sequencing Panels beyond Gene Fusions to Complex, Intragenic Structural Rearrangements" Cancers 15, no. 17: 4394. https://doi.org/10.3390/cancers15174394
APA StyleSchieffer, K. M., Moccia, A., Bucknor, B. A., Stonerock, E., Jayaraman, V., Jenkins, H., McKinney, A., Koo, S. C., Mathew, M. T., Mardis, E. R., Lee, K., Reshmi, S. C., & Cottrell, C. E. (2023). Expanding the Clinical Utility of Targeted RNA Sequencing Panels beyond Gene Fusions to Complex, Intragenic Structural Rearrangements. Cancers, 15(17), 4394. https://doi.org/10.3390/cancers15174394







