Detection and Mitigation of Neurovascular Uncoupling in Brain Gliomas
Simple Summary
Abstract
1. Basics of BOLD fMRI
2. BOLD fMRI Acquisition
3. Preoperative BOLD fMRI
4. Clinical Evaluation of Brain Gliomas with Task-Based BOLD fMRI
5. Resting-State Functional Connectivity and Neuronal Activity
6. Neurovascular Uncoupling (NVU) and Its Impact on Clinical fMRI
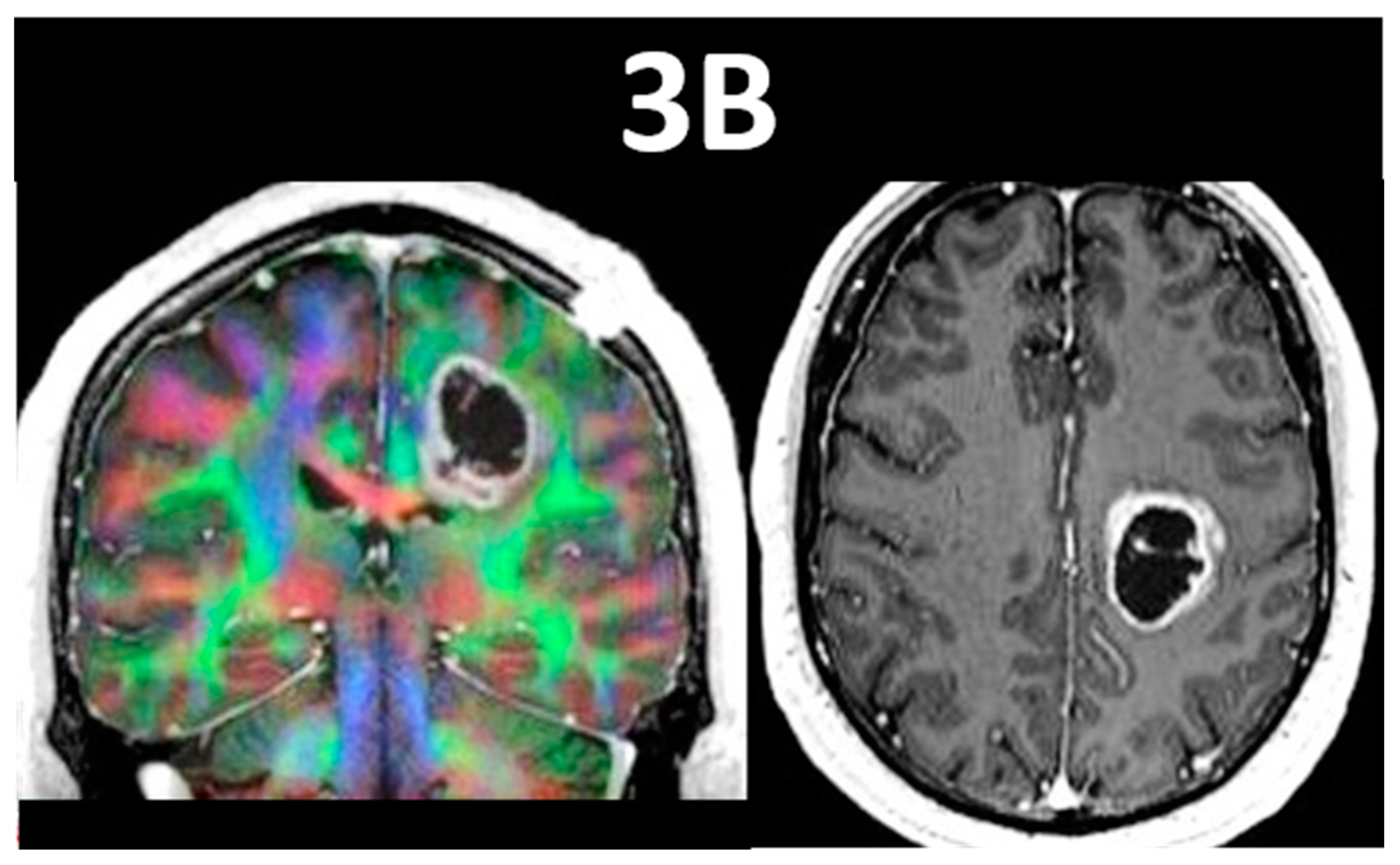
7. NVU Assessment on Resting-State fMRI
8. Cerebrovascular Reactivity Mapping (CVR) for NVU Detection in Brain Tumors
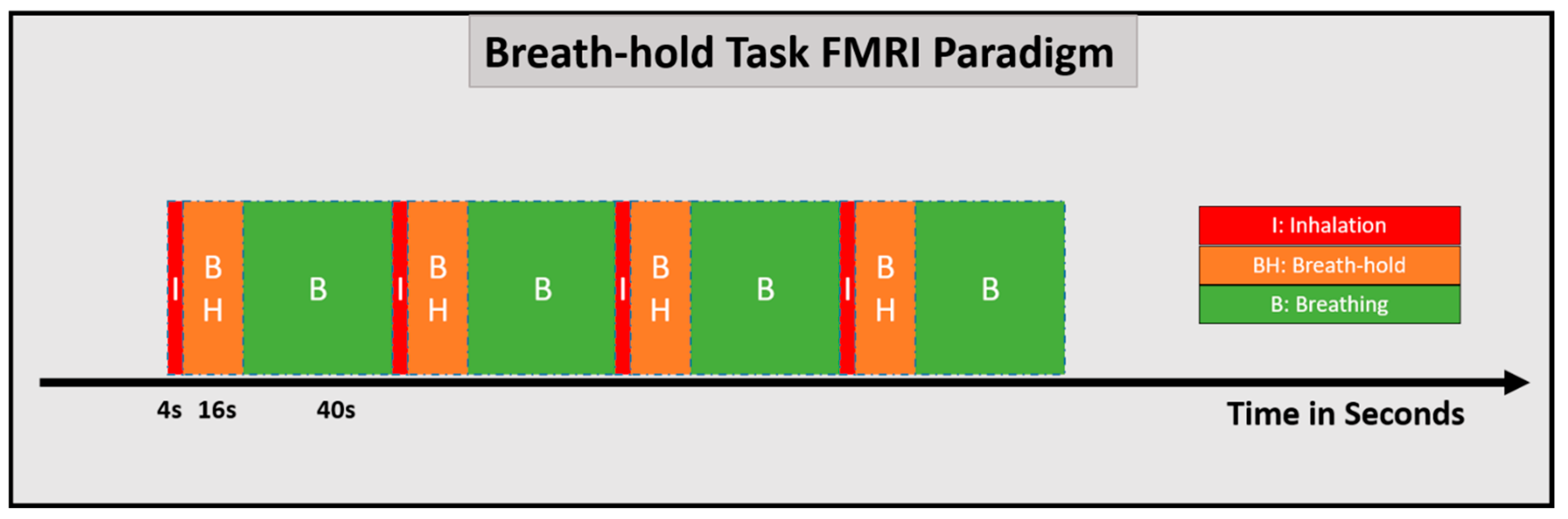
8.1. CVR Mapping Using Breath-Hold Methods
8.2. CVR Mapping Using Exogenous Gas Delivery Approaches
8.3. CVR Mapping Using rs-fMRI
9. Other Approaches for NVU Assessment
10. Methodological Approaches for Mitigation of Brain Tumor Induced NVU
10.1. Using rs-fMRI
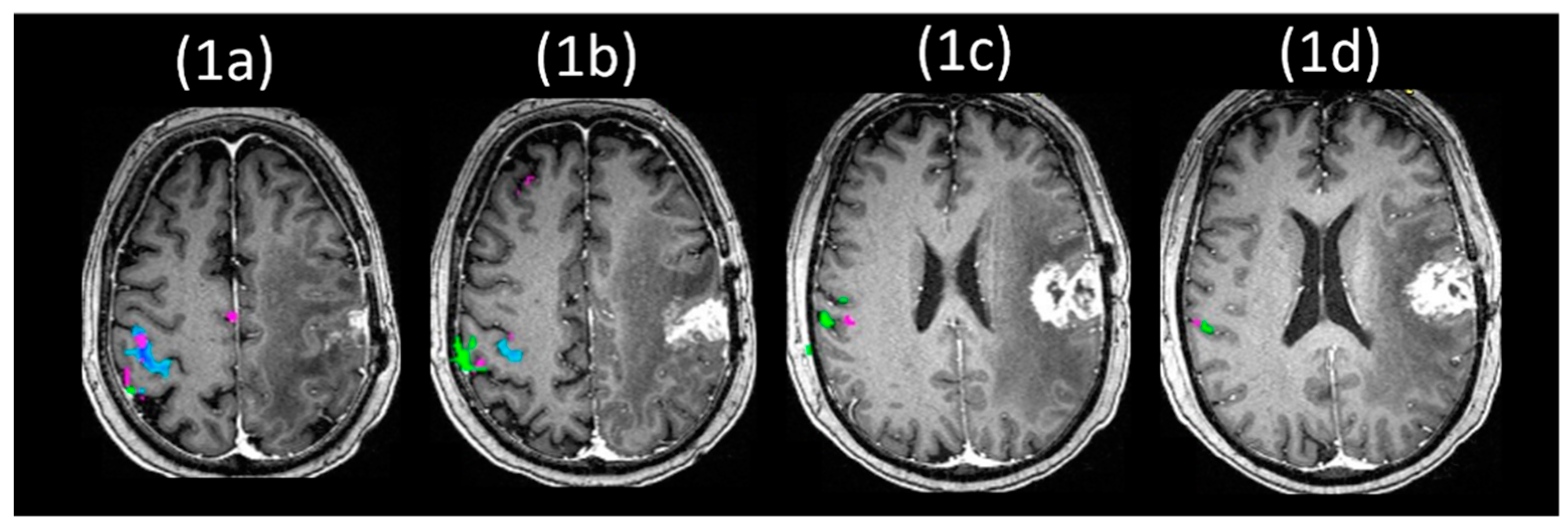
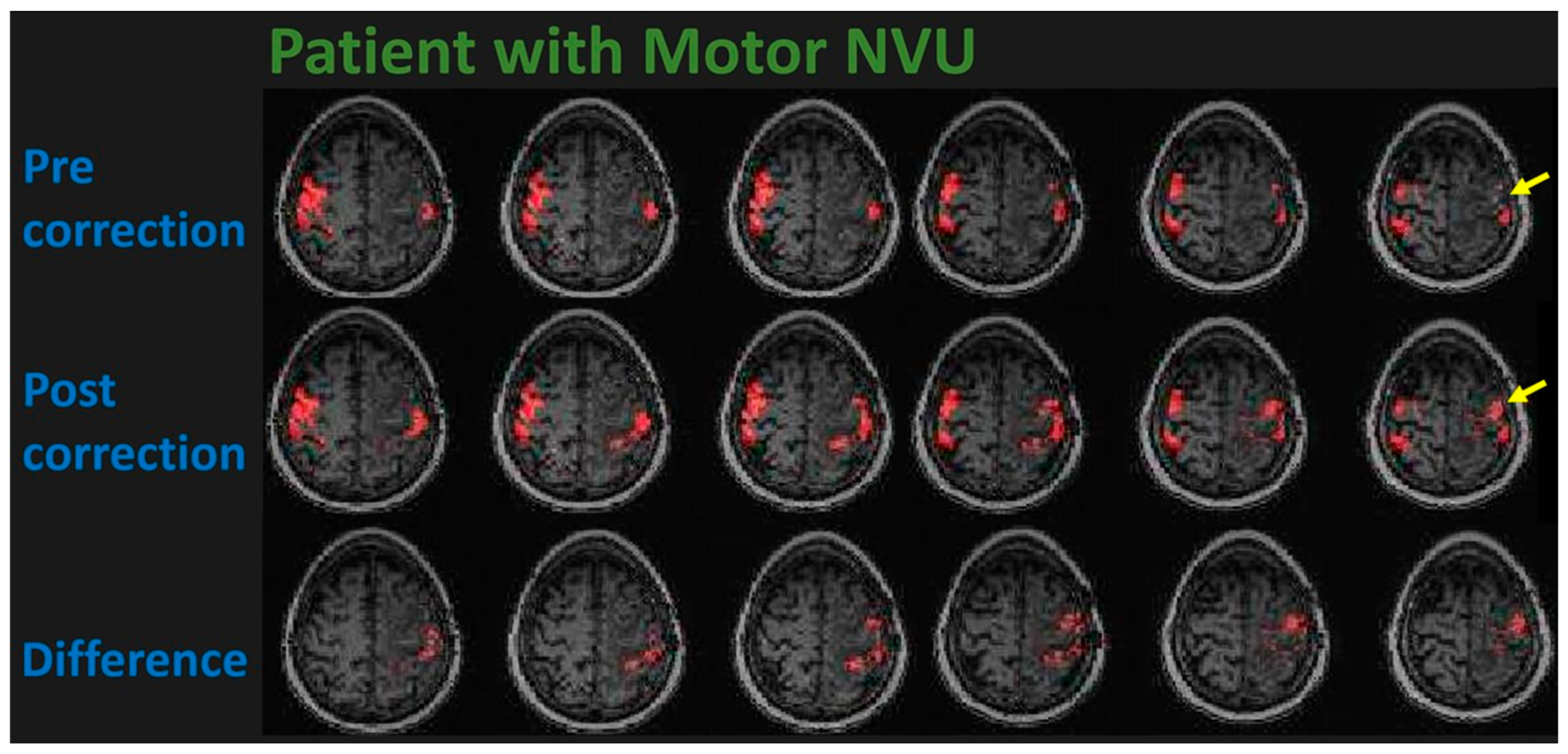
10.2. Using BH-fMRI
10.3. Cerebrovascular Dynamics of Gliomas vs. Temporal Dynamics of BOLD Signal
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Disclosure
References
- Ogawa, S.; Tank, D.W.; Menon, R.; Ellermann, J.M.; Kim, S.G.; Merkle, H.; Ugurbil, K. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 1992, 89, 5951–5955. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.J.; Beall, E.B. Selection of Optimal Pulse Sequences for fMRI. In fMRI Techniques and Protocols; Filippi, M., Ed.; Springer: New York, NY, USA, 2016; pp. 69–111. [Google Scholar] [CrossRef]
- Chen, J.E.; Glover, G.H. Functional Magnetic Resonance Imaging Methods. Neuropsychol. Rev. 2015, 25, 289–313. [Google Scholar] [CrossRef]
- Elster, A.D. Gradient-echo MR imaging: Techniques and acronyms. Radiology 1993, 186, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Poustchi-Amin, M.; Mirowitz, S.A.; Brown, J.J.; McKinstry, R.C.; Li, T. Principles and applications of echo-planar imaging: A review for the general radiologist. Radiographics 2001, 21, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, D.A.; Setsompop, K. Ultra-fast MRI of the human brain with simultaneous multi-slice imaging. J. Magn. Reson. 2013, 229, 90–100. [Google Scholar] [CrossRef]
- Pillai, J.J. The evolution of clinical functional imaging during the past 2 decades and its current impact on neurosurgical planning. Am. J. Neuroradiol. 2010, 31, 219–225. [Google Scholar] [CrossRef]
- Gupta, A.; Shah, A.; Young, R.J.; Holodny, A.I. Imaging of brain tumors: Functional magnetic resonance imaging and diffusion tensor imaging. Neuroimaging Clin. N. Am. 2010, 20, 379–400. [Google Scholar] [CrossRef]
- DeYoe, E.A.; Bandettini, P.; Neitz, J.; Miller, D.; Winans, P. Functional magnetic resonance imaging (FMRI) of the human brain. J. Neurosci. Methods 1994, 54, 171–187. [Google Scholar] [CrossRef]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef]
- Fox, M.D.; Corbetta, M.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. USA 2006, 103, 10046–10051. [Google Scholar] [CrossRef]
- Vincent, J.L.; Kahn, I.; Snyder, A.Z.; Raichle, M.E.; Buckner, R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008, 100, 3328–3342. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Miller-Thomas, M.M.; Benzinger, T.L.; Marcus, D.S.; Hacker, C.D.; Leuthardt, E.C.; Shimony, J.S. Clinical Resting-state fMRI in the Preoperative Setting: Are We Ready for Prime Time? Top. Magn. Reson. Imaging 2016, 25, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Johnston, J.M.; Fox, M.D.; Leuthardt, E.C.; Grubb, R.L.; Chicoine, M.R.; Smyth, M.D.; Snyder, A.Z.; Raichle, M.E.; Shimony, J.S. Preoperative sensorimotor mapping in brain tumor patients using spontaneous fluctuations in neuronal activity imaged with functional magnetic resonance imaging: Initial experience. Neurosurgery 2009, 65, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, S.M.; Nikkinen, J.; Remes, J.; Kantola, J.; Starck, T.; Haapea, M.; Tuominen, J.; Tervonen, O.; Kiviniemi, V. Preoperative localization of the sensorimotor area using independent component analysis of resting-state fMRI. Magn. Reson. Imaging 2009, 27, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.J.; Hacker, C.D.; Breshears, J.D.; Szrama, N.P.; Sharma, M.; Bundy, D.T.; Pahwa, M.; Corbetta, M.; Snyder, A.Z.; Shimony, J.S.; et al. A novel data-driven approach to preoperative mapping of functional cortex using resting-state functional magnetic resonance imaging. Neurosurgery 2013, 73, 969–982, discussion 982–963. [Google Scholar] [CrossRef] [PubMed]
- Ghinda, D.C.; Wu, J.S.; Duncan, N.W.; Northoff, G. How much is enough-Can resting state fMRI provide a demarcation for neurosurgical resection in glioma? Neurosci. Biobehav. Rev. 2018, 84, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Zaca, D.; Corsini, F.; Rozzanigo, U.; Dallabona, M.; Avesani, P.; Annicchiarico, L.; Zigiotto, L.; Faraca, G.; Chioffi, F.; Jovicich, J.; et al. Whole-Brain Network Connectivity Underlying the Human Speech Articulation as Emerged Integrating Direct Electric Stimulation, Resting State fMRI and Tractography. Front. Hum. Neurosci. 2018, 12, 405. [Google Scholar] [CrossRef] [PubMed]
- Zaca, D.; Jovicich, J.; Corsini, F.; Rozzanigo, U.; Chioffi, F.; Sarubbo, S. ReStNeuMap: A tool for automatic extraction of resting-state functional MRI networks in neurosurgical practice. J. Neurosurg. 2018, 131, 764–771. [Google Scholar] [CrossRef]
- Qiu, T.M.; Gong, F.Y.; Gong, X.; Wu, J.S.; Lin, C.P.; Biswal, B.B.; Zhuang, D.X.; Yao, C.J.; Zhang, X.L.; Lu, J.F.; et al. Real-Time Motor Cortex Mapping for the Safe Resection of Glioma: An Intraoperative Resting-State fMRI Study. Am. J. Neuroradiol. 2017, 38, 2146–2152. [Google Scholar] [CrossRef]
- Waheed, S.H.; Mirbagheri, S.; Agarwal, S.; Kamali, A.; Yahyavi-Firouz-Abadi, N.; Chaudhry, A.; DiGianvittorio, M.; Gujar, S.K.; Pillai, J.J.; Sair, H.I. Reporting of Resting-State Functional Magnetic Resonance Imaging Preprocessing Methodologies. Brain Connect. 2016, 6, 663–668. [Google Scholar] [CrossRef]
- Gujar, S.K.; Manzoor, K.; Wongsripuemtet, J.; Wang, G.; Ryan, D.; Agarwal, S.; Lindquist, M.; Caffo, B.; Pillai, J.J.; Sair, H.I. Identification of the Language Network from Resting-State fMRI in Patients with Brain Tumors: How Accurate Are Experts? Am. J. Neuroradiol. 2023, 44, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, C.F.; DeLuca, M.; Devlin, J.T.; Smith, S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.S.; Rombouts, S.A.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Beckmann, C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13848–13853. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.F.; He, Y.; Zhu, C.Z.; Cao, Q.J.; Sui, M.Q.; Liang, M.; Tian, L.X.; Jiang, T.Z.; Wang, Y.F. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007, 29, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Jiang, T.; Lu, Y.; He, Y.; Tian, L. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004, 22, 394–400. [Google Scholar] [CrossRef]
- Logothetis, N.K. What we can do and what we cannot do with fMRI. Nature 2008, 453, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; Macvicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, N.K. Neurovascular Uncoupling: Much Ado about Nothing. Front. Neuroenergetics 2010, 2, 2. [Google Scholar] [CrossRef]
- Venkat, P.; Chopp, M.; Chen, J. New insights into coupling and uncoupling of cerebral blood flow and metabolism in the brain. Croat. Med. J. 2016, 57, 223–228. [Google Scholar] [CrossRef]
- Lee, J.; Lund-Smith, C.; Borboa, A.; Gonzalez, A.M.; Baird, A.; Eliceiri, B.P. Glioma-induced remodeling of the neurovascular unit. Brain Res. 2009, 1288, 125–134. [Google Scholar] [CrossRef]
- Pak, R.W.; Hadjiabadi, D.H.; Senarathna, J.; Agarwal, S.; Thakor, N.V.; Pillai, J.J.; Pathak, A.P. Implications of neurovascular uncoupling in functional magnetic resonance imaging (fMRI) of brain tumors. J. Cereb. Blood Flow. Metab. 2017, 37, 3475–3478. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.K.; Kim, S.H.; Dovas, A.; Zhao, H.T.; Goldberg, A.R.; Xu, W.; Yagielski, A.J.; Cambareri, M.K.; Patel, K.B.; Mela, A.; et al. Glioma-Induced Alterations in Neuronal Activity and Neurovascular Coupling during Disease Progression. Cell Rep. 2020, 31, 107500. [Google Scholar] [CrossRef] [PubMed]
- Hosford, P.S.; Christie, I.N.; Niranjan, A.; Aziz, Q.; Anderson, N.; Ang, R.; Lythgoe, M.F.; Wells, J.A.; Tinker, A.; Gourine, A.V. A critical role for the ATP-sensitive potassium channel subunit KIR6.1 in the control of cerebral blood flow. J. Cereb. Blood Flow. Metab. 2019, 39, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.L.; Bradbury, M.; Peck, K.K.; Petrovich, N.M.; Gutin, P.H.; Holodny, A.I. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. Neuroimage 2006, 32, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Krainik, A.; David, O.; Salon, C.; Tropres, I.; Hoffmann, D.; Pannetier, N.; Barbier, E.L.; Bombin, E.R.; Warnking, J.; et al. Impaired fMRI activation in patients with primary brain tumors. Neuroimage 2010, 52, 538–548. [Google Scholar] [CrossRef]
- Pelligrino, D.A.; Vetri, F.; Xu, H.L. Purinergic mechanisms in gliovascular coupling. Semin. Cell Dev. Biol. 2011, 22, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 4196. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, G.V.; Minagar, A.; Alexander, J.S. Neuronal and astrocytic interactions modulate brain endothelial properties during metabolic stresses of in vitro cerebral ischemia. Cell Commun. Signal 2014, 12, 7. [Google Scholar] [CrossRef]
- Agarwal, S.; Sair, H.I.; Pillai, J.J. The Problem of Neurovascular Uncoupling. Neuroimaging Clin. N. Am. 2021, 31, 53–67. [Google Scholar] [CrossRef]
- Fierstra, J.; Mikulis, D.J. Neurovascular Uncoupling in Functional MRI. In Functional Neuroradiology: Principles and Clinical Applications; Faro, S.H., Mohamed, F.B., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 511–520. [Google Scholar] [CrossRef]
- Pillai, J.; Zaca, D.; Choudhri, A. Clinical impact of integrated physiologic brain tumor imaging. Technol. Cancer Res. Treat. 2010, 9, 359–380. [Google Scholar] [CrossRef]
- Ulmer, J.L.; Krouwer, H.G.; Mueller, W.M.; Ugurel, M.S.; Kocak, M.; Mark, L.P. Pseudo-reorganization of language cortical function at fMR imaging: A consequence of tumor-induced neurovascular uncoupling. Am. J. Neuroradiol. 2003, 24, 213–217. [Google Scholar] [PubMed]
- Li, Q.; Dong, J.W.; Del Ferraro, G.; Petrovich Brennan, N.; Peck, K.K.; Tabar, V.; Makse, H.A.; Holodny, A.I. Functional Translocation of Broca’s Area in a Low-Grade Left Frontal Glioma: Graph Theory Reveals the Novel, Adaptive Network Connectivity. Front. Neurol. 2019, 10, 702. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, R.A.; Jost, E.; Shaw, K.; Brennan, N.P.; Peck, K.K.; Holodny, A.I. Cortical Plasticity in the Setting of Brain Tumors. Top. Magn. Reson. Imaging 2016, 25, 25–30. [Google Scholar] [CrossRef]
- Agarwal, S.; Lu, H.; Pillai, J.J. Value of Frequency Domain Resting-State Functional Magnetic Resonance Imaging Metrics Amplitude of Low-Frequency Fluctuation and Fractional Amplitude of Low-Frequency Fluctuation in the Assessment of Brain Tumor-Induced Neurovascular Uncoupling. Brain Connect. 2017, 7, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sair, H.I.; Airan, R.; Hua, J.; Jones, C.K.; Heo, H.Y.; Olivi, A.; Lindquist, M.A.; Pekar, J.J.; Pillai, J.J. Demonstration of Brain Tumor-Induced Neurovascular Uncoupling in Resting-State fMRI at Ultrahigh Field. Brain Connect. 2016, 6, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sair, H.I.; Gujar, S.; Hua, J.; Lu, H.; Pillai, J.J. Functional Magnetic Resonance Imaging Activation Optimization in the Setting of Brain Tumor-Induced Neurovascular Uncoupling Using Resting-State Blood Oxygen Level-Dependent Amplitude of Low Frequency Fluctuations. Brain Connect. 2019, 9, 241–250. [Google Scholar] [CrossRef]
- Agarwal, S.; Sair, H.I.; Yahyavi-Firouz-Abadi, N.; Airan, R.; Pillai, J.J. Neurovascular uncoupling in resting state fMRI demonstrated in patients with primary brain gliomas. J. Magn. Reson. Imaging 2016, 43, 620–626. [Google Scholar] [CrossRef]
- Biswal, B.B.; Kannurpatti, S.S.; Rypma, B. Hemodynamic scaling of fMRI-BOLD signal: Validation of low-frequency spectral amplitude as a scalability factor. Magn. Reson. Imaging 2007, 25, 1358–1369. [Google Scholar] [CrossRef]
- Mallela, A.N.; Peck, K.K.; Petrovich-Brennan, N.M.; Zhang, Z.; Lou, W.; Holodny, A.I. Altered Resting-State Functional Connectivity in the Hand Motor Network in Glioma Patients. Brain Connect. 2016, 6, 587–595. [Google Scholar] [CrossRef]
- Pillai, J.J.; Mikulis, D.J. Cerebrovascular reactivity mapping: An evolving standard for clinical functional imaging. Am. J. Neuroradiol. 2015, 36, 7–13. [Google Scholar] [CrossRef]
- Catchlove, S.J.; Pipingas, A.; Hughes, M.E.; Macpherson, H. Magnetic resonance imaging for assessment of cerebrovascular reactivity and its relationship to cognition: A systematic review. BMC Neurosci. 2018, 19, 21. [Google Scholar] [CrossRef]
- Pinto, J.; Bright, M.G.; Bulte, D.P.; Figueiredo, P. Cerebrovascular Reactivity Mapping Without Gas Challenges: A Methodological Guide. Front. Physiol. 2020, 11, 608475. [Google Scholar] [CrossRef] [PubMed]
- Solis-Barquero, S.M.; Echeverria-Chasco, R.; Calvo-Imirizaldu, M.; Cacho-Asenjo, E.; Martinez-Simon, A.; Vidorreta, M.; Dominguez, P.D.; Garcia de Eulate, R.; Fernandez-Martinez, M.; Fernandez-Seara, M.A. Breath-Hold Induced Cerebrovascular Reactivity Measurements Using Optimized Pseudocontinuous Arterial Spin Labeling. Front. Physiol. 2021, 12, 621720. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.L.; Kwong, K.K.; Weisskoff, R.M.; Rosen, B.R. Calibrated functional MRI: Mapping the dynamics of oxidative metabolism. Proc. Natl. Acad. Sci. USA 1998, 95, 1834–1839. [Google Scholar] [CrossRef] [PubMed]
- Kastrup, A.; Krüger, G.; Neumann-Haefelin, T.; Moseley, M.E. Assessment of cerebrovascular reactivity with functional magnetic resonance imaging: Comparison of CO2 and breath holding. Magn. Reson. Imaging 2001, 19, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sleight, E.; Stringer, M.S.; Marshall, I.; Wardlaw, J.M.; Thrippleton, M.J. Cerebrovascular Reactivity Measurement Using Magnetic Resonance Imaging: A Systematic Review. Front. Physiol. 2021, 12, 643468. [Google Scholar] [CrossRef] [PubMed]
- Hare, H.V.; Germuska, M.; Kelly, M.E.; Bulte, D.P. Comparison of CO2 in air versus carbogen for the measurement of cerebrovascular reactivity with magnetic resonance imaging. J. Cereb. Blood Flow. Metab. 2013, 33, 1799–1805. [Google Scholar] [CrossRef]
- Donahue, M.J.; Dethrage, L.M.; Faraco, C.C.; Jordan, L.C.; Clemmons, P.; Singer, R.; Mocco, J.; Shyr, Y.; Desai, A.; O’Duffy, A.; et al. Routine clinical evaluation of cerebrovascular reserve capacity using carbogen in patients with intracranial stenosis. Stroke 2014, 45, 2335–2341. [Google Scholar] [CrossRef]
- Zhou, Y.; Rodgers, Z.B.; Kuo, A.H. Cerebrovascular reactivity measured with arterial spin labeling and blood oxygen level dependent techniques. Magn. Reson. Imaging 2015, 33, 566–576. [Google Scholar] [CrossRef]
- van der Zande, F.H.; Hofman, P.A.; Backes, W.H. Mapping hypercapnia-induced cerebrovascular reactivity using BOLD MRI. Neuroradiology 2005, 47, 114–120. [Google Scholar] [CrossRef]
- Pillai, J.J.; Zaca, D. Clinical utility of cerebrovascular reactivity mapping in patients with low grade gliomas. World J. Clin. Oncol. 2011, 2, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.J.; Zaca, D. Comparison of BOLD cerebrovascular reactivity mapping and DSC MR perfusion imaging for prediction of neurovascular uncoupling potential in brain tumors. Technol. Cancer Res. Treat. 2012, 11, 361–374. [Google Scholar] [CrossRef]
- Zacà, D.; Agarwal, S.; Pillai, J.J. Breath-Hold Cerebrovascular ReactivityCerebrovascular reactivity (CVR) Mapping for Neurovascular UncouplingNeurovascular uncoupling (NVU) Assessment in Primary GliomasGliomas. In Cerebrovascular Reactivity: Methodological Advances and Clinical Applications; Chen, J., Fierstra, J., Eds.; Springer: New York, NY, USA, 2022; pp. 167–183. [Google Scholar] [CrossRef]
- Zaca, D.; Hua, J.; Pillai, J.J. Cerebrovascular reactivity mapping for brain tumor presurgical planning. World J. Clin. Oncol. 2011, 2, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Zaca, D.; Jovicich, J.; Nadar, S.R.; Voyvodic, J.T.; Pillai, J.J. Cerebrovascular reactivity-based calibration of presurgical motor activation maps to improve detectability of the BOLD signal in patients with perirolandic brain tumors. In Proceedings of the International Society for Magnetic Resonance in Medicine (ISMRM) 21st Annual Meeting, Salt Lake City, UT, USA, 20–26 April 2013; p. Abstract 3554. [Google Scholar]
- Zaca, D.; Jovicich, J.; Nadar, S.R.; Voyvodic, J.T.; Pillai, J.J. Cerebrovascular reactivity mapping in patients with low grade gliomas undergoing presurgical sensorimotor mapping with BOLD fMRI. J. Magn. Reson. Imaging 2014, 40, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Birn, R.M.; Smith, M.A.; Jones, T.B.; Bandettini, P.A. The respiration response function: The temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage 2008, 40, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Iranmahboob, A.; Peck, K.K.; Brennan, N.P.; Karimi, S.; Fisicaro, R.; Hou, B.; Holodny, A.I. Vascular Reactivity Maps in Patients with Gliomas Using Breath-Holding BOLD fMRI. J. Neuroimaging 2016, 26, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Hsu, Y.Y.; Chang, C.N.; Jung, S.M.; Lim, K.E.; Huang, J.C.; Fang, S.Y.; Liu, H.L. Blood oxygenation level-dependent MRI of cerebral gliomas during breath holding. J. Magn. Reson. Imaging 2004, 19, 160–167. [Google Scholar] [CrossRef]
- Peacock, J.; Black, D.; DeLone, D.; Welker, K. Use of a simple breath-holding task for cerebrovascular reactivity scans in clinical functional MR imaging. Neurographics 2016, 6, 213–218. [Google Scholar] [CrossRef]
- Cohen, A.D.; Jagra, A.S.; Visser, N.J.; Yang, B.; Fernandez, B.; Banerjee, S.; Wang, Y. Improving the Breath-Holding CVR Measurement Using the Multiband Multi-Echo EPI Sequence. Front. Physiol. 2021, 12, 619714. [Google Scholar] [CrossRef]
- Parkes, M.J. Breath-holding and its breakpoint. Exp. Physiol. 2006, 91, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Moreton, F.C.; Dani, K.A.; Goutcher, C.; O’Hare, K.; Muir, K.W. Respiratory challenge MRI: Practical aspects. Neuroimage Clin. 2016, 11, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Thomason, M.E.; Glover, G.H. Controlled inspiration depth reduces variance in breath-holding-induced BOLD signal. Neuroimage 2008, 39, 206–214. [Google Scholar] [CrossRef]
- Li, T.Q.; Kastrup, A.; Takahashi, A.M.; Moseley, M.E. Functional MRI of human brain during breath holding by BOLD and FAIR techniques. Neuroimage 1999, 9, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Thomason, M.E.; Burrows, B.E.; Gabrieli, J.D.; Glover, G.H. Breath holding reveals differences in fMRI BOLD signal in children and adults. Neuroimage 2005, 25, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Bright, M.G.; Murphy, K. Reliable quantification of BOLD fMRI cerebrovascular reactivity despite poor breath-hold performance. Neuroimage 2013, 83, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Magon, S.; Basso, G.; Farace, P.; Ricciardi, G.K.; Beltramello, A.; Sbarbati, A. Reproducibility of BOLD signal change induced by breath holding. Neuroimage 2009, 45, 702–712. [Google Scholar] [CrossRef]
- Scouten, A.; Schwarzbauer, C. Paced respiration with end-expiration technique offers superior BOLD signal repeatability for breath-hold studies. Neuroimage 2008, 43, 250–257. [Google Scholar] [CrossRef]
- Roberts, P.; Jezzard, P.; Bulte, D. Comparison of Breath Holding Techniques for the Calibration of FMRI Measurements of Oxygen Metabolism. Proc. Intl. Soc. Mag. Reson. Med. 2009, 17, 1532. [Google Scholar]
- Kastrup, A.; Li, T.Q.; Glover, G.H.; Moseley, M.E. Cerebral blood flow-related signal changes during breath-holding. Am. J. Neuroradiol. 1999, 20, 1233–1238. [Google Scholar]
- Bright, M.G.; Bulte, D.P.; Jezzard, P.; Duyn, J.H. Characterization of regional heterogeneity in cerebrovascular reactivity dynamics using novel hypocapnia task and BOLD fMRI. Neuroimage 2009, 48, 166–175. [Google Scholar] [CrossRef] [PubMed]
- van Niftrik, C.H.; Piccirelli, M.; Bozinov, O.; Pangalu, A.; Valavanis, A.; Regli, L.; Fierstra, J. Fine tuning breath-hold-based cerebrovascular reactivity analysis models. Brain Behav. 2016, 6, e00426. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.A. The CO2 stimulus for cerebrovascular reactivity: Fixing inspired concentrations vs. targeting end-tidal partial pressures. J. Cereb. Blood Flow. Metab. 2016, 36, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.A.; Sobczyk, O.; Crawley, A.; Poublanc, J.; Dufort, P.; Venkatraghavan, L.; Sam, K.; Mikulis, D.; Duffin, J. Assessing cerebrovascular reactivity by the pattern of response to progressive hypercapnia. Hum. Brain Mapp. 2017, 38, 3415–3427. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; De Vis, J.B.; Lu, H. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: A technical review. Neuroimage 2019, 187, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, P.; Yezhuvath, U.; Cheng, Y.; Marshall, O.; Ge, Y. MRI mapping of cerebrovascular reactivity via gas inhalation challenges. J. Vis. Exp. 2014, 94, e52306. [Google Scholar] [CrossRef]
- Poublanc, J.; Crawley, A.P.; Sobczyk, O.; Montandon, G.; Sam, K.; Mandell, D.M.; Dufort, P.; Venkatraghavan, L.; Duffin, J.; Mikulis, D.J.; et al. Measuring cerebrovascular reactivity: The dynamic response to a step hypercapnic stimulus. J. Cereb. Blood Flow. Metab. 2015, 35, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
- Mandell, D.M.; Han, J.S.; Poublanc, J.; Crawley, A.P.; Stainsby, J.A.; Fisher, J.A.; Mikulis, D.J. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in Patients with arterial steno-occlusive disease: Comparison with arterial spin labeling MRI. Stroke 2008, 39, 2021–2028. [Google Scholar] [CrossRef]
- Fierstra, J.; van Niftrik, C.; Warnock, G.; Wegener, S.; Piccirelli, M.; Pangalu, A.; Esposito, G.; Valavanis, A.; Buck, A.; Luft, A.; et al. Staging Hemodynamic Failure With Blood Oxygen-Level-Dependent Functional Magnetic Resonance Imaging Cerebrovascular Reactivity: A Comparison Versus Gold Standard ((15)O-)H(2)O-Positron Emission Tomography. Stroke 2018, 49, 621–629. [Google Scholar] [CrossRef]
- Tancredi, F.B.; Lajoie, I.; Hoge, R.D. A simple breathing circuit allowing precise control of inspiratory gases for experimental respiratory manipulations. BMC Res. Notes 2014, 7, 235. [Google Scholar] [CrossRef]
- Prisman, E.; Slessarev, M.; Azami, T.; Nayot, D.; Milosevic, M.; Fisher, J. Modified oxygen mask to induce target levels of hyperoxia and hypercarbia during radiotherapy: A more effective alternative to carbogen. Int. J. Radiat. Biol. 2007, 83, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Mark, C.I.; Slessarev, M.; Ito, S.; Han, J.; Fisher, J.A.; Pike, G.B. Precise control of end-tidal carbon dioxide and oxygen improves BOLD and ASL cerebrovascular reactivity measures. Magn. Reson. Med. 2010, 64, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Duffin, J. Measuring the respiratory chemoreflexes in humans. Respir. Physiol. Neurobiol. 2011, 177, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.A.; Iscoe, S.; Duffin, J. Sequential gas delivery provides precise control of alveolar gas exchange. Respir. Physiol. Neurobiol. 2016, 225, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, H.; Brodrick, P.M.; Taylor, N.J.; Abdelatti, M.O.; Jordan, L.C.; Vasudevan, A.S.; Phillips, H.; Saunders, M.I.; Hoskin, P.J. Gas exchange parameters in radiotherapy patients during breathing of 2%, 3.5% and 5% carbogen gas mixtures. Br. J. Radiol. 2000, 73, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.G.; Pattinson, K.T.; Bulte, D.P.; Chiarelli, P.A.; Mayhew, S.D.; Balanos, G.M.; O’Connor, D.F.; Pragnell, T.R.; Robbins, P.A.; Tracey, I.; et al. Dynamic forcing of end-tidal carbon dioxide and oxygen applied to functional magnetic resonance imaging. J. Cereb. Blood Flow. Metab. 2007, 27, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Slessarev, M.; Han, J.; Mardimae, A.; Prisman, E.; Preiss, D.; Volgyesi, G.; Ansel, C.; Duffin, J.; Fisher, J.A. Prospective targeting and control of end-tidal CO2 and O2 concentrations. J. Physiol. 2007, 581, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.A.; Mikulis, D.J. Cerebrovascular Reactivity: Purpose, Optimizing Methods, and Limitations to Interpretation—A Personal 20-Year Odyssey of (Re)searching. Front. Physiol. 2021, 12, 629651. [Google Scholar] [CrossRef]
- Champagne, A.A.; Bhogal, A.A. Insights Into Cerebral Tissue-Specific Response to Respiratory Challenges at 7T: Evidence for Combined Blood Flow and CO2-Mediated Effects. Front. Physiol. 2021, 12, 601369. [Google Scholar] [CrossRef]
- Kannurpatti, S.S.; Biswal, B.B. Detection and scaling of task-induced fMRI-BOLD response using resting state fluctuations. Neuroimage 2008, 40, 1567–1574. [Google Scholar] [CrossRef]
- Kannurpatti, S.S.; Motes, M.A.; Biswal, B.B.; Rypma, B. Assessment of unconstrained cerebrovascular reactivity marker for large age-range FMRI studies. PLoS ONE 2014, 9, e88751. [Google Scholar] [CrossRef] [PubMed]
- Lipp, I.; Murphy, K.; Caseras, X.; Wise, R.G. Agreement and repeatability of vascular reactivity estimates based on a breath-hold task and a resting state scan. Neuroimage 2015, 113, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, Y.; Pinho, M.; Park, D.C.; Welch, B.G.; Lu, H. Cerebrovascular reactivity mapping without gas challenges. Neuroimage 2017, 146, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Yezhuvath, U.S.; Lewis-Amezcua, K.; Varghese, R.; Xiao, G.; Lu, H. On the assessment of cerebrovascular reactivity using hypercapnia BOLD MRI. NMR Biomed. 2009, 22, 779–786. [Google Scholar] [CrossRef]
- Liu, P.; Liu, G.; Pinho, M.C.; Lin, Z.; Thomas, B.P.; Rundle, M.; Park, D.C.; Huang, J.; Welch, B.G.; Lu, H. Cerebrovascular Reactivity Mapping Using Resting-State BOLD Functional MRI in Healthy Adults and Patients with Moyamoya Disease. Radiology 2021, 299, 419–425. [Google Scholar] [CrossRef]
- Yeh, M.Y.; Chen, H.S.; Hou, P.; Kumar, V.A.; Johnson, J.M.; Noll, K.R.; Prabhu, S.S.; Ferguson, S.D.; Schomer, D.F.; Peng, H.H.; et al. Cerebrovascular Reactivity Mapping Using Resting-State Functional MRI in Patients With Gliomas. J. Magn. Reson. Imaging 2022, 56, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Golestani, A.M.; Wei, L.L.; Chen, J.J. Quantitative mapping of cerebrovascular reactivity using resting-state BOLD fMRI: Validation in healthy adults. Neuroimage 2016, 138, 147–163. [Google Scholar] [CrossRef]
- Fesharaki, N.J.; Mathew, A.B.; Mathis, J.R.; Huddleston, W.E.; Reuss, J.L.; Pillai, J.J.; DeYoe, E.A. Effects of Thresholding on Voxel-Wise Correspondence of Breath-Hold and Resting-State Maps of Cerebrovascular Reactivity. Front. Neurosci. 2021, 15, 654957. [Google Scholar] [CrossRef]
- Hou, X.; Guo, P.; Wang, P.; Liu, P.; Lin, D.D.M.; Fan, H.; Li, Y.; Wei, Z.; Lin, Z.; Jiang, D.; et al. Deep-learning-enabled brain hemodynamic mapping using resting-state fMRI. npj Digit. Med. 2023, 6, 116. [Google Scholar] [CrossRef]
- DeYoe, E.A.; Ulmer, J.L.; Mueller, W.M.; Sabsevitz, D.S.; Reitsma, D.C.; Pillai, J.J. Imaging of the Functional and Dysfunctional Visual System. Semin. Ultrasound CT MR 2015, 36, 234–248. [Google Scholar] [CrossRef][Green Version]
- Voss, H.U.; Peck, K.K.; Petrovich Brennan, N.M.; Pogosbekyan, E.L.; Zakharova, N.E.; Batalov, A.I.; Pronin, I.N.; Potapov, A.A.; Holodny, A.I. A vascular-task response dependency and its application in functional imaging of brain tumors. J. Neurosci. Methods 2019, 322, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Pronin, I.N.; Batalov, A.I.; Zakharova, N.E.; Fadeeva, L.M.; Pogosbekyan, E.L.; Goryaynov, S.A.; Buklina, S.B.; Ogurtsova, A.A.; Kulikov, A.S.; Rodionov, P.V.; et al. Evaluation of vascular reactivity to overcome limitations of neurovascular uncoupling in BOLD fMRI of malignant brain tumors. Zh Vopr. Neirokhir Im. N. N. Burdenko 2018, 82, 21–29. [Google Scholar] [CrossRef]
- Agarwal, S.; Airan, R.; Gujar, S.K.; Sair, H.I.; Pillai, J.J. Calibration of BOLD fMRI motor activation maps using BOLD breath hold cerebrovascular reactivity mapping for effective compensation of brain tumor-related neurovascular uncoupling. In Proceedings of the International Society for Magnetic Resonance in Medicine (ISMRM) 23rd Annual Meeting, Toronto, ON, Canada, 30 May–5 June 2015; p. Abstract 0216. [Google Scholar]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, A.; Zimmermann, M.; Heinz, G.; Oberndorfer, S.; Doerfler, A.; Buchfelder, M.; Rössler, K. Magnetic resonance imaging biomarkers for clinical routine assessment of microvascular architecture in glioma. J. Cereb. Blood Flow. Metab. 2017, 37, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Englander, Z.K.; Horenstein, C.I.; Bowden, S.G.; Chow, D.S.; Otten, M.L.; Lignelli, A.; Bruce, J.N.; Canoll, P.; Grinband, J. Extent of BOLD Vascular Dysregulation Is Greater in Diffuse Gliomas without Isocitrate Dehydrogenase 1 R132H Mutation. Radiology 2018, 287, 965–972. [Google Scholar] [CrossRef]
- Hahn, A.; Bode, J.; Krüwel, T.; Solecki, G.; Heiland, S.; Bendszus, M.; Tews, B.; Winkler, F.; Breckwoldt, M.O.; Kurz, F.T. Glioblastoma multiforme restructures the topological connectivity of cerebrovascular networks. Sci. Rep. 2019, 9, 11757. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Zuo, Z.; Ying, J.; Jin, L.; Kang, J.; Gui, S.; Wang, R.; Li, C. Structural and Functional Alterations in the Contralesional Medial Temporal Lobe in Glioma Patients. Front. Neurosci. 2020, 14, 10. [Google Scholar] [CrossRef]
- Tong, Y.; Frederick, B. Concurrent fNIRS and fMRI processing allows independent visualization of the propagation of pressure waves and bulk blood flow in the cerebral vasculature. Neuroimage 2012, 61, 1419–1427. [Google Scholar] [CrossRef]
- Tong, Y.; Hocke, L.M.; Frederick, B.B. Low Frequency Systemic Hemodynamic “Noise” in Resting State BOLD fMRI: Characteristics, Causes, Implications, Mitigation Strategies, and Applications. Front. Neurosci. 2019, 13, 787. [Google Scholar] [CrossRef]
- Tong, Y.; Hocke, L.M.; Fan, X.; Janes, A.C.; Frederick, B. Can apparent resting state connectivity arise from systemic fluctuations? Front. Hum. Neurosci. 2015, 9, 285. [Google Scholar] [CrossRef]
- Tong, Y.; Lindsey, K.P.; Hocke, L.M.; Vitaliano, G.; Mintzopoulos, D.; Frederick, B.D. Perfusion information extracted from resting state functional magnetic resonance imaging. J. Cereb. Blood Flow. Metab. 2017, 37, 564–576. [Google Scholar] [CrossRef]
- Gupta, L.; Gupta, R.K.; Postma, A.A.; Sahoo, P.; Gupta, P.K.; Patir, R.; Ahlawat, S.; Saha, I.; Backes, W.H. Advanced and amplified BOLD fluctuations in high-grade gliomas. J. Magn. Reson. Imaging 2018, 47, 1616–1625. [Google Scholar] [CrossRef]
- Cai, S.; Shi, Z.; Zhou, S.; Liang, Y.; Wang, L.; Wang, K.; Zhang, L. Cerebrovascular Dysregulation in Patients with Glioma Assessed with Time-shifted BOLD fMRI. Radiology 2022, 304, 155–163. [Google Scholar] [CrossRef]
- Petridis, P.D.; Horenstein, C.I.; Pereira, B.; Wu, P.B.; Samanamud, J.; Marie, T.; Boyett, D.; Sudhakar, T.D.; Sheth, S.A.; McKhann, G.M.; et al. BOLD asynchrony elucidates tumor burden in IDH-mutated gliomas. Neuro Oncol. 2022, 24, 78–87. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, S.; Welker, K.M.; Black, D.F.; Little, J.T.; DeLone, D.R.; Messina, S.A.; Passe, T.J.; Bettegowda, C.; Pillai, J.J. Detection and Mitigation of Neurovascular Uncoupling in Brain Gliomas. Cancers 2023, 15, 4473. https://doi.org/10.3390/cancers15184473
Agarwal S, Welker KM, Black DF, Little JT, DeLone DR, Messina SA, Passe TJ, Bettegowda C, Pillai JJ. Detection and Mitigation of Neurovascular Uncoupling in Brain Gliomas. Cancers. 2023; 15(18):4473. https://doi.org/10.3390/cancers15184473
Chicago/Turabian StyleAgarwal, Shruti, Kirk M. Welker, David F. Black, Jason T. Little, David R. DeLone, Steven A. Messina, Theodore J. Passe, Chetan Bettegowda, and Jay J. Pillai. 2023. "Detection and Mitigation of Neurovascular Uncoupling in Brain Gliomas" Cancers 15, no. 18: 4473. https://doi.org/10.3390/cancers15184473
APA StyleAgarwal, S., Welker, K. M., Black, D. F., Little, J. T., DeLone, D. R., Messina, S. A., Passe, T. J., Bettegowda, C., & Pillai, J. J. (2023). Detection and Mitigation of Neurovascular Uncoupling in Brain Gliomas. Cancers, 15(18), 4473. https://doi.org/10.3390/cancers15184473





