Targeting MET Amplification: Opportunities and Obstacles in Therapeutic Approaches
Abstract
Simple Summary
Abstract
1. Introduction
2. Gene Amplification and Protein Overexpression
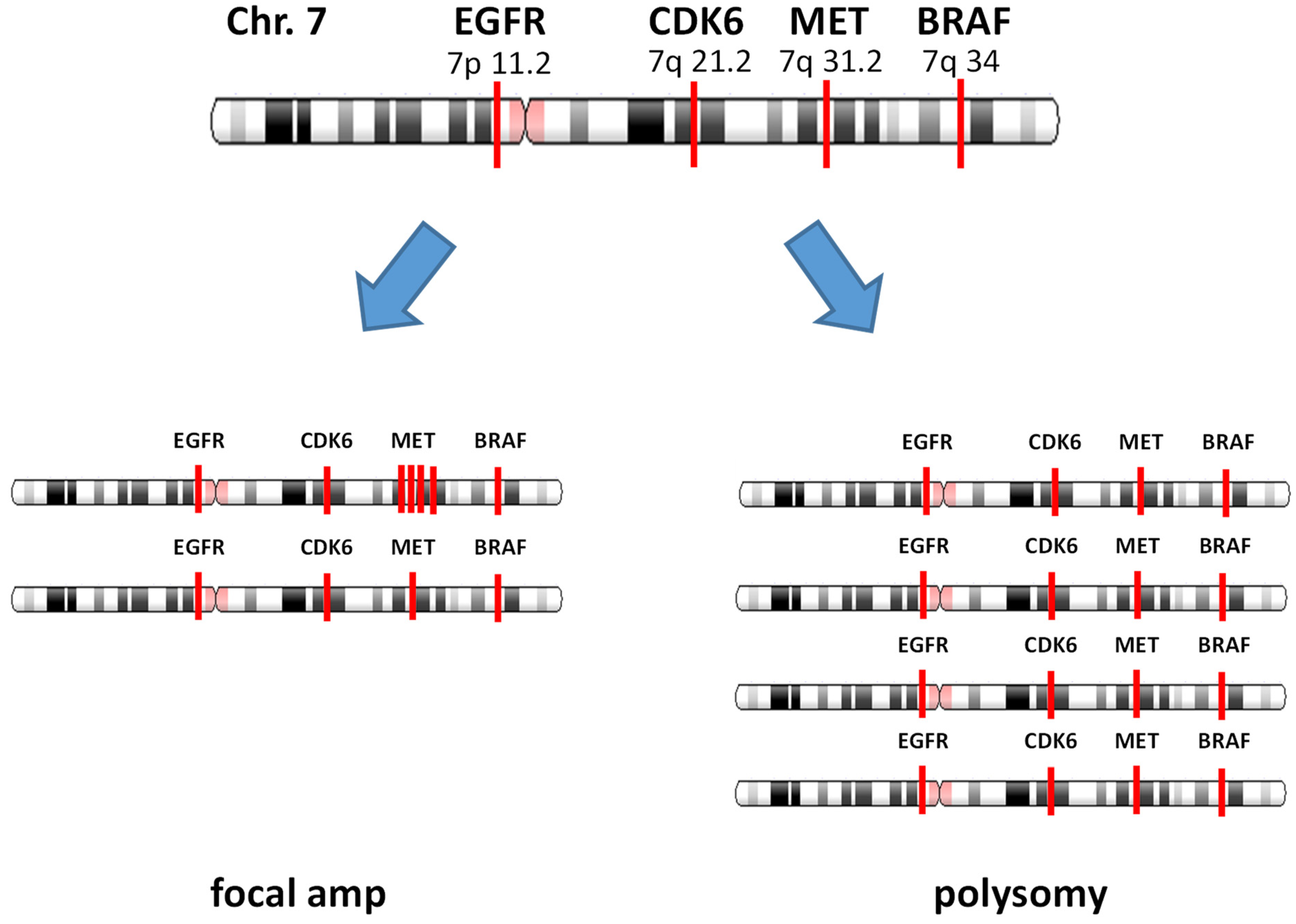
3. Focal Gene Amplification and Polysomy
4. MET Amplification Detection by FISH
5. MET Amplification Detection by NGS
- (a)
- MET copy number ≥ 2.2.
- (b)
- MET is amplified without co-amplification of CDK6 and BRAF. Co-amplification status was defined as “increased together” when the copy number of the other gene (CDK6 or BRAF) ≥ 2.2, and the difference with MET amplification is within +/−0.5.
- (c)
- MET amplification that satisfies both (a) and (b) is defined as focal.
6. MET Amplification as an Acquired Resistance Mechanism
7. Treatment Option Targeting for MET Amplification
7.1. Monotherapy
7.2. Combination Therapy
7.3. Ongoing Study
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Bono, J.S.; Yap, T.A. c-MET: An exciting new target for anticancer therapy. Ther. Adv. Med. Oncol. 2011, 3 (Suppl. S1), S3–S5. [Google Scholar] [CrossRef]
- Van Der Steen, N.; Pauwels, P.; Gil-Bazo, I.; Castañon, E.; Raez, L.; Cappuzzo, F.; Rolfo, C. cMET in NSCLC: Can We Cut off the Head of the Hydra? From the Pathway to the Resistance. Cancers 2015, 7, 556–573. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhu, Y.; Wang, Q.; Gao, J.; Li, Y.; Ge, S.; Shen, L. Prognostic significance of MET amplification and expression in gastric cancer: A systematic review with meta-analysis. PLoS ONE 2014, 9, e84502. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Okamoto, I. MET-targeted therapy for gastric cancer: The importance of a biomarker-based strategy. Gastric Cancer 2016, 19, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Parizadeh, S.M.; Jafarzadeh-Esfehani, R.; Fazilat-Panah, D.; Hassanian, S.M.; Shahidsales, S.; Khazaei, M.; Parizadeh, S.M.R.; Ghayour-Mobarhan, M.; Ferns, G.A.; Avan, A. The potential therapeutic and prognostic impacts of the c-MET/HGF signaling pathway in colorectal cancer. IUBMB Life 2019, 71, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Linehan, W.M.; Spellman, P.T.; Ricketts, C.J.; Creighton, C.J.; Fei, S.S.; Davis, C.; Wheeler, D.A.; Murray, B.A.; Schmidt, L.; Vocke, C.D.; et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N. Engl. J. Med. 2016, 374, 135–145. [Google Scholar]
- Qi, X.S.; Guo, X.Z.; Han, G.H.; Li, H.Y.; Chen, J. MET inhibitors for treatment of advanced hepatocellular carcinoma: A review. World J. Gastroenterol. 2015, 21, 5445–5453. [Google Scholar] [CrossRef]
- Gastaldi, S.; Comoglio, P.M.; Trusolino, L. The Met oncogene and basal-like breast cancer: Another culprit to watch out for? Breast Cancer Res. 2010, 12, 208. [Google Scholar] [CrossRef]
- Ho-Yen, C.M.; Jones, J.L.; Kermorgant, S. The clinical and functional significance of c-Met in breast cancer: A review. Breast Cancer Res. 2015, 17, 52. [Google Scholar] [CrossRef]
- Frampton, G.M.; Ali, S.M.; Rosenzweig, M.; Chmielecki, J.; Lu, X.; Bauer, T.M.; Akimov, M.; Bufill, J.A.; Lee, C.; Jentz, D.; et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015, 5, 850–859. [Google Scholar] [CrossRef]
- Schrock, A.B.; Frampton, G.M.; Suh, J.; Chalmers, Z.R.; Rosenzweig, M.; Erlich, R.L.; Halmos, B.; Goldman, J.; Forde, P.; Leuenberger, K.; et al. Characterization of 298 Patients with Lung Cancer Harboring MET Exon 14 Skipping Alterations. J. Thorac. Oncol. 2016, 11, 1493–1502. [Google Scholar] [CrossRef]
- Organ, S.L.; Tsao, M.S. An overview of the c-MET signaling pathway. Ther. Adv. Med. Oncol. 2011, 3 (Suppl. S1), S7–S19. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, F.; Rabe, D.C.; Bottaro, D. Targeting the HGF/Met signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Garajová, I.; Giovannetti, E.; Biasco, G.; Peters, G.J. c-Met as a Target for Personalized Therapy. Transl. Oncogenomics 2015, 7 (Suppl. S1), 13–31. [Google Scholar] [PubMed]
- De Silva, D.M.; Roy, A.; Kato, T.; Cecchi, F.; Lee, Y.H.; Matsumoto, K.; Bottaro, D.P. Targeting the hepatocyte growth factor/Met pathway in cancer. Biochem. Soc. Trans. 2017, 45, 855–870. [Google Scholar] [CrossRef]
- Kawakami, H.; Okamoto, I.; Okamoto, W.; Tanizaki, J.; Nakagawa, K.; Nishio, K. Targeting MET Amplification as a New Oncogenic Driver. Cancers 2014, 6, 1540–1552. [Google Scholar] [CrossRef]
- Liu, L.; Kalyani, F.S.; Yang, H.; Zhou, C.; Xiong, Y.; Zhu, S.; Yang, N.; Qu, J. Prognosis and Concurrent Genomic Alterations in Patients with Advanced NSCLC Harboring MET Amplification or MET Exon 14 Skipping Mutation Treated with MET Inhibitor: A Retrospective Study. Front. Oncol. 2021, 11, 649766. [Google Scholar] [CrossRef]
- Wolf, J.; Seto, T.; Han, J.Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef]
- Mazieres, J.; Paik, P.K.; Garassino, M.C.; Le, X.; Sakai, H.; Veillon, R.; Smit, E.F.; Cortot, A.B.; Raskin, J.; Viteri, S.; et al. Tepotinib Treatment in Patients with MET Exon 14-Skipping Non-Small Cell Lung Cancer: Long-term Follow-up of the VISION Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2023. [Google Scholar] [CrossRef]
- Kawakami, H.; Okamoto, I.; Arao, T.; Okamoto, W.; Matsumoto, K.; Taniguchi, H.; Kuwata, K.; Yamaguchi, H.; Nishio, K.; Nakagawa, K.; et al. MET amplification as a potential therapeutic target in gastric cancer. Oncotarget 2013, 4, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Botting, G.M.; Rastogi, I.; Chhabra, G.; Nlend, M.; Puri, N. Mechanism of Resistance and Novel Targets Mediating Resistance to EGFR and c-Met Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. PLoS ONE 2015, 10, e0136155. [Google Scholar] [CrossRef] [PubMed]
- Bean, J.; Brennan, C.; Shih, J.Y.; Riely, G.; Viale, A.; Wang, L.; Chitale, D.; Motoi, N.; Szoke, J.; Broderick, S.; et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl. Acad. Sci. USA 2007, 104, 20932–20937. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.M.; Zhao, X.; Christensen, J.; et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007, 316, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, S.; Wang, K.; Sun, S.Y. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J. Hematol. Oncol. 2019, 12, 63. [Google Scholar] [CrossRef]
- Coleman, N.; Hong, L.; Zhang, J.; Heymach, J.; Hong, D.; Le, X. Beyond epidermal growth factor receptor: MET amplification as a general resistance driver to targeted therapy in oncogene-driven non-small-cell lung cancer. ESMO Open 2021, 6, 100319. [Google Scholar] [CrossRef]
- Schwab, M. Oncogene amplification in solid tumors. Semin. Cancer Biol. 1999, 9, 319–325. [Google Scholar] [CrossRef]
- Albertson, D.G. Gene amplification in cancer. Trends Genet. 2006, 22, 447–455. [Google Scholar] [CrossRef]
- Matsui, A.; Ihara, T.; Suda, H.; Mikami, H.; Semba, K. Gene amplification: Mechanisms and involvement in cancer. Biomol. Concepts 2013, 4, 567–582. [Google Scholar] [CrossRef]
- Krishnamurti, U.; Silverman, J.F. HER2 in breast cancer: A review and update. Adv. Anat. Pathol. 2014, 21, 100–107. [Google Scholar] [CrossRef]
- Pauletti, G.; Godolphin, W.; Press, M.F.; Slamon, D.J. Detection and quantitation of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene 1996, 13, 63–72. [Google Scholar] [PubMed]
- Kobayashi, M.; Ooi, A.; Oda, Y.; Nakanishi, I. Protein overexpression and gene amplification of c-erbB-2 in breast carcinomas: A comparative study of immunohistochemistry and fluorescence in situ hybridization of formalin-fixed, paraffin-embedded tissues. Hum. Pathol. 2002, 33, 21–28. [Google Scholar] [CrossRef]
- Kreutzfeldt, J.; Rozeboom, B.; Dey, N.; De, P. The trastuzumab era: Current and upcoming targeted HER2+ breast cancer therapies. Am. J. Cancer Res. 2020, 10, 1045–1067. [Google Scholar] [PubMed]
- Onozato, R.; Kosaka, T.; Kuwano, H.; Sekido, Y.; Yatabe, Y.; Mitsudomi, T. Activation of MET by Gene Amplification or by Splice Mutations Deleting the Juxtamembrane Domain in Primary Resected Lung Cancers. J. Thorac. Oncol. 2009, 4, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Sasaki, H.; Yukiue, H.; Yano, M.; Fujii, Y. Met gene copy number predicts the prognosis for completely resected non-small cell lung cancer. Cancer Sci. 2008, 99, 2280–2285. [Google Scholar] [CrossRef]
- Cappuzzo, F.; Marchetti, A.; Skokan, M.; Rossi, E.; Gajapathy, S.; Felicioni, L.; Del Grammastro, M.; Sciarrotta, M.G.; Buttitta, F.; Incarbone, M.; et al. Increased MET Gene Copy Number Negatively Affects Survival of Surgically Resected Non–Small-Cell Lung Cancer Patients. J. Clin. Oncol. 2009, 27, 1667–1674. [Google Scholar] [CrossRef]
- Schildhaus, H.-U.; Schultheis, A.M.; Rüschoff, J.; Binot, E.; Merkelbach-Bruse, S.; Fassunke, J.; Schulte, W.; Ko, Y.D.; Schlesinger, A.; Bos, M.; et al. MET Amplification Status in Therapy-Naïve Adeno- and Squamous Cell Carcinomas of the Lung. Clin. Cancer Res. 2015, 21, 907–915. [Google Scholar] [CrossRef]
- Graziano, F.; Galluccio, N.; Lorenzini, P.; Ruzzo, A.; Canestrari, E.; D’Emidio, S.; Catalano, V.; Sisti, V.; Ligorio, C.; Andreoni, F.; et al. Genetic activation of the MET pathway and prognosis of patients with high-risk, radically resected gastric cancer. J. Clin. Oncol. 2011, 29, 4789–4795. [Google Scholar] [CrossRef]
- Peng, Z.; Li, Z.; Gao, J.; Lu, M.; Gong, J.; Tang, E.T.; Oliner, K.S.; Hei, Y.J.; Zhou, H.; Shen, L. Tumor MET Expression and Gene Amplification in Chinese Patients with Locally Advanced or Metastatic Gastric or Gastroesophageal Junction Cancer. Mol. Cancer Ther. 2015, 14, 2634–2641. [Google Scholar] [CrossRef]
- Raghav, K.; Morris, V.; Tang, C.; Morelli, P.; Amin, H.M.; Chen, K.; Manyam, G.C.; Broom, B.; Overman, M.J.; Shaw, K.; et al. MET amplification in metastatic colorectal cancer: An acquired response to EGFR inhibition, not a de novo phenomenon. Oncotarget 2016, 7, 54627–54631. [Google Scholar] [CrossRef]
- Zhang, M.; Li, G.; Sun, X.; Ni, S.; Tan, C.; Xu, M.; Huang, D.; Ren, F.; Li, D.; Wei, P.; et al. MET amplification, expression, and exon 14 mutations in colorectal adenocarcinoma. Hum. Pathol. 2018, 77, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Ali, S.M.; Yakirevich, E.; Geynisman, D.M.; Karam, J.A.; Elvin, J.A.; Frampton, G.M.; Huang, X.; Lin, D.I.; Rosenzweig, M.; et al. Characterization of Clinical Cases of Advanced Papillary Renal Cell Carcinoma via Comprehensive Genomic Profiling. Eur. Urol. 2018, 73, 71–78. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Chen, H.; Karuturi, M.S.; Chavez-MacGregor, M.; Tsavachidis, S.; Meric-Bernstam, F.; Do, K.A.; Hortobagyi, G.N.; Thompson, P.A.; Mills, G.B.; et al. Frequency of mesenchymal-epithelial transition factor gene (MET) and the catalytic subunit of phosphoinositide-3-kinase (PIK3CA) copy number elevation and correlation with outcome in patients with early stage breast cancer. Cancer 2013, 119, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Gunia, S.; Erbersdobler, A.; Hakenberg, O.W.; Koch, S.; May, M. C-MET is expressed in the majority of penile squamous cell carcinomas and correlates with polysomy-7 but is not associated with MET oncogene amplification, pertinent histopathologic parameters, or with cancer-specific survival. Pathol. Res. Pr. 2013, 209, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Mignard, X.; Ruppert, A.M.; Antoine, M.; Vasseur, J.; Girard, N.; Mazières, J.; Moro-Sibilot, D.; Fallet, V.; Rabbe, N.; Thivolet-Bejui, F.; et al. c-MET Overexpression as a Poor Predictor of MET Amplifications or Exon 14 Mutations in Lung Sarcomatoid Carcinomas. J. Thorac. Oncol. 2018, 13, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Berry, L.D.; Aisner, D.L.; Sheren, J.; Boyle, T.; Bunn, P.A.; Johnson, B.E.; Kwiatkowski, D.J.; Drilon, A.; Sholl, L.M.; et al. MET IHC Is a Poor Screen for MET Amplification or MET Exon 14 Mutations in Lung Adenocarcinomas: Data from a Tri-Institutional Cohort of the Lung Cancer Mutation Consortium. J. Thorac. Oncol. 2019, 14, 1666–1671. [Google Scholar] [CrossRef]
- Lai, G.G.Y.; Lim, T.H.; Lim, J.; Liew, P.J.R.; Kwang, X.L.; Nahar, R.; Aung, Z.W.; Takano, A.; Lee, Y.Y.; Lau, D.P.X.; et al. Clonal MET Amplification as a Determinant of Tyrosine Kinase Inhibitor Resistance in Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2019, 37, 876–884. [Google Scholar] [CrossRef]
- Spigel, D.R.; Edelman, M.J.; O’Byrne, K.; Paz-Ares, L.; Mocci, S.; Phan, S.; Shames, D.S.; Smith, D.; Yu, W.; Paton, V.E.; et al. Results from the Phase III Randomized Trial of Onartuzumab Plus Erlotinib Versus Erlotinib in Previously Treated Stage IIIB or IV Non-Small-Cell Lung Cancer: METLung. J. Clin. Oncol. 2017, 35, 412–420. [Google Scholar] [CrossRef]
- Neal, J.W.; Dahlberg, S.E.; Wakelee, H.A.; Aisner, S.C.; Bowden, M.; Huang, Y.; Carbone, D.P.; Gerstner, G.J.; Lerner, R.E.; Rubin, J.L.; et al. Erlotinib, cabozantinib, or erlotinib plus cabozantinib as second-line or third-line treatment of patients with EGFR wild-type advanced non-small-cell lung cancer (ECOG-ACRIN 1512): A randomised, controlled, open-label, multicentre, phase 2 trial. Lancet Oncol. 2016, 17, 1661–1671. [Google Scholar] [CrossRef]
- Camidge, D.R.; Barlesi, F.; Goldman, J.W.; Morgensztern, D.; Heist, R.; Vokes, E.; Spira, A.; Angevin, E.; Su, W.C.; Hong, D.S.; et al. Phase Ib Study of Telisotuzumab Vedotin in Combination with Erlotinib in Patients with c-Met Protein-Expressing Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2023, 41, 1105–1115. [Google Scholar] [CrossRef]
- Orsaria, M.; Khelifa, S.; Buza, N.; Kamath, A.; Hui, P. Chromosome 17 polysomy: Correlation with histological parameters and HER2NEU gene amplification. J. Clin. Pathol. 2013, 66, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Vanden Bempt, I.; Van Loo, P.; Drijkoningen, M.; Neven, P.; Smeets, A.; Christiaens, M.R.; Paridaens, R.; De Wolf-Peeters, C. Polysomy 17 in breast cancer: Clinicopathologic significance and impact on HER-2 testing. J. Clin. Oncol. 2008, 26, 4869–4874. [Google Scholar] [CrossRef] [PubMed]
- Yosepovich, A.; Avivi, C.; Bar, J.; Polak-Charcon, S.; Mardoukh, C.; Barshack, I. Breast cancer HER2 equivocal cases: Is there an alternative to FISH testing? A pilot study using two different antibodies sequentially. Isr. Med. Assoc. J. 2010, 12, 353–356. [Google Scholar] [PubMed]
- Kong, H.; Bai, Q.; Li, A.; Zhou, X.; Yang, W. Characteristics of HER2-negative breast cancers with FISH-equivocal status according to 2018 ASCO/CAP guideline. Diagn. Pathol. 2022, 17, 5. [Google Scholar] [CrossRef]
- Lim, T.H.; Lim, A.S.; Thike, A.A.; Tien, S.L.; Tan, P.H. Implications of the Updated 2013 American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations on Human Epidermal Growth Factor Receptor 2 Gene Testing Using Immunohistochemistry and Fluorescence In Situ Hybridization for Breast Cancer. Arch. Pathol. Lab. Med. 2016, 140, 140–147. [Google Scholar]
- Kumaki, Y.; Olsen, S.; Suenaga, M.; Nakagawa, T.; Uetake, H.; Ikeda, S. Comprehensive Genomic Profiling of Circulating Cell-Free DNA Distinguishes Focal MET Amplification from Aneuploidy in Diverse Advanced Cancers. Curr. Oncol. 2021, 28, 3717–3728. [Google Scholar] [CrossRef]
- Guo, R.; Luo, J.; Chang, J.; Rekhtman, N.; Arcila, M.; Drilon, A. MET-dependent solid tumours—Molecular diagnosis and targeted therapy. Nat. Rev. Clin. Oncol. 2020, 17, 569–587. [Google Scholar] [CrossRef]
- Camidge, D.R.; Davies, K.D. MET Copy Number as a Secondary Driver of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Resistance in EGFR-Mutant Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2019, 37, 855–857. [Google Scholar] [CrossRef]
- Jørgensen, J.T.; Mollerup, J. Companion Diagnostics and Predictive Biomarkers for MET-Targeted Therapy in NSCLC. Cancers 2022, 14, 2150. [Google Scholar] [CrossRef]
- Castiglione, R.; Alidousty, C.; Holz, B.; Duerbaum, N.; Wittersheim, M.; Binot, E.; Merkelbach-Bruse, S.; Friedrichs, N.; Dettmer, M.S.; Bosse, A.; et al. MET-FISH Evaluation Algorithm: Proposal of a Simplified Method. J. Cancer Sci. Clin. Ther. 2022, 6, 411–427. [Google Scholar] [CrossRef]
- Chrzanowska, N.M.; Kowalewski, J.; Lewandowska, M.A. Use of Fluorescence In Situ Hybridization (FISH) in Diagnosis and Tailored Therapies in Solid Tumors. Molecules 2020, 25, 1864. [Google Scholar] [CrossRef] [PubMed]
- Jardim, D.L.F.; Tang, C.; Gagliato, D.e.M.; Falchook, G.S.; Hess, K.; Janku, F.; Fu, S.; Wheler, J.J.; Zinner, R.G.; Naing, A.; et al. Analysis of 1115 Patients Tested for MET Amplification and Therapy Response in the MD Anderson Phase I Clinic. Clin. Cancer Res. 2014, 20, 6336–6345. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.H.; Yeung, S.F.; Chan, A.W.; Chung, L.Y.; Chau, S.L.; Lung, R.W.; Tong, C.Y.; Chow, C.; Tin, E.K.; Yu, Y.H.; et al. MET Amplification and Exon 14 Splice Site Mutation Define Unique Molecular Subgroups of Non-Small Cell Lung Carcinoma with Poor Prognosis. Clin. Cancer Res. 2016, 22, 3048–3056. [Google Scholar] [CrossRef] [PubMed]
- Noonan, S.A.; Berry, L.; Lu, X.; Gao, D.; Barón, A.E.; Chesnut, P.; Sheren, J.; Aisner, D.L.; Merrick, D.; Doebele, R.C.; et al. Identifying the Appropriate FISH Criteria for Defining MET Copy Number-Driven Lung Adenocarcinoma through Oncogene Overlap Analysis. J. Thorac. Oncol. 2016, 11, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R.; Yen, C.T.; Coudry, R.; Ou, S.I.; Varella-Garcia, M.; Camidge, D.R.; de Castro, G. Responses to Crizotinib Can Occur in High-Level MET-Amplified Non-Small Cell Lung Cancer Independent of MET Exon 14 Alterations. J. Thorac. Oncol. 2017, 12, 141–144. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Wang, Y.; Zhang, B.; Xu, J.; Cao, S.; Zhong, H. Characteristics and response to crizotinib in lung cancer patients with MET amplification detected by next-generation sequencing. Lung Cancer 2020, 149, 17–22. [Google Scholar] [CrossRef]
- Peng, L.X.; Jie, G.L.; Li, A.N.; Liu, S.Y.; Sun, H.; Zheng, M.M.; Zhou, J.Y.; Zhang, J.T.; Zhang, X.C.; Zhou, Q.; et al. MET amplification identified by next-generation sequencing and its clinical relevance for MET inhibitors. Exp. Hematol. Oncol. 2021, 10, 52. [Google Scholar] [CrossRef]
- Schubart, C.; Stöhr, R.; Tögel, L.; Fuchs, F.; Sirbu, H.; Seitz, G.; Seggewiss-Bernhardt, R.; Leistner, R.; Sterlacci, W.; Vieth, M.; et al. MET Amplification in Non-Small Cell Lung Cancer (NSCLC)-A Consecutive Evaluation Using Next-Generation Sequencing (NGS) in a Real-World Setting. Cancers 2021, 13, 5023. [Google Scholar] [CrossRef]
- Morsberger, L.; Pallavajjala, A.; Long, P.; Hardy, M.; Park, R.; Parish, R.; Nozari, A.; Zou, Y.S. HER2 amplification by next-generation sequencing to identify HER2-positive invasive breast cancer with negative HER2 immunohistochemistry. Cancer Cell Int. 2022, 22, 350. [Google Scholar] [CrossRef]
- Turke, A.B.; Zejnullahu, K.; Wu, Y.L.; Song, Y.; Dias-Santagata, D.; Lifshits, E.; Toschi, L.; Rogers, A.; Mok, T.; Sequist, L.; et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell 2010, 17, 77–88. [Google Scholar] [CrossRef]
- Drilon, A.; Cappuzzo, F.; Ou, S.I.; Camidge, D.R. Targeting MET in Lung Cancer: Will Expectations Finally Be MET? J. Thorac. Oncol. 2017, 12, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Benedettini, E.; Sholl, L.M.; Peyton, M.; Reilly, J.; Ware, C.; Davis, L.; Vena, N.; Bailey, D.; Yeap, B.Y.; Fiorentino, M.; et al. Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. Am. J. Pathol. 2010, 177, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Morán, T.; Majem, M.; Reguart, N.; Dalmau, E.; Márquez-Medina, D.; Lianes, P. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: A new era begins. Cancer Treat. Rev. 2014, 40, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.I.; Agarwal, N.; Ali, S.M. High MET amplification level as a resistance mechanism to osimertinib (AZD9291) in a patient that symptomatically responded to crizotinib treatment post-osimertinib progression. Lung Cancer 2016, 98, 59–61. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Yoda, S.; Lennerz, J.K.; Langenbucher, A.; Lin, J.J.; Rooney, M.M.; Prutisto-Chang, K.; Oh, A.; Adams, N.A.; Yeap, B.Y.; et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin. Cancer Res. 2020, 26, 2535–2545. [Google Scholar] [CrossRef]
- Lin, J.J.; Liu, S.V.; McCoach, C.E.; Zhu, V.W.; Tan, A.C.; Yoda, S.; Peterson, J.; Do, A.; Prutisto-Chang, K.; Dagogo-Jack, I.; et al. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer. Ann. Oncol. 2020, 31, 1725–1733. [Google Scholar] [CrossRef]
- Rosen, E.Y.; Johnson, M.L.; Clifford, S.E.; Somwar, R.; Kherani, J.F.; Son, J.; Bertram, A.A.; Davare, M.A.; Gladstone, E.; Ivanova, E.V.; et al. Overcoming MET-Dependent Resistance to Selective RET Inhibition in Patients with RET Fusion-Positive Lung Cancer by Combining Selpercatinib with Crizotinib. Clin. Cancer Res. 2021, 27, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Tyler, L.C.; Le, A.T.; Chen, N.; Nijmeh, H.; Bao, L.; Wilson, T.R.; Chen, D.; Simmons, B.; Turner, K.M.; Perusse, D.; et al. MET gene amplification is a mechanism of resistance to entrectinib in ROS1+ NSCLC. Thorac. Cancer 2022, 13, 3032–3041. [Google Scholar] [CrossRef]
- Cocco, E.; Schram, A.M.; Kulick, A.; Misale, S.; Won, H.H.; Yaeger, R.; Razavi, P.; Ptashkin, R.; Hechtman, J.F.; Toska, E.; et al. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat. Med. 2019, 25, 1422–1427. [Google Scholar] [CrossRef]
- Awad, M.M.; Liu, S.; Rybkin, I.I.; Arbour, K.C.; Dilly, J.; Zhu, V.W.; Johnson, M.L.; Heist, R.S.; Patil, T.; Riely, G.J.; et al. Acquired Resistance to KRAS(G12C) Inhibition in Cancer. N. Engl. J. Med. 2021, 384, 2382–2393. [Google Scholar] [CrossRef]
- Angevin, E.; Spitaleri, G.; Rodon, J.; Dotti, K.; Isambert, N.; Salvagni, S.; Moreno, V.; Assadourian, S.; Gomez, C.; Harnois, M.; et al. A first-in-human phase I study of SAR125844, a selective MET tyrosine kinase inhibitor, in patients with advanced solid tumours with MET amplification. Eur. J. Cancer 2017, 87, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Karaszewska, B.; Kang, Y.K.; Chung, H.C.; Shankaran, V.; Siena, S.; Go, N.F.; Yang, H.; Schupp, M.; Cunningham, D. A Multicenter Phase II Study of AMG 337 in Patients with MET-Amplified Gastric/Gastroesophageal Junction/Esophageal Adenocarcinoma and Other MET-Amplified Solid Tumors. Clin. Cancer Res. 2019, 25, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; LoRusso, P.; Hamid, O.; Janku, F.; Kittaneh, M.; Catenacci, D.V.T.; Chan, E.; Bekaii-Saab, T.; Gadgeel, S.M.; Loberg, R.D.; et al. Phase I Study of AMG 337, a Highly Selective Small-molecule MET Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2019, 25, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Otterson, G.A.; Clark, J.W.; Ignatius Ou, S.H.; Weiss, J.; Ades, S.; Shapiro, G.I.; Socinski, M.A.; Murphy, D.A.; Conte, U.; et al. Crizotinib in Patients with MET-Amplified NSCLC. J. Thorac. Oncol. 2021, 16, 1017–1029. [Google Scholar] [CrossRef]
- Landi, L.; Chiari, R.; Tiseo, M.; D’Incà, F.; Dazzi, C.; Chella, A.; Delmonte, A.; Bonanno, L.; Giannarelli, D.; Cortinovis, D.L.; et al. Crizotinib in MET-Deregulated or ROS1-Rearranged Pretreated Non-Small Cell Lung Cancer (METROS): A Phase II, Prospective, Multicenter, Two-Arms Trial. Clin. Cancer Res. 2019, 25, 7312–7319. [Google Scholar] [CrossRef]
- Moro-Sibilot, D.; Cozic, N.; Pérol, M.; Mazières, J.; Otto, J.; Souquet, P.J.; Bahleda, R.; Wislez, M.; Zalcman, G.; Guibert, S.D.; et al. Crizotinib in c-MET- or ROS1-positive NSCLC: Results of the AcSé phase II trial. Ann. Oncol. 2019, 30, 1985–1991. [Google Scholar] [CrossRef]
- Schuler, M.; Berardi, R.; Lim, W.T.; de Jonge, M.; Bauer, T.M.; Azaro, A.; Gottfried, M.; Han, J.Y.; Lee, D.H.; Wollner, M.; et al. Molecular correlates of response to capmatinib in advanced non-small-cell lung cancer: Clinical and biomarker results from a phase I trial. Ann. Oncol. 2020, 31, 789–797. [Google Scholar] [CrossRef]
- Qin, S.; Chan, S.L.; Sukeepaisarnjaroen, W.; Han, G.; Choo, S.P.; Sriuranpong, V.; Pan, H.; Yau, T.; Guo, Y.; Chen, M.; et al. A phase II study of the efficacy and safety of the MET inhibitor capmatinib (INC280) in patients with advanced hepatocellular carcinoma. Ther. Adv. Med. Oncol. 2019, 11, 1758835919889001. [Google Scholar] [CrossRef]
- Wu, Y.L.; Zhang, L.; Kim, D.W.; Liu, X.; Lee, D.H.; Yang, J.C.; Ahn, M.J.; Vansteenkiste, J.F.; Su, W.C.; Felip, E.; et al. Phase Ib/II Study of Capmatinib (INC280) Plus Gefitinib After Failure of Epidermal Growth Factor Receptor (EGFR) Inhibitor Therapy in Patients with EGFR-Mutated, MET Factor-Dysregulated Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3101–3109. [Google Scholar] [CrossRef]
- Wu, Y.L.; Cheng, Y.; Zhou, J.; Lu, S.; Zhang, Y.; Zhao, J.; Kim, D.W.; Soo, R.A.; Kim, S.W.; Pan, H.; et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): An open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir. Med. 2020, 8, 1132–1143. [Google Scholar] [CrossRef]
- Sequist, L.V.; Han, J.Y.; Ahn, M.J.; Cho, B.C.; Yu, H.; Kim, S.W.; Yang, J.C.; Lee, J.S.; Su, W.C.; Kowalski, D.; et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: Interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020, 21, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Fang, J.; Shu, Y.Q.; Chang, J.H.; Chen, G.Y.; He, J.X.; Li, W.; Liu, X.Q.; Yang, N.; Zhou, C.; et al. A phase Ib study of the highly selective MET-TKI savolitinib plus gefitinib in patients with EGFR-mutated, MET-amplified advanced non-small-cell lung cancer. Investig. New Drugs 2021, 39, 477–487. [Google Scholar] [CrossRef] [PubMed]
| Drug | Cancer Type | Study | MET Amplification Criteria | Clinical Outcome | |
|---|---|---|---|---|---|
| SAR125844 | Solid tumors | Phase I (n = 72) Angevin et al., 2017 [81] | GCN > 4 and MET/CEP ≥ 2.0 by FISH (or IHC 2+/3+) | ORR 17% (5/29) | |
| AMG337 | Gastric cancers | Phase II (n = 60) Van Cutsem et al., 2019 [82] | MET/CEP ≥ 2.0 by FISH | ORR 18% (8/45) | |
| Solid tumors | Phase I (n = 111) Hong et al., 2019 [83] | MET/CEP ≥ 2.0 by FISH | 4 > MET/CEP | ORR 0% (0/2) | |
| MET/CEP ≥ 4 | ORR 60% (6/10) | ||||
| crizotinib | NSCLC | Phase I (n = 38) [PROFILE 1001] Camidge et al., 2021 [84] | MET/CEP ≥ 1.8 by FISH | 2.2 ≥ MET/CEP ≥ 1.8 | ORR 33% (1/3) mPFS 1.8 months |
| 4.0 > MET/CEP > 2.2 | ORR 14% (2/14) mPFS 1.9 months | ||||
| MET/CEP ≥ 4.0 | ORR 38% (8/21) mPFS 6.7 months | ||||
| Phase II (n = 26) [METROS] Landi et al., 2019 [85] | MET/CEP > 2.2 by FISH | 5.0 > MET/CEP > 2.2 | ORR 36% (5/14) | ||
| MET/CEP ≥ 5.0 | ORR 0% (0/2) | ||||
| Phase II (n = 25) [AcSe] Moro-Sibilot et al., 2019 [86] | GCN ≥ 6 by FISH | ORR 16% (4/25) mPFS 3.2 months | |||
| capmatinib | NSCLC | Phase I (n = 44) Schuler et al., 2020 [87] | GCN ≥ 5 or MET/CEP ≥ 2.0 by FISH (or IHC 2+/3+ or H-score ≥ 150) | 4 > GCN | ORR 0% (0/17) |
| 6 > GCN ≥ 4 | ORR 17% (2/12) | ||||
| GCN ≥ 6 | ORR 47% (7/15) mPFS 9.3 months | ||||
| Phase II (n = 195) [GEOMETRY mono-1] Wolf et al., 2020 [18] | determined by FISH, NGS | 4 > GCN | ORR 7% (2/30) mPFS 3.6 months | ||
| GCN 4 or 5 | ORR 9% (5/54) mPFS 2.7 months | ||||
| GCN 6 to 9 | ORR 12% (5/42) mPFS 2.7 months | ||||
| GCN ≥ 10 | ORR 29% (20/69) mPFS 4.1 months | ||||
| Hepatocellular carcinoma | Phase II (n = 30) Qin et al., 2019 [88] | GCN ≥ 5 or MET/CEP ≥ 2.0 by FISH (or IHC 2+/3+) | ORR 10% (3/30) | ||
| Drug | Study | Patient/MET Amplification Criteria | Clinical Outcome | |
|---|---|---|---|---|
| capmatinib/gefitinib | Phase II (n = 100) Wu et al., 2018 [89] | acquired resistance to EGFR-TKI GCN ≥ 4 by FISH (or IHC 3+) | 4 > GCN | ORR 12% (5/41) mPFS 3.9 months |
| 6 > GCN ≥ 4 | ORR 22% (4/18) mPFS 5.4 months | |||
| GCN ≥ 6 | ORR 47% (17/36) mPFS 5.5 months | |||
| tepotinib/gefitinib | Phase II (n = 12) [INSIGHIT] Wu et al., 2020 [90] | acquired resistance to EGFR-TKI and T790 negative GCN ≥ 5 or MET/CEP ≥ 2.0 by FISH (or IHC 2+/3+) | ORR 67% (8/12) mPFS 16.6mo (vs. 4.2 months with chemotherapy, HR = 0.13, 90% CI = 0.04–0.43) OS 37.3mo (vs. 13.1 months with chemotherapy, HR = 0.08, 90% CI = 0.01–0.51) | |
| savolitinib/osimertinib | Phase Ib (n = 174) [TATTON] Sequist et al., 2020 [91] | acquired resistance to EGFR-TKI GCN ≥ 5 or MET/CEP ≥ 2.0 by FISH or GCN ≥ 5 by NGS | Cohort B1 *; after 3rd gen. EGFR-TKI and T790 negative | ORR 30% (21/69) mPFS 5.4 months |
| Cohort B2 *; after 1st/2nd gen. EGFR-TKI and T790 negative | ORR 65% (33/51) mPFS 9.0 months | |||
| Cohort B3 *; after 1st/2nd gen. EGFR-TKI and T790 positive | ORR 67% (12/18) mPFS 11.0 months | |||
| Cohort D **; after 1st/2nd gen. EGFR-TKI and T790 negative | ORR 64% (23/36) mPFS 9.1 months | |||
| savolitinib/gefitinib | Phase I (n = 57) Yang et al., 2021 [92] | acquired resistance to EGFR-TKI and T790 negative GCN ≥ 5 or MET/CEP ≥ 2.0 by FISH | ORR 52% (12/23) | |
| Telisotuzumab vedotin/erlotinib | Phase Ib (n = 28) *** Camidge et al., 2023 [50] | acquired resistance to EGFR-TKI (H-score ≥ 150 by IHC) | ORR 32% (9/28) mPFS 5.9 months | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumaki, Y.; Oda, G.; Ikeda, S. Targeting MET Amplification: Opportunities and Obstacles in Therapeutic Approaches. Cancers 2023, 15, 4552. https://doi.org/10.3390/cancers15184552
Kumaki Y, Oda G, Ikeda S. Targeting MET Amplification: Opportunities and Obstacles in Therapeutic Approaches. Cancers. 2023; 15(18):4552. https://doi.org/10.3390/cancers15184552
Chicago/Turabian StyleKumaki, Yuichi, Goshi Oda, and Sadakatsu Ikeda. 2023. "Targeting MET Amplification: Opportunities and Obstacles in Therapeutic Approaches" Cancers 15, no. 18: 4552. https://doi.org/10.3390/cancers15184552
APA StyleKumaki, Y., Oda, G., & Ikeda, S. (2023). Targeting MET Amplification: Opportunities and Obstacles in Therapeutic Approaches. Cancers, 15(18), 4552. https://doi.org/10.3390/cancers15184552







