Resveratrol-Laden Nano-Systems in the Cancer Environment: Views and Reviews
Abstract
:Simple Summary
Abstract
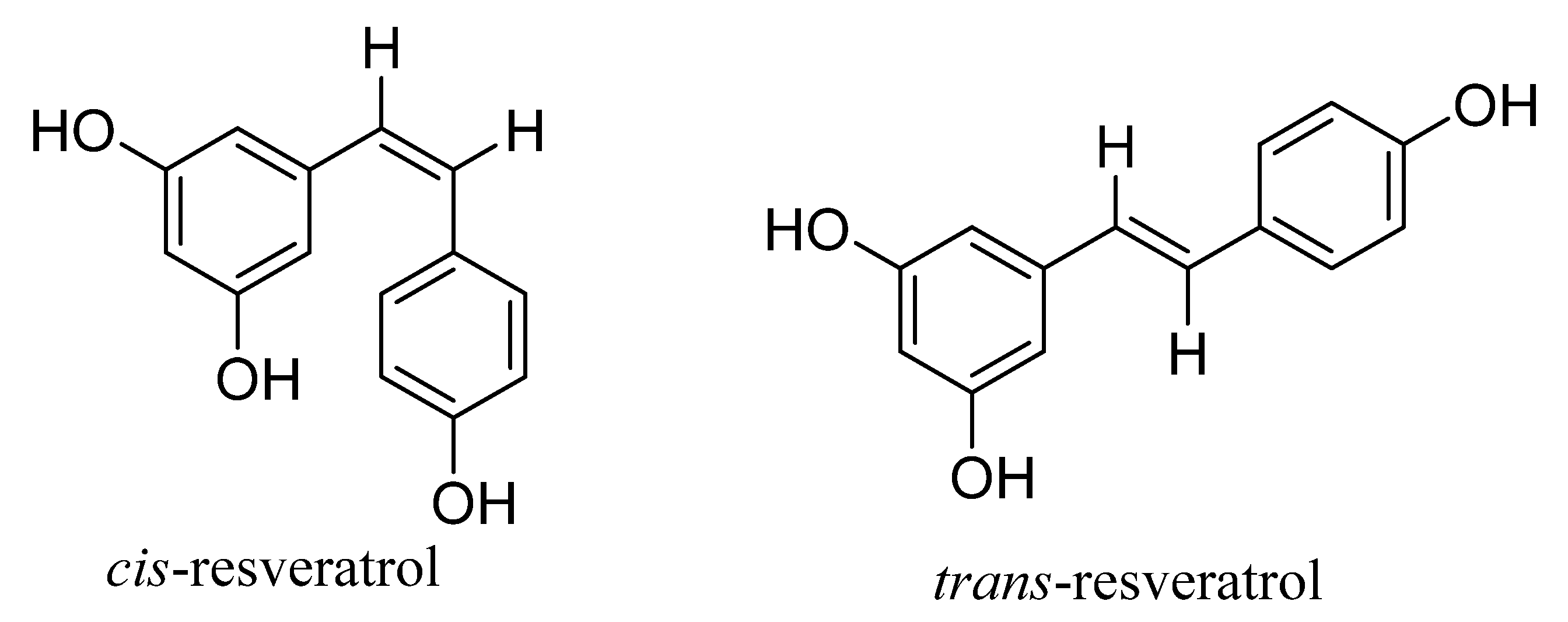
1. Introduction
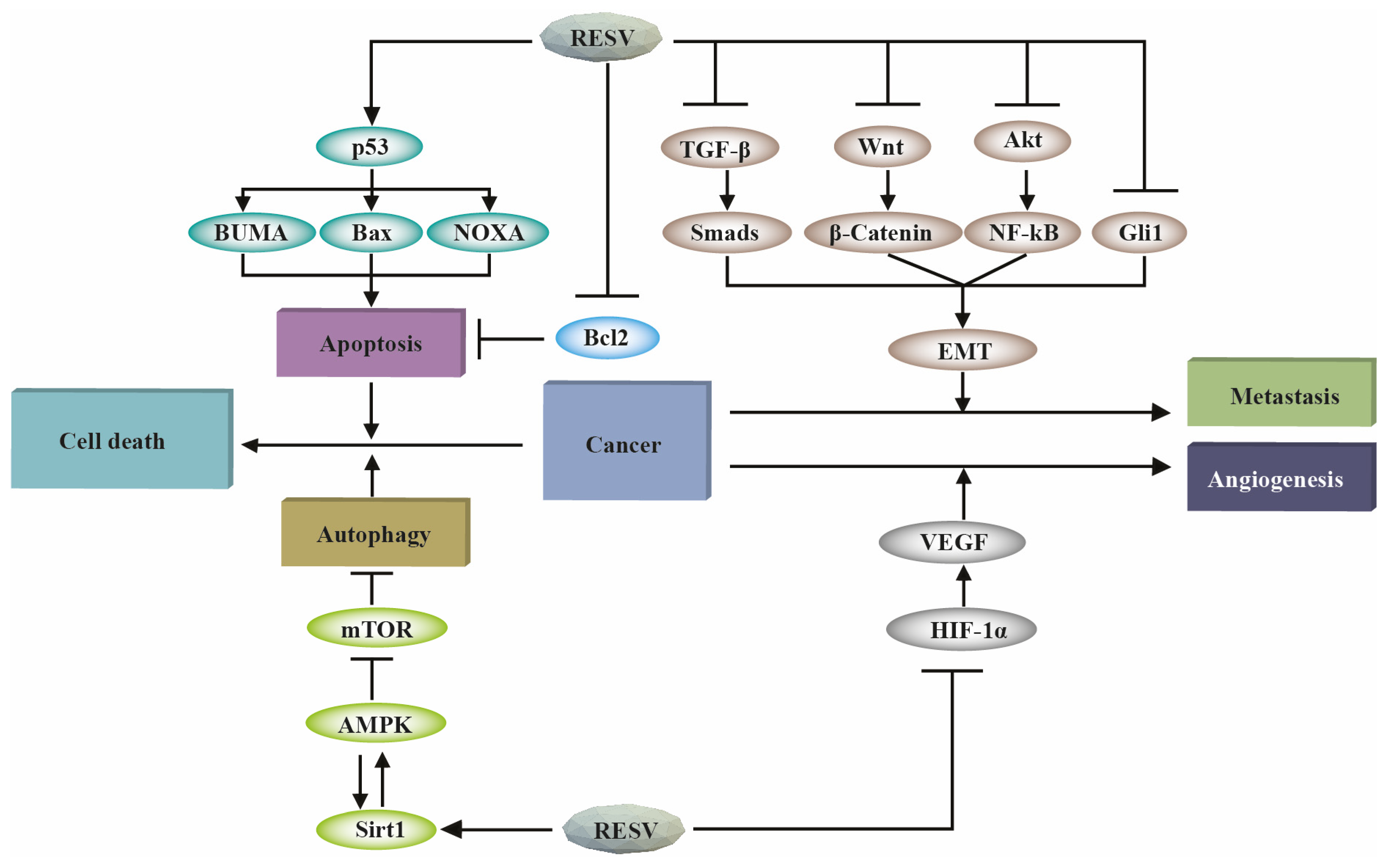
2. Mode of Action of Resveratrol
| Mode of Action | Description | Ref. |
|---|---|---|
| Inhibition of metastasis | Decreases the expression levels of MMP-2 and MMP-9, fibronectin, and α-smooth muscle actin (α-SMA) | [30] |
| Inhibition of angiogenesis | Inhibition of endothelial cell adhesion and migration by reducing MMP-2 activity during neo-angiogenesis | [31] |
| Induction of autophagy | Increased activation and expression of sirtuin1 (SIRT1) and the inhibition of the protein kinase B/mammalian target of rapamycin (Akt/mTOR) | [32] |
| Induction of apoptosis | Activation of the extracellular signal-regulated kinase (ERK)1/2 via MAPK-kinase | [33] |
| Reprogramming of cancer cell metabolism | Regulates the enzymatic activity of pyruvate dehydrogenase (PDH) in obtaining coenzyme A | [34] |
2.1. Resveratrol-Mediated Induction of Autophagy
2.2. Resveratrol-Facilitated Induction of Apoptosis
2.3. Resveratrol-Assisted Inhibition of Angiogenesis
2.4. Resveratrol-Enabled Inhibition of Metastasis
2.5. Resveratrol-Aided Reprogramming of Metabolism in Cancer Cells
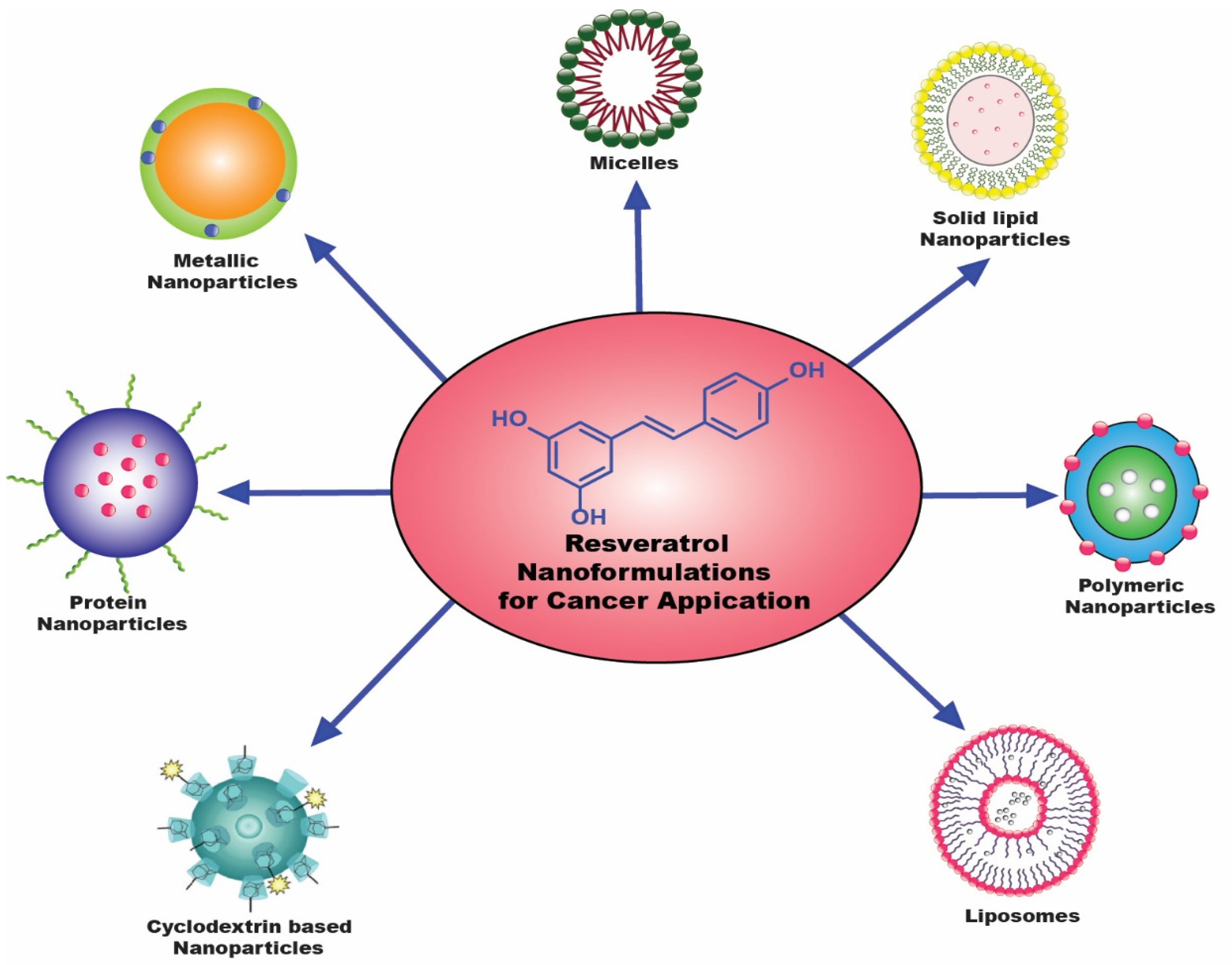
3. Resveratrol-Entrapped Nanosystems
3.1. Enhanced Stability: In Vitro Studies
3.2. Enhanced Therapeutic Potential
3.3. Toxicity Studies
3.4. New Patent Literature: Innovative Formulations and Technological Advancements
4. Challenges and Future Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karakaya, S.; Koca, M.; Yılmaz, S.V.; Yıldırım, K.; Pınar, N.M.; Demirci, B.; Brestic, M.; Sytar, O. Molecular Docking Studies of Coumarins Isolated from Extracts and Essential Oils of Zosima Absinthifolia Link as Potential Inhibitors for Alzheimer’s Disease. Molecules 2019, 24, 722. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as plant-based nutraceuticals: Health effects, encapsulation, nano-delivery, and application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary anti-aging polyphenols and potential mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7661. [Google Scholar] [CrossRef] [PubMed]
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol Nanoformulations: Challenges and Opportunities. Int. J. Pharm. 2015, 479, 282–290. [Google Scholar] [CrossRef]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of Resveratrol: What Formulation Solutions to Bioavailability Limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; McClements, D.J. Nutraceutical Delivery Systems: Resveratrol Encapsulation in Grape Seed Oil Nanoemulsions Formed by Spontaneous Emulsification. Food Chem. 2015, 167, 205–212. [Google Scholar] [CrossRef]
- Rossi, D.; Guerrini, A.; Bruni, R.; Brognara, E.; Borgatti, M.; Gambari, R.; Maietti, S.; Sacchetti, G. Trans-Resveratrol in Nutraceuticals: Issues in Retail Quality and Effectiveness. Molecules 2012, 17, 12393–12405. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Farhan, F.; Al Kury, L.T. Resveratrol and tumor microenvironment: Mechanistic basis and therapeutic targets. Molecules 2020, 25, 4282. [Google Scholar] [CrossRef]
- Patnaik, S.; Gorain, B.; Padhi, S.; Choudhury, H.; Gabr, G.A.; Md, S.; Kumar Mishra, D.; Kesharwani, P. Recent Update of Toxicity Aspects of Nanoparticulate Systems for Drug Delivery. Eur. J. Pharm. Biopharm. 2021, 161, 100–119. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Behera, A.; Hasnain, M.S.; Nayak, A.K. Chitosan-Based Drug Delivery Systems in Cancer Therapeutics. In Chitosan Drug Delivery, 1st ed.; Hasnain, M.S., Beg, S., Nayak, A.K., Eds.; Academic Press: Massachusetts, MA, USA, 2022; Volume 1, pp. 159–193. ISBN 978-0-12-819336-5. [Google Scholar]
- Padhi, S.; Dash, M.; Behera, A. Nanophytochemicals for the Treatment of Type II Diabetes Mellitus: A Review. Environ. Chem. Lett. 2021, 19, 4349–4373. [Google Scholar] [CrossRef]
- Padhi, S.; Nayak, A.K.; Behera, A. Type II Diabetes Mellitus: A Review on Recent Drug Based Therapeutics. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Fenizia, S.; Vassalle, C. Sphingolipid Levels and Signaling via Resveratrol and Antioxidant Actions in Cardiometabolic Risk and Disease. Antioxidants 2023, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Pala, N.; Sechi, M. Targeted Therapy Using Nanotechnology: Focus on Cancer. Int. J. Nanomed. 2014, 9, 467. [Google Scholar] [CrossRef]
- Bohara, R.A.; Tabassum, N.; Singh, M.P.; Gigli, G.; Ragusa, A.; Leporatti, S. Recent overview of resveratrol’s beneficial effects and its nano-delivery systems. Molecules 2022, 27, 5154. [Google Scholar] [CrossRef] [PubMed]
- Karve, S.; Werner, M.E.; Sukumar, R.; Cummings, N.D.; Copp, J.A.; Wang, E.C.; Li, C.; Sethi, M.; Chen, R.C.; Pacold, M.E.; et al. Revival of the Abandoned Therapeutic Wortmannin by Nanoparticle Drug Delivery. Proc. Natl. Acad. Sci. USA 2012, 109, 8230–8235. [Google Scholar] [CrossRef]
- Annaji, M.; Poudel, I.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Resveratrol-Loaded Nanomedicines for Cancer Applications. Cancer Rep. 2021, 4, e1353. [Google Scholar] [CrossRef]
- Blanco, E.; Hsiao, A.; Mann, A.P.; Landry, M.G.; Meric-Bernstam, F.; Ferrari, M. Nanomedicine in Cancer Therapy: Innovative Trends and Prospects. Cancer Sci. 2011, 102, 1247–1252. [Google Scholar] [CrossRef]
- Behera, A.; Padhi, S. Passive and Active Targeting Strategies for the Delivery of the Camptothecin Anticancer Drug: A Review. Environ. Chem. Lett. 2020, 18, 1557–1567. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.; Rodriguez-Torres, M.D.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Kapoor, R.; Verma, D.; Panda, A.K.; Iqbal, Z. Formulation and Optimization of Topotecan Nanoparticles: In Vitro Characterization, Cytotoxicity, Cellular Uptake and Pharmacokinetic Outcomes. J. Photochem. Photobiol. B Biol. 2018, 183, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Mirza, M.A.; Verma, D.; Khuroo, T.; Panda, A.K.; Talegaonkar, S.; Khar, R.K.; Iqbal, Z. Revisiting the Nanoformulation Design Approach for Effective Delivery of Topotecan in Its Stable Form: An Appraisal of Its In Vitro Behavior and Tumor Amelioration Potential. Drug Deliv. 2015, 23, 2827–2837. [Google Scholar] [CrossRef]
- Kundu, A.; Padhi, S.; Behera, A.; Hasnain, M.S.; Nayak, A.K. Tumor Targeting Strategies by Chitosan-Based Nanocarriers. In Chitosan in Biomedical Applications, 1st ed.; Hasnain, M.S., Beg, S., Nayak, A.K., Eds.; Academic Press: Massachusetts, MA, USA, 2022; Volume 1, pp. 163–188. ISBN 978-0-12-821058-1. [Google Scholar]
- Vervandier-Fasseur, D.; Latruffe, N. The Potential Use of Resveratrol for Cancer Prevention. Molecules 2019, 24, 4506. [Google Scholar] [CrossRef] [PubMed]
- Berardi, V.; Ricci, F.; Castelli, M.; Galati, G.; Risuleo, G. Resveratrol exhibits a strong cytotoxic activity in cultured cells and has an antiviral action against polyomavirus: Potential clinical use. J. Exp. Clin. Cancer Res. 2009, 28, 96. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.M.; Lu, Y.Y.; Zhang, H.; Chen, Q.L.; Zhao, M.; Su, S.B. Resveratrol Inhibits the Migration and Metastasis of MDA-MB-231 Human Breast Cancer by Reversing TGF-Β1-Induced Epithelial-Mesenchymal Transition. Molecules 2019, 24, 1131. [Google Scholar] [CrossRef]
- Trapp, V.; Parmakhtiar, B.; Papazian, V.; Willmott, L.; Fruehauf, J.P. Anti-Angiogenic Effects of Resveratrol Mediated by Decreased VEGF and Increased TSP1 Expression in Melanoma-Endothelial Cell Co-Culture. Angiogenesis 2010, 13, 305–315. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Cao, N.; Li, Z.; Han, J.; Li, L. Resveratrol, an Activator of SIRT1, Induces Protective Autophagy in Non-Small-Cell Lung Cancer via Inhibiting Akt/MTOR and Activating P38-MAPK. OncoTargets Ther. 2018, 11, 7777. [Google Scholar] [CrossRef]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An Overview of Its Anti-Cancer Mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Mukazhanova, Z.; Knut, E.; Turgumbayeva, A.; Kipchakbayeva, A.; Seitimova, G.; Mahomoodally, M.F.; Lobine, D.; Koay, A.; et al. Resveratrol-Based Nanoformulations as an Emerging Therapeutic Strategy for Cancer. Front. Mol. Biosci. 2021, 8, 222. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Kohli, L.; Roth, K.A. Autophagy: Cerebral home cooking. Am. J. Pathol. 2010, 176, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Karantza-Wadsworth, V.; White, E. Role of Autophagy in Cancer. Nat. Rev. Cancer 2007, 7, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, S.; Gao, K.; Zhou, Z.; Wang, C.; Shen, Z.; Guo, Y.; Li, Z.; Wan, Z.; Liu, C.; et al. Resveratrol Protects against Spinal Cord Injury by Activating Autophagy and Inhibiting Apoptosis Mediated by the SIRT1/AMPK Signaling Pathway. Neuroscience 2017, 348, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Qin, Z.; Li, F.; Zhang, H.; Fang, Z.; Hao, E. Apoptotic Cell Death Induced by Resveratrol Is Partially Mediated by the Autophagy Pathway in Human Ovarian Cancer Cells. PLoS ONE 2015, 10, e0129196. [Google Scholar] [CrossRef]
- Chang, C.H.; Lee, C.Y.; Lu, C.C.; Tsai, F.J.; Hsu, Y.M.; Tsao, J.W.; Juan, Y.N.; Chiu, H.Y.; Yang, J.S.; Wang, C.C. Resveratrol-Induced Autophagy and Apoptosis in Cisplatin-Resistant Human Oral Cancer CAR Cells: A Key Role of AMPK and Akt/MTOR Signaling. Int. J. Oncol. 2017, 50, 873–882. [Google Scholar] [CrossRef]
- Bao, L.; Jaramillo, M.C.; Zhang, Z.; Zheng, Y.; Yao, M.; Zhang, D.D.; Yi, X. Induction of autophagy contributes to cisplatin resistance in human ovarian cancer cells. Mol. Med. Rep. 2015, 11, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Liu, Y.; Li, Q.; Guo, X.; Gu, L.; Ma, Z.G.; Zhu, Y.P. Resveratrol Induces Apoptosis and Autophagy in T-Cell Acute Lymphoblastic Leukemia Cells by Inhibiting Akt/MTOR and Activating P38-MAPK. Biomed. Environ. Sci. 2013, 26, 902–911. [Google Scholar] [CrossRef]
- Joe, A.K.; Liu, H.; Suzui, M.; Vural, M.E.; Xiao, D.; Weinstein, I.B. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin. Cancer Res. 2002, 8, 893–903. [Google Scholar]
- Liu, Z.; Li, Y.; Yang, R. Effects of Resveratrol on Vascular Endothelial Growth Factor Expression in Osteosarcoma Cells and Cell Proliferation. Oncol. Lett. 2012, 4, 837–839. [Google Scholar] [CrossRef] [PubMed]
- Kasiotis, K.M.; Pratsinis, H.; Kletsas, D.; Haroutounian, S.A. Resveratrol and Related Stilbenes: Their Anti-Aging and Anti-Angiogenic Properties. Food Chem. Toxicol. 2013, 61, 112–120. [Google Scholar] [CrossRef]
- Cao, Z.; Fang, J.; Xia, C.; Shi, X.; Jiang, B.H. Trans-3,4,5′-Trihydroxystibene Inhibits Hypoxia-Inducible Factor 1α and Vascular Endothelial Growth Factor Expression in Human Ovarian Cancer Cells. Clin. Cancer Res. 2004, 10, 5253–5263. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Liu, X.; Han, Z.; Zhou, L.; Sui, H.; Yan, L.; Jiang, H.; Ren, J.; Cai, J.; Li, Q. Resveratrol Suppresses Epithelial-to-Mesenchymal Transition in Colorectal Cancer through TGF-Β1/Smads Signaling Pathway Mediated Snail/E-Cadherin Expression. BMC Cancer 2015, 15, 97. [Google Scholar] [CrossRef]
- Ji, Q.; Liu, X.; Fu, X.; Zhang, L.; Sui, H.; Zhou, L.; Sun, J.; Cai, J.; Qin, J.; Ren, J.; et al. Resveratrol Inhibits Invasion and Metastasis of Colorectal Cancer Cells via MALAT1 Mediated Wnt/β-Catenin Signal Pathway. PLoS ONE 2013, 8, e78700. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Cheng, L.; Jiang, Z.; Chen, K.; Zhou, C.; Sun, L.; Cao, J.; Qian, W.; Li, J.; Shan, T.; et al. Resveratrol inhibits ROS-promoted activation and glycolysis of pancreatic stellate cells via suppression of miR-21. Oxid. Med. Cell. Longev. 2018, 2018, 1346958. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, L.; Luo, Y.; Li, X.; Chen, G.; Wang, Y. Resveratrol inhibits the proliferation and induces the apoptosis in ovarian cancer cells via inhibiting glycolysis and targeting AMPK/mTOR signaling pathway. J. Cell. Biochem. 2018, 119, 6162–6172. [Google Scholar] [CrossRef]
- Robinson, K.; Mock, C.; Liang, D. Pre-Formulation Studies of Resveratrol. Drug Dev. Ind. Pharm. 2014, 41, 1464–1469. [Google Scholar] [CrossRef]
- Yu, C.; Geun Shin, Y.; Chow, A.; Li, Y.; Kosmeder, J.W.; Sup Lee, Y.; Hirschelman, W.H.; Pezzuto, J.M.; Mehta, R.G.; Van Breemen, R.B. Human, Rat, and Mouse Metabolism of Resveratrol. Pharm. Res. 2002, 19, 1907–1914. [Google Scholar] [CrossRef]
- Chung, J.H.; Lee, J.S.; Lee, H.G. Resveratrol-Loaded Chitosan–γ-Poly(Glutamic Acid) Nanoparticles: Optimization, Solubility, UV Stability, and Cellular Antioxidant Activity. Colloids Surf. B Biointerfaces 2020, 186, 110702. [Google Scholar] [CrossRef]
- Hu, W.H.; Chan, G.K.L.; Duan, R.; Wang, H.Y.; Kong, X.P.; Dong, T.T.X.; Tsim, K.W.K. Synergy of Ginkgetin and Resveratrol in Suppressing VEGF-Induced Angiogenesis: A Therapy in Treating Colorectal Cancer. Cancers 2019, 11, 1828. [Google Scholar] [CrossRef] [PubMed]
- Behera, A.; Padhi, S.; Nayak, A.K. Engineered Liposomes as Drug Delivery and Imaging Agents. Des. Appl. Theranostic Nanomed. 2022, 13, 75. [Google Scholar]
- Coimbra, M.; Isacchi, B.; Van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.F.; Storm, G.; et al. Improving Solubility and Chemical Stability of Natural Compounds for Medicinal Use by Incorporation into Liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef]
- Isailović, B.D.; Kostić, I.T.; Zvonar, A.; Dordević, V.B.; Gašperlin, M.; Nedović, V.A.; Bugarski, B.M. Resveratrol Loaded Liposomes Produced by Different Techniques. Innov. Food Sci. Emerg. Technol. 2013, 19, 181–189. [Google Scholar] [CrossRef]
- Doane, T.L.; Chuang, C.H.; Hill, R.J.; Burda, C. Nanoparticle ζ-Potentials. Acc. Chem. Res. 2012, 45, 317–326. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Matencio, A.; Caldera, F.; Argenziano, M.; Cavalli, R.; Dianzani, C.; Zanetti, M.; López-Nicolás, J.M.; Trotta, F. Comparative Evaluation of Solubility, Cytotoxicity and Photostability Studies of Resveratrol and Oxyresveratrol Loaded Nanosponges. Pharmaceutics 2019, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, B.; Li, Y.; Li, H.; Ji, S.; Xia, Q. pH-responsive Pickering emulsions-pectin hydrogel beads for loading of resveratrol: Preparation, characterization, and evaluation. J. Drug Deliv. Sci. Technol. 2023, 79, 104008. [Google Scholar] [CrossRef]
- Kumar, S.; Pooja; Trotta, F.; Rao, R. Encapsulation of Babchi Oil in Cyclodextrin-Based Nanosponges: Physicochemical Characterization, Photodegradation, and In Vitro Cytotoxicity Studies. Pharmaceutics 2018, 10, 169. [Google Scholar] [CrossRef]
- Baek, Y.; Jeong, E.W.; Lee, H.G. Encapsulation of resveratrol within size-controlled nanoliposomes: Impact on solubility, stability, cellular permeability, and oral bioavailability. Colloids Surf. B 2023, 224, 113205. [Google Scholar] [CrossRef]
- Tang, Y.; Yu, Z.; Lu, X.; Fan, Q.; Huang, W. Overcoming vascular barriers to improve the theranostic outcomes of nanomedicines. Adv. Sci. 2022, 9, 2103148. [Google Scholar] [CrossRef]
- Ahmad, E.; Ali, A.; Fatima, M.T.; Kumar, A.; Sumi, M.P.; Sattar, R.S.; Mahajan, B.; Saluja, S.S. Ligand decorated biodegradable nanomedicine in the treatment of cancer. Pharmacol. Res. 2021, 167, 105544. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An Overview of Active and Passive Targeting Strategies to Improve the Nanocarriers Efficiency to Tumour Sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
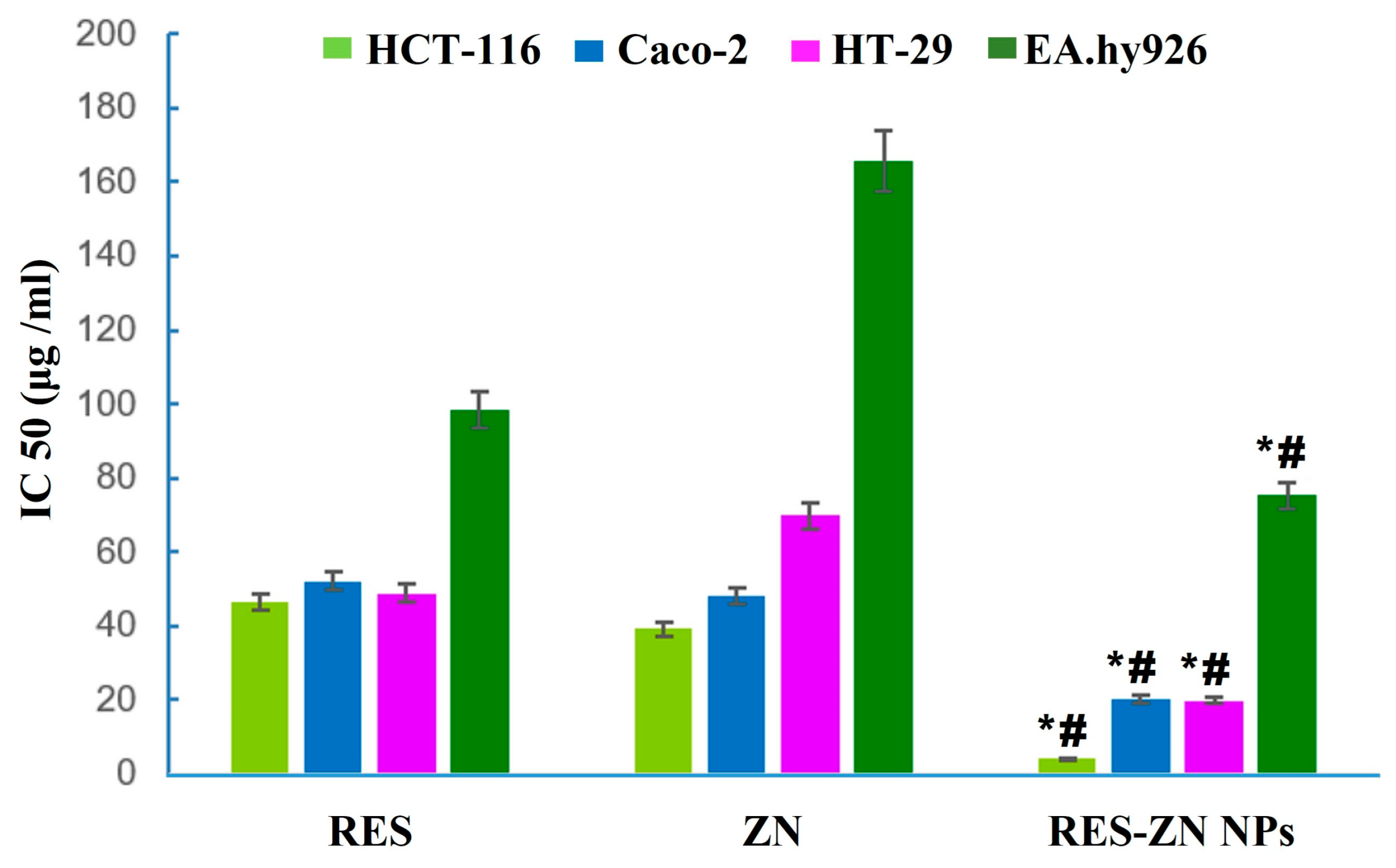
- Khayat, M.T.; Zarka, M.A.; El-Telbany, D.F.A.; El-Halawany, A.M.; Kutbi, H.I.; Elkhatib, W.F.; Noreddin, A.M.; Khayyat, A.N.; El-Telbany, R.F.A.; Hammad, S.F.; et al. Intensification of Resveratrol Cytotoxicity, pro-Apoptosis, Oxidant Potentials in Human Colorectal Carcinoma HCT-116 Cells Using Zein Nanoparticles. Sci. Rep. 2022, 12, 15235. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic Efficacy of Curcumin and Resveratrol against Cancer: Chemoprevention, Chemoprotection, Drug Synergism and Clinical Pharmacokinetics. Semin. Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef]
- Sudha, T.; El-Far, A.H.; Mousa, D.S.; Mousa, S.A. Resveratrol and Its Nanoformulation Attenuate Growth and the Angiogenesis of Xenograft and Orthotopic Colon Cancer Models. Molecules 2020, 25, 1412. [Google Scholar] [CrossRef] [PubMed]
- Bozorgi, A.; Haghighi, Z.; Khazaei, M.R.; Bozorgi, M.; Khazaei, M. The anti-cancer effect of chitosan/resveratrol polymeric nanocomplex against triple-negative breast cancer; an in vitro assessment. IET Nanobiotechnol. 2023, 17, 91–102. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Aghaz, F.; Khazaei, M.; Raygani, A.V. The anti-cancer effect of resveratrol nano-encapsulated supplements against breast cancer via the regulation of oxidative stress. J. Microencapsul. 2023, 40, 318–329. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A.; Höckel, M. Impact of Hemoglobin Levels on Tumor Oxygenation: The Higher, the Better? Strahlenther. Und Onkol. 2006, 182, 63–71. [Google Scholar] [CrossRef]
- Xiang, S.; Zhang, K.; Yang, G.; Gao, D.; Zeng, C.; He, M. Mitochondria-Targeted and Resveratrol-Loaded Dual-Function Titanium Disulfide Nanosheets for Photothermal-Triggered Tumor Chemotherapy. Nanoscale Res. Lett. 2019, 14, 211. [Google Scholar] [CrossRef]
- Sanna, V.; Siddiqui, I.A.; Sechi, M.; Mukhtar, H. Resveratrol-Loaded Nanoparticles Based on Poly(Epsiloncaprolactone) and Poly(D,L-Lactic-Co-Glycolic Acid)-Poly(Ethylene Glycol) Blend for Prostate Cancer Treatment. Mol. Pharm. 2013, 10, 3871–3881. [Google Scholar] [CrossRef]
- Peñalva, R.; Morales, J.; González-Navarro, C.J.; Larrañeta, E.; Quincoces, G.; Peñuelas, I.; Irache, J.M. Increased Oral Bioavailability of Resveratrol by Its Encapsulation in Casein Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2816. [Google Scholar] [CrossRef]
- Wu, J.M.; Hsieh, T.; Wang, Z. Cardioprotection by Resveratrol: A Review of Effects/Targets in Cultured Cells and Animal Tissues. Am. J. Cardiovasc. Dis. 2011, 1, 38. [Google Scholar] [PubMed]
- Mankowski, R.T.; You, L.; Buford, T.W.; Leeuwenburgh, C.; Manini, T.M.; Schneider, S.; Qiu, P.; Anton, S.D. Higher Dose of Resveratrol Elevated Cardiovascular Disease Risk Biomarker Levels in Overweight Older Adults—A Pilot Study. Exp. Gerontol. 2020, 131, 110821. [Google Scholar] [CrossRef]
- Tome-Carneiro, J.; Larrosa, M.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A.; Teresa Garcia-Conesa, M.; Carlos Espin, J. Resveratrol and Clinical Trials: The Crossroad from In Vitro Studies to Human Evidence. Curr. Pharm. Des 2013, 19, 6064–6093. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Zeng, J.; Li, Z.; Zuo, H.; Huang, C.; Zhao, X. Nano-Gold Loaded with Resveratrol Enhance the Anti-Hepatoma Effect of Resveratrol In Vitro and In Vivo. J. Biomed. Nanotechnol. 2019, 15, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, M.R.; Kumari, L.; Patel, K.K.; Vuddanda, P.R.; Vajanthri, K.Y.; Mahto, S.K.; Singh, S. Intravenous Administration of Trans-Resveratrol-Loaded TPGS-Coated Solid Lipid Nanoparticles for Prolonged Systemic Circulation, Passive Brain Targeting and Improved in Vitro Cytotoxicity against C6 Glioma Cell Lines. RSC Adv. 2016, 6, 50336–50348. [Google Scholar] [CrossRef]
- Abdelgawad, I.Y.; Grant, M.K.O.; Zordoky, B.N. Leveraging the Cardio-Protective and Anticancer Properties of Resveratrol in Cardio-Oncology. Nutrients 2019, 11, 627. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol Commonly Displays Hormesis: Occurrence and Biomedical Significance. Hum. Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.S.; Garland, L.L.; Hsu, C.H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol Modulates Drug- and Carcinogen-Metabolizing Enzymes in a Healthy Volunteer Study. Cancer Prev. Res. 2010, 3, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Afzal, N.; Khan, J.A.; Hussain, Z.; Qureshi, A.S.; Anwar, H.; Jamil, Y. Antioxidant Resveratrol Protects against Copper Oxide Nanoparticle Toxicity in Vivo. Naunyn. Schmiedeberg’s Arch. Pharmacol. 2018, 391, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.; Zhao, X.; Ma, M.; Zhu, G.; Yin, L. Resveratrol-Loaded Albumin Nanoparticles with Prolonged Blood Circulation and Improved Biocompatibility for Highly Effective Targeted Pancreatic Tumor Therapy. Nanoscale Res. Lett. 2017, 12, 437. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Cao, Y.N.; Sun, J.; Wu, Q.; Cui, S.H.; Zhi, D.F.; Zhang, S.B.; Liang, Z.; Zhen, Y.H.; Guo, S.T. Anti-Breast Cancer Activity of Resveratrol Encapsulated in Liposomes. J. Mater. Chem. B 2019, 8, 27–37. [Google Scholar] [CrossRef]
- Rostami, M.; Ghorbani, M.; Aman Mohammadi, M.; Delavar, M.; Tabibiazar, M.; Ramezani, S. Development of Resveratrol Loaded Chitosan-Gellan Nanofiber as a Novel Gastrointestinal Delivery System. Int. J. Biol. Macromol. 2019, 135, 698–705. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, T.; Yang, C.L. Development of PLGA-Lipid Nanoparticles with Covalently Conjugated Indocyanine Green as a Versatile Nanoplatform for Tumor-Targeted Imaging and Drug Delivery. Int. J. Nanomed. 2016, 11, 5807. [Google Scholar] [CrossRef]
- Lian, B.; Wu, M.; Feng, Z.; Deng, Y.; Zhong, C.; Zhao, X. Folate-Conjugated Human Serum Albumin-Encapsulated Resveratrol Nanoparticles: Preparation, Characterization, Bioavailability and Targeting of Liver Tumors. Artif. Cells Nanomed. Biotechnol. 2019, 47, 154–165. [Google Scholar] [CrossRef]
- Jadhav, P.; Bothiraja, C.; Pawar, A. Resveratrol-Piperine Loaded Mixed Micelles: Formulation, Characterization, Bioavailability, Safety and in Vitro Anticancer Activity. RSC Adv. 2016, 6, 112795–112805. [Google Scholar] [CrossRef]
- Suktham, K.; Koobkokkruad, T.; Wutikhun, T.; Surassmo, S. Efficiency of Resveratrol-Loaded Sericin Nanoparticles: Promising Bionanocarriers for Drug Delivery. Int. J. Pharm. 2018, 537, 48–56. [Google Scholar] [CrossRef]
- Hao, J.; Tong, T.; Jin, K.; Zhuang, Q.; Han, T.; Bi, Y.; Wang, J.; Wang, X. Folic Acid-Functionalized Drug Delivery Platform of Resveratrol Based on Pluronic 127/D-α-Tocopheryl Polyethylene Glycol 1000 Succinate Mixed Micelles. Int. J. Nanomed. 2017, 12, 2279. [Google Scholar] [CrossRef]
- Chauhan, A.S.; Newenhouse, E.A.; Gerhardt, A.H. Compositions Comprising a Dendrimer-Resveratrol Complex and Methods for Making and Using the Same. U.S. Patent US11110068B2, 7 September 2021. [Google Scholar]
- Dang, H.N.T.; Lai, N.H. Process for Producing a Nano Resveratrol Microemulsion System. U.S. Patent US10882012B2, 5 January 2021. [Google Scholar]
- Mingxing, D.; Huafeng, Z. Resveratrol Phospholipid Composite Nano-Emulsion and Preparation Method and Application Thereof. Chinese Patent CN101579291B, 15 June 2011. [Google Scholar]
- Mingxing, D.; Huafeng, Z. Resveratrol Phospholipid Composite Nano-Emulsion and Preparation Method and Application Thereof. Chinese Patent Application Publication CN101579291A, 18 November 2009. [Google Scholar]
- Chen, W.; Liao, W. Nano Resveratrol-Coated Compound As Well As Preparation Method and Application Thereof. Chinese Patent Application Publication CN112773728A, 11 May 2021. [Google Scholar]
- Tripathi, V. Colloidally Stable Resveratrol Nanoparticles with Improved Bioavailability and Half-Life and Synthesis Thereof. PCT Patent Application Publication WO2017137957A1, 17 August 2017. [Google Scholar]
- Zhao, X.; Huang, C.; Zhang, D.; Zhang, J. Preparation Method of Gold Nano-Loaded Resveratrol. Chinese Patent CN108159427B, 17 December 2019. [Google Scholar]
- Zhao, X.; Zhang, J.; Zhang, D. Application of gold nanometer load resveratrol. Chinese Patent Application Publication CN108114286A, 5 June 2018. [Google Scholar]
- Shi, Q.; Jin, R.; Chen, J.; Chen, M.; Li, H.; Lv, L.; Gao, B. Flexible Liposome of Resveratrol and Preparation Method Thereof. European Patent Application Publication EP2431023A1, 21 March 2012. [Google Scholar]
- Wang, S.; Jin, C.; Xu, T.; Hu, Y.; Zhang, X.; Mao, B.; Bao, S. Resveratrol Nanoethosome and Preparation Method and Application Thereof. Chinese Patent CN110200829B, 1 April 2022. [Google Scholar]
- Chen, J.; Zhang, X.; Le, Y.; Wang, J.; Huang, H.; Geng, Y.; Zhang, Z.; Zhu, W. Resveratrol Nanoscale Dispersoid and Preparation Method Thereof. Chinese Patent CN102614127B, 30 July 2014. [Google Scholar]
- Ouyangwuqing; Baoping, Y. Resveratrol Nano Emulsion Anti-Cancer Medicine. Chinese Patent Application Publication CN101214225A, 9 July 2008.
- Wu, Y.; Sun, Z.; Chang, J.; Fu, P.; Li, J. Resveratrol Nano-Liposome As Well As Preparation Method and Application Thereof. Chinese Patent Application Publication CN111920771A, 13 November 2020. [Google Scholar]
- Zhang, W.; Liang, X.; Qin, Y.; Zhu, H. Resveratrol-Coated Nano Solid Lipid Carrier and Preparation Method Thereof. Chinese Patent Application Publication CN105534724A, 4 May 2016. [Google Scholar]
- Wang, Q.; Liu, H.; Liu, L.; Chen, Q.; Hu, H. Resveratrol Nano-Liposome and Preparation Method Thereof. Chinese Patent CN103040754B, 20 May 2015. [Google Scholar]
- Wang, Z.; Zhu, K.; Wang, B.; Cai, F.; Ren, J.; Zhang, T.; Zhang, Q.; Zong, S. Resveratrol Solid Lipid Nanoparticles and Preparation Method Thereof. Chinese Patent CN104688715B, 10 October 2017. [Google Scholar]
- Xiao, C.; Chen, X.; Ding, J.; Zhuang, X.; Chen, X. Resveratrol Nano-Particles and Preparation Method Thereof. Chinese Patent CN105126116B, 1 June 2018. [Google Scholar]
- Ren, X.; Hu, D.; Gu, T.; Yu, J.; Zhang, X.; Xu, B.; Liang, Q. Method for Preparing Zein Nanometer Particles for Embedding Resveratrol through Ultrasonic Waves. Chinese Patent Application Publication CN106954861A, 18 July 2017. [Google Scholar]
- Xia, Q.; Zhao, W. Resveratrol Nanostructured Lipid Carrier and Preparation Method Thereof. Chinese Patent CN102614091B, 22 January 2014. [Google Scholar]
- Kim, C.T.; Kim, C.J.; Cho, Y.J.; Choi, A.J. Nanoemulsion, Nanoparticle Containing Resveratrol and Method of Production Thereof. Korean Patent Application Publication KR20090132357A, 30 December 2009. [Google Scholar]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kurmi, B.D.; Singh, A.; Singh, D. Potential role of resveratrol and its nano-formulation as anti-cancer agent. Explor Target Antitumor Ther. 2022, 3, 643. [Google Scholar] [CrossRef] [PubMed]
- Houacine, C.; Singh, K.K. 10 Nano resveratrol: A promising future nanonutraceutical. NanoNutraceuticals 2018, 6, 165–181. [Google Scholar]
- Taghavi, S.M.; Momenpour, M.; Azarian, M.; Ahmadian, M.; Souri, F.; Taghavi, S.A.; Sadeghain, M.; Karchani, M. Effects of nanoparticles on the environment and outdoor workplaces. Electron. Physician 2013, 5, 706. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Sanna, V.; Ahmad, N.; Sechi, M.; Mukhtar, H. Resveratrol nanoformulation for cancer prevention and therapy. Ann. N. Y. Acad. Sci. 2015, 1348, 20–31. [Google Scholar] [CrossRef]
- Singh, C.K.; Ndiaye, M.A.; Ahmad, N. Resveratrol and cancer: Challenges for clinical translation. Biochim. Biophys. Acta. Mol. Basis Dis. 2015, 1852, 1178–1185. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Keutzer, L.; You, H.; Farnoud, A.; Nyberg, J.; Wicha, S.G.; Maher-Edwards, G.; Vlasakakis, G.; Moghaddam, G.K.; Svensson, E.M.; Menden, M.P.; et al. Machine learning and pharmacometrics for prediction of pharmacokinetic data: Differences, similarities and challenges illustrated with rifampicin. Pharmaceutics 2022, 14, 1530. [Google Scholar] [CrossRef]
- Bannigan, P.; Aldeghi, M.; Bao, Z.; Häse, F.; Aspuru-Guzik, A.; Allen, C. Machine learning directed drug formulation development. Adv. Drug Deliv. Rev. 2021, 175, 113806. [Google Scholar] [CrossRef]
- Saleem, Z.; Rehman, K.; Hamid Akash, M.S. Role of Drug Delivery System in Improving the Bioavailability of Resveratrol. Curr. Pharm. Des. 2022, 28, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Furxhi, I.; Murphy, F. Predicting in vitro neurotoxicity induced by nanoparticles using machine learning. Int. J. Mol. Sci. 2020, 21, 5280. [Google Scholar] [CrossRef] [PubMed]
- Eicher, T.; Kinnebrew, G.; Patt, A.; Spencer, K.; Ying, K.; Ma, Q.; Machiraju, R.; Mathé, E.A. Metabolomics and multi-omics integration: A survey of computational methods and resources. Metabolites 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]

| NCT Identifier | Condition | Intervention | Measures of Outcome | Status |
|---|---|---|---|---|
| NCT00256334 | Colon cancer | Resveratrol | Test the hypothesis that resveratrol modulates Wnt signaling in vivo in colon cancer and normal colonic mucosa | Phase I (completed) |
| NCT02261844 | Liver cancer | Resveratrol; Placebo | Improve metabolic profile of liver cells; decrease cell growth and proliferation; and decrease hepatic inflammation | Phase I/II (withdrawn due to lack of funding) |
| NCT00098969 | Adult solid tumor | Resveratrol | Determine the concentration of resveratrol and its metabolites in healthy participants’ plasma, urine, and feces; correlate the dose with the systemic concentration of this drug and its metabolites in these participants; and determine the drug’s safety | Phase I (completed) |
| NCT01476592 | Neuroendocrine tumor | Dietary supplement; Resveratrol | Notch1 activation in post-treatment tumor biopsy specimens when compared to pretreatment levels | Phase not mentioned (completed) |
| NCT04266353 | Chemoprevention | Dietary supplement; Resveratrol | IGF2 assessment using ELISA assays | Phase not mentioned (suspended due to COVID-19) |
| NCT00433576 | Adenocarcinoma of the colon; adenocarcinoma of the rectum | Resveratrol | Pharmacodynamics of resveratrol; concentrations of biomarkers | Phase I (completed) |
| NCT00920556 | Multiple myeloma | SRT501; Bortezomib | Last Observed Response (LOR); Number of Participants with Stable Disease (SD) as Best Response (BR); Number of Participants with Stable Disease (SD) as LOR; Number of Participants with Progressive Disease (PD) PD as Best Response (BR); Time to Disease Progression; and Change From Baseline in Hematology | Phase II (withdrawn) |
| Formulation | Cancer Type | Cell Line; In Vitro Cytotoxicity | In Vivo Toxicity | Ref. |
|---|---|---|---|---|
| Chitosan-gellan nanofibers | Colorectal | HT29 cells; when contrasted to native resveratrol, the said nano-formulation had a nearly identical cytotoxicity profile | NA | [88] |
| Indocyanin- and folic-acid-conjugated nanoparticles | Tumor | U87 cell; cytotoxicity of targeted formulation vs. native drug: 81.4 ± 2.1% vs. 53.1 ± 1.1% | The blood measurements showed that the heart, liver, spleen, lungs, and kidneys were significantly unharmed, with selective tumor uptake; no remarkable weight loss in the studied mice | [89] |
| Folate-conjugated HAS nanoparticles | Liver | HePG2 cells; targeted NPs displayed a slower resveratrol release and enhanced cytotoxicity than the non-targeted formulation | No toxic effects on the major organs were marked | [90] |
| Piperine-loaded mixed micelles | Breast | MCF-7; superior cytotoxicity by the nano-formulation was noted | No significant tissue toxicity was noted in the heart, kidney, and lungs | [91] |
| Sericin nanoparticles | Colorectal | CRL-2522 and Caco-2 cells; cytotoxic action against Caco-2 cells | Not mentioned | [92] |
| Folic-acid-targeted micelles | Breast | MCF-7; dummy NPs displayed no cytotoxicity on the cells, and the nano-formulation sustained the release of entrapped resveratrol, leading to enhanced cytotoxicity | Reduced accumulation in the heart and kidneys due to tissue dispersion | [93] |
| Patent/Application Number/Applicant | Summary |
|---|---|
| US11110068B2 (Concordia University, United States) | The patent discusses several compositions containing a dendrimer–resveratrol complex. For the treatment of various diseases, including malignancies, the composition may come in the form of a dispersion, aqueous solution, solid, semi-solid, nanosuspension, or other combinations [94]. |
| US10882012B2 (Wakamono Corp, Vietnam) | This patent finding is connected to generating a resveratrol nano/microemulsion system. This invention might be used to treat cancers [95]. |
| CN101579291B (Tsinghua University, China) | The invention describes a nano-emulsion composed of resveratrol and phospholipids and its preparation technique and applications. The particle size of the resveratrol phospholipid composite in this newly discovered formulation was under 200 nm. The formulation efficiently transports resveratrol to the cancer’s target site [96]. |
| CN101579291A (Tsinghua University, China) | This invention revealed the method of the preparation of a resveratrol phospholipid composite nano-emulsion. This method produced particles with a size of less than 200 nm, which can then be applied to the treatment of cancer [97]. |
| CN112773728A (Jiangxi Science and Technology Normal University, China) | In this discovery, the resveratrol is encased in lecithin nanoparticles to achieve the effects of continuously and gradually releasing resveratrol and the good effect of promoting the transdermal penetration of the resveratrol. This prolongs the resveratrol’s retention time on the skin and enhances its bioavailability [98]. |
| WO2017137957A1 (Tripathi Vinaykumar, India) | This invention combines resveratrol with tree fat in a unique resveratrol delivery system. This procedure created nanoparticles with a particle size of less than 100 nm. The nanoparticles can maintain their stability and prevent phase 2 metabolism in the mammalian body [99]. |
| CN108159427B (Southwest University, China) | The invention discloses a preparation method for gold nano-load resveratrol, in which resveratrol was facilitated to react with a chloroauric acid solution to afford the gold nano-loaded resveratrol for treating hepatic carcinoma [100]. |
| CN108114286A (Southwest University, Chongqing, China) | The application of resveratrol in tumors is disclosed in the invention, along with the preparation of the gold nano-loaded resveratrol and its application in treating liver cancer [101]. This invention is the extended work of patent CN108159427B [100]. |
| EP2431023A1 (Shanghai Jahwa United Company Limited, China) | The resveratrol flexible liposome is the subject of the current innovation. Due to the resveratrol flexible liposome’s strong penetrability in the current invention, the active ingredient’s transdermal absorption is significantly improved. This method could be used to topically administer chemotherapy drugs such as resveratrol [102]. |
| CN110200829B (Hunan University of Humanities Science and Technology, China) | The application of a resveratrol nano ethosome through the skin to address bioavailability problems is the subject of the innovation. The discovery could be applied to the topical administration of resveratrol for treating skin cancer [103]. |
| CN102614127B (Beijing Fuyuan Pharmaceuticals, China) | The resveratrol nanoscale dispersoid and a technique for making it are the subjects of the invention. When the resveratrol nanoscale dispersoid is dispersed in cold water, a transparent solution with nano-resveratrol particles smaller than 150 nanometers is obtained. The process of creating resveratrol nanoscale dispersoids with a high water dispersibility and consistency can be used to produce medications, health items, food, and cosmetics [104]. |
| CN101214225A (Northwest A&F University, China) | The current invention describes an anti-malignant nano-emulsion containing resveratrol. The produced resveratrol nano-emulsion had particle sizes ranging from 1nm to 100 nm. The nano-emulsion had a homogeneous dispersion, high fluidity, and low viscidity. The produced nano-emulsion had a stronger anti-cancer effect and longer half-life. Moreover, the formulation mentioned above increases resveratrol’s stability by preventing the oxygenation of the compound [105]. |
| CN111920771A (Henan University, China) | The resveratrol nano-liposome is made using the film dispersion method in this invention. This new technique claims to increase the drug’s solubility in water by about 172 times and increase its stability. It was also said to have a great encapsulation efficiency and small particle size. As per the claim, the preparation procedure is straightforward, the preparation duration is brief, the production expenses are reasonable, and industrial output can be scaled up. The applications of resveratrol in healthcare products, foods, and medicines are promoted [106]. |
| CN105534724A (Shanghai Institute of Technology, Shanghai, China) | This invention relates to a nano-solid lipid carrier with particles of 50–200 nm, coated with resveratrol, and capable of penetrating a cuticle well. The invention did not mention the pharmacological application of this sort of nanocarrier. However, it is predicted that the formed resveratrol-coated nano-solid lipid carrier can be employed for treating skin cancer [107]. |
| CN103040754B (Institute of Food Science and Technology of CAAS, China) | A resveratrol nano-liposome and its production method are described in this invention. The produced resveratrol nano-liposome exhibits a high encapsulation efficiency, good stability, tiny grain size, uniformity, and consistency. The resveratrol nano-liposome can be employed as a chemotherapy drug to treat liver cancer [108]. |
| CN104688715B (Shanghai Traditional Chinese medicine hospital, China) | The subject of this invention is a solid lipid nanogranule made of resveratrol and a way to make it. The current invention’s resveratrol solid lipid nanogranules offer the following advantages: a small particle size, high bioavailability, rapid drug absorption, a high drug loading rate, ease of preparation, low toxicity, and so on [109]. |
| CN105126116B (Changchun Institute of Applied Chemistry of CAS, China) | The current invention produces a nanoparticle form of resveratrol utilizing polyglycol monomethyl ether. The experimental results showed that the resveratrol nano-particle has good water solubility, and the resveratrol-loaded formulation better fragment tumor cells [110]. |
| CN106954861A (Jiangsu University, China) | This discovery produces resveratrol nanoparticles with zeins incorporated using ultrasonic waves. The created zeins nanoparticles of the current invention have several benefits, including a good stability, good biocompatibility, gradual release, prolonged active period, and the capacity to be used in various industries, including food, health products, medicine, and cosmetics [111]. |
| CN102614091B (Xia Qiang, China) | This innovation discloses a nanostructured lipid carrier for resveratrol. The resveratrol carrier system is stable and water-soluble. The mentioned formulation is prepared controllably and easily, and the method can be repeatable. Manufacturers of resveratrol-containing cosmetics can make use of this delivery system. This preparation can also effectively deliver resveratrol into the cancer microenvironment to treat various cancers [112]. |
| KR20090132357A (Korea Food Research Institute, Korea) | In this disclosure, a nanoparticle and nano-emulsion and their synthesis methods are offered to improve the bioavailability of resveratrol, which is only marginally soluble in water. The nano-emulsions and nanoparticles may deliver resveratrol chemotherapeutics in various tumor milieus [113]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarfraz, M.; Arafat, M.; Zaidi, S.H.H.; Eltaib, L.; Siddique, M.I.; Kamal, M.; Ali, A.; Asdaq, S.M.B.; Khan, A.; Aaghaz, S.; et al. Resveratrol-Laden Nano-Systems in the Cancer Environment: Views and Reviews. Cancers 2023, 15, 4499. https://doi.org/10.3390/cancers15184499
Sarfraz M, Arafat M, Zaidi SHH, Eltaib L, Siddique MI, Kamal M, Ali A, Asdaq SMB, Khan A, Aaghaz S, et al. Resveratrol-Laden Nano-Systems in the Cancer Environment: Views and Reviews. Cancers. 2023; 15(18):4499. https://doi.org/10.3390/cancers15184499
Chicago/Turabian StyleSarfraz, Muhammad, Mosab Arafat, Syeda Huma H. Zaidi, Lina Eltaib, Muhammad Irfan Siddique, Mehnaz Kamal, Abuzer Ali, Syed Mohammed Basheeruddin Asdaq, Abida Khan, Shams Aaghaz, and et al. 2023. "Resveratrol-Laden Nano-Systems in the Cancer Environment: Views and Reviews" Cancers 15, no. 18: 4499. https://doi.org/10.3390/cancers15184499
APA StyleSarfraz, M., Arafat, M., Zaidi, S. H. H., Eltaib, L., Siddique, M. I., Kamal, M., Ali, A., Asdaq, S. M. B., Khan, A., Aaghaz, S., Alshammari, M. S., & Imran, M. (2023). Resveratrol-Laden Nano-Systems in the Cancer Environment: Views and Reviews. Cancers, 15(18), 4499. https://doi.org/10.3390/cancers15184499










