The Clinical Validation of Modulated Electro-Hyperthermia (mEHT)
Abstract
Simple Summary
Abstract
1. Introduction
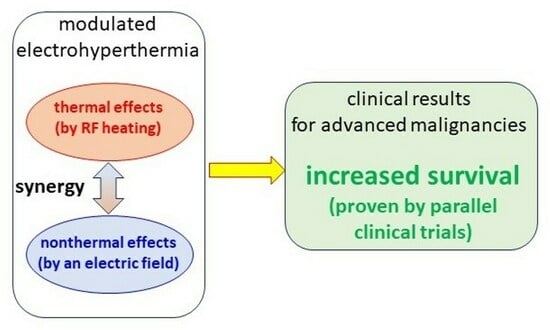
- Thermal effects occur in the form of heat and temperature increase. Thermal effects are mostly unselective; the heat spreads all over the volume seeking thermal equilibrium. The temperature characterizes the homogeneous distribution as average energy of the heat-absorbers.
- Nonthermal processes are electron excitations, generating chemical reactions. The nonthermal impact may change the intercellular membrane, and intracellular processes select them by the dielectric and conductive heterogeneity of the target.
- Due to the intensive metabolism of the malignant cells, the ionic species of the nutrients and waste molecules (such as lactate) have high concentrations in the tumor microenvironment (TME), and together with the extended volume of the extracellular matrix (ECM), create a significantly higher electric conductivity of the microenvironment of malignant cells and the entire tumor [13,14]. This conduction difference drives the RF current to the area [15].
- The malignant cells break their networking connections (e.g., adherent connections and junctions [16]), and became autonomic. This cellular individualism makes the tumor microenvironment different, causing a higher dielectric permittivity of the tumor microenvironment (TME) than it was in the networking conditions [17,18]. The high dielectric permittivity favors conducting the radiofrequency (RF), making an additional selective factor for tumor cells.
- The broken connections leave numerous transmembrane proteins on the membrane of the malignant cells. These membrane-embedded proteins and their lipid-enriched clusters (membrane rafts) have significantly higher energy absorption from the RF current than their surrounding lipid layer [19]. This makes these proteins particularly heatable and chemically excitable.
- The malignancy had lost its healthy homeostatic control, and so it has locally modified physiologic regulations [20]. The arising structural and pathological modifications appear as an additional selectivity factor.
- The frequency dispersion has an optimal range of RF application for the above selection. However, the requested optimal frequency range of the membrane energy absorption/excitation and the driving of the molecular changes during the excitation need different frequencies which must be coordinated. The selection absorption optimum is near 10 MHz [23], while the desired molecular changes happen with a frequency less than 10 kHz. This 1/1000 ratio may be solved by modulation. The carrier is the approved medical frequency 13.56 MHz, and the modulation is a spectrum in the 10 Hz–10 kHz region [24]. The modulation spectrum is the physiologic noise of healthy homeostasis (its power density depends on the reciprocal value of the frequency) [25], and so forces the homeostatic control.
- The applied modulated RF current kills the malignant cells in an apoptotic way, producing a damage-associated molecular pattern (DAMP) [26], realizing an immunogenic cell death (ICD) [27]. The ICD secrete calreticulin (CRT) [28] and heat-shock protein (HSP) on the malignant membrane [29] and attract the natural killer cells [30]; this is proven with mEHT too [31]. The ICD liberates the high mobility group box 1 (HMGB1) molecules [32] together with HSP70, HSP90 [33], and ATP [34] into the ECM. The membrane HSP-s activate the natural killer cells, and the other DAMP molecules maturate the dendritic cells, producing antigen presentation which creates immune reactions. The rising tumor-specific killer and helper T-cells activate antitumoral processes all over the body, acting on distant micro- and macro-metastases (abscopal effect) [35].
2. Clinical Validation
2.1. Crossroads of Clinical Applications
2.1.1. Change of Paradigm
2.1.2. Clinical Challenges
- Patients who cannot receive either surgery, chemo, or radiotherapy (conventional gold standards) according to various contraindicated aspects such as:
- They have a comorbidity that contraindicates the conventional oncotherapy procedures.
- There is no effective conventional procedure for the given tumor.
- Conventional curative procedures are no longer available and usually get a palliative setting only.
- Patients with relapsed locally far-advanced tumors and no alternative standard curative therapy exists.
- Conventional therapy cannot be continued due to organ failure or low blood count.
- Despite standard treatments, patients show intense progression, relapse, and broad malignant dissemination.
- Severe metastatic activity does not allow conventional treatment, salvage, or terminal state.
2.1.3. Study Challenges
2.2. Clinical Results
2.2.1. Safety
2.2.2. Survival Time
2.2.3. Comparison of Survival Curves
2.2.4. Quality of Life
2.2.5. Immunogenic Effects
3. Discussion
- As natural nanoparticles in the membrane, the rafts are heat-sensitive molecular clusters. The too-large absorbed energy destroys the rafts by overheating. The massive distortion of the rafts may degrade the membrane integrity and cause necrosis, losing the apoptotic “harmony” with the homeostasis, which is suboptimal.
- The selected energy absorption of rafts heats the TME and tissues to a lesser extent. The standard applied SAR in nanoparticles, considering their weight heating, is 0.1–1.5 MW/kg. The approximation of the absorbed power of rafts in selection is SAR > 1 MW/kg [19], which is similar to the standard MNP energies [160]. The increased diffusion redistributes the initial spacing with nanoparticles [161]. The electric field impacts the diffusion of the charged and dipole particles, modifying the electrokinetics of the effusion [162] and the angiogenesis [163]. In case of electric field heating at mEHT, the electrodiffusion modifies the allocation of the gold NP-s too, positions them to the volumes of high electric field, thus promoting the heat on the TME [162]. The heating of the NPs shares the energy, reducing the effect on the membrane rafts, and, despite the increase of temperature, the apoptosis decreases [164]. The distribution of magnetic NP-s, which are modified by the increased diffusion with the temperature [165], has to be impacted too by electrophoresis and electroosmosis, and so the electric field in low frequencies (modulation frequencies of mEHT) regroups them on the same way such as in the case of the non-magnetic metallic NPs [164].
- The thermal effect happens in nanoscopic local “points”, the rafts. These molecular clusters are sensitive to overheating. When the absorbed energy is too large, it destroys the rafts and the mEHT loses its largest advantage, producing immunogenic cell death (ICD).
- The large energy absorption extensively forces the spread of heat, and the selection of microscopic differences vanishes. A macroscopic average will characterize the target, and the cellular selection with intended molecular excitations will vanish. The thermal component will become dominant, and the selection mechanisms cannot prevail. A limited thermal component ensures the selection of rafts.
- For human adults the surface heat-loss is floss≅0.15 at rest in the 0 ≤ floss ≤ 1 scale [166], so the heat exchange is intensive enough even by intense local heating. Consequently, the bloodstream in the tumor maintains massive cooling efficacy. The cooling is inhomogeneous; it depends on the vascularization. As such, the thermal factor of mEHT is less homogenous than the nonthermal one.
- The thermally induced vasoconstriction regulates the blood perfusion and heat conduction in tumors [167,168,169], while the heated healthy tissues in the surroundings have vasodilation. The relative blood flow could promote vascular invasion of the tumor border, reducing the prognostic expectations [170]. The special nano-selectivity and the applied low incident power (about 1/8th of the other conventional local hyperthermia therapies) produce a moderate (fever level) temperature in the tumor mass and its surrounding tissues, which causes much less increase of the blood perfusion than the conventional hyperthermia methods, Figure 11. The thermal damage, which is usually calculated [171], is not considered in mEHT, the nonthermal factor makes the apoptosis, ICD, and immune effects also have a gradient on the tumor border, but it helps focus on the denser tumor by refraction angle.
- The thermally promoted intensive metabolic activity deprives the ATP sources [172]. However, the massive energy demand of proliferation requests enormous ATP production, which induces anaerobic metabolism, improving the intensive proliferation [173], promoting the malignant processes [174], and leading the growth direction by an acidic invasion front [175].
- A positive process of ATP deprivation may cause protein aggregation in the cytosol [129,176], destroying the cytoskeleton order. The collapsing cytoskeleton destabilizes the plasma membrane, and the cell necrotizes [176]. Increased temperatures can slow down or even block DNA replication [177], and the DNA strand breaks [178], which completes radiotherapy [179].
- The appropriate frequency is accurately selected around 10 MHz [182,183]. When the frequency is larger (>15 MHz), the membrane impedance becomes too small to select the disordered TME. The current will flow through the entire cell almost equally, neglecting the selection factor of dielectric permittivity.
- The electromagnetic nano-targeting of rafts has similarities to the molecular targeting of drugs at cancer cells. The chemo dose is limited by poisoning. When the rafts are overheated, the raft protein may coagulate, and no selective heating is possible thereafter.
- The approaching of the contact current by mEHT has further limitations. RF safety standards specify the exposure limits [186]. The SAR could be extremely high in the small cross-section when the applicator does not smoothly cover the treatment area and the current flows through a small area which may burn in this touching. The challenge grows when the interface between the skin and electrode has a conductive layer such as sweat, saline, or other aqueous solution. The thin layer may be heated dangerously quickly, so the skin surface must be kept dry.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duffell, E. Curative power of fever. Lancet 2001, 358, 1276. [Google Scholar] [CrossRef] [PubMed]
- Szasz, O.; Szasz, A. Approaching complexity: Hyperthermia dose and its possible measurement in oncology. Op. J. Biophys. 2021, 11, 68–132. [Google Scholar] [CrossRef]
- Seel, M.; Ladik, J. The Tragicomedy of Modern Theoretical Biology, Chapter 1. In Advances in Quantum Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 81, pp. 1–13. [Google Scholar] [CrossRef]
- Vaupel, P.; Hammersen, F. Mikrozirkulation in Malignen Tumoren. In 6. Jahrestagung der Gesellschaft für Mikrozirkulation E.V., München; Karger: Basel, Switzerland, 1982. [Google Scholar]
- Charkoudian, N. Skin Blood Flow in Adult Human Thermoregulation: How It Works, When It Does Not, and Why. Mayo Clin. Proc. 2003, 78, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Szasz, A. Stimulation and control of homeostasis. Open J. Biophys. 2022, 12, 89–131. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Fiorentini, G.; Szasz, A.M.; Szigeti, G.; Szasz, A.; Minnaar, C.A. Quo vadis oncological hyperthermia (2020)? Front. Oncol. 2020, 10, 1690. [Google Scholar] [CrossRef]
- Szasz, A. Thermal and nonthermal effects of radiofrequency on living state and applications as an adjuvant with radiation therapy. J. Radiat. Cancer Res. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Szasz, A. Heterogeneous heat absorption is complementary to radiotherapy. Cancers 2022, 14, 901. [Google Scholar] [CrossRef]
- Minnaar, C.A.; Szasz, A. Forcing the antitumor effects of HSPs using a modulated electric field. Cells 2022, 11, 1838. [Google Scholar] [CrossRef]
- Minnaar, C.A.; Szasz, A.; Lee, S.Y.; Szigeti, G.P.; Szasz, A.M.; Mathe, D. Supportive and palliative care in cancer therapies—Path from tumor-driven therapies to patient-driven ones. Int. J. Clin. Med. 2022, 13, 287–359. [Google Scholar] [CrossRef]
- Lee, S.Y.; Szasz, A. Immunogenic Effect of Modulated Electro-Hyperthermia (mEHT) in Solid Tumors. In Interdisciplinary Cancer Research; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Muftuler, T.L.; Hamamura, M.J.; Birgul, O.; Nalcioglu, O. In Vivo MRI Electrical Impedance Tomography (MREIT) of Tumors. Technol. Cancer Res. Treat. 2006, 5, 381–387. [Google Scholar]
- Smith, S.R.; Foster, K.R.; Wolf, G.L. Dielectric Properties of VX-2 Carcinoma Versus Normal Liver Tissue. IEEE Trans. Biomed. Eng. 1986, 33, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Joy, M.; Scott, G.; Henkelman, M. In vivo detection of applied electric currents by magnetic resonance imaging. Magn. Reson. Imaging 1989, 7, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, W.R.; Kanno, Y. Intercellular communication and tissue growth. J. Cell Biol. 1967, 33, 225–234. [Google Scholar] [CrossRef]
- Foster, K.R.; Schepps, J.L. Dielectric properties of tumor and normal tissues at radio through microwave frequencies. J. Microw. Power 1981, 16, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Szentgyorgyi, A. Electronic Biology and Cancer; Marcel Dekkerm: New York, NY, USA, 1998. [Google Scholar]
- Papp, E.; Vancsik, T.; Kiss, E.; Szasz, O. Energy absorption by the membrane rafts in the modulated electro-hyperthermia (mEHT). Open J. Biophys. 2017, 7, 216–229. [Google Scholar] [CrossRef][Green Version]
- Baish, J.W.; Jain, R.K. Fractals and Cancers. Cancer Res. 2000, 60, 3683–3688. [Google Scholar]
- Andocs, G.; Rehman, M.U.; Zhao, Q.L.; Papp, E.; Kondo, T.; Szasz, A. Nanoheating without Artificial Nanoparticles Part II. Experimental support of the nanoheating concept of the modulated electro-hyperthermia method, using U937 cell suspension model. Biol. Med. 2015, 7, 247. [Google Scholar] [CrossRef]
- Andocs, G.; Rehman, M.U.; Zhao, Q.-L.; Tabuchi, Y.; Kanamori, M.; Kondo, T. Comparison of biological effects of modulated electro-hyperthermia and conventional heat treatment in human lymphoma U937 cell. Cell Death Discov. 2016, 2, 16039. [Google Scholar] [CrossRef]
- Martinsen, O.G.; Grimnes, S.; Schwan, H.P. Interface Phenomena and Dielectric Properties of Biological Tissue. In Encyclopedia of Surface and Colloid Science; CRC: Boca Raton, Fl, USA, 2002; pp. 2643–2652. [Google Scholar]
- Schwan, H.P.; Takashima, S. Dielectric behavior of biological cells and membranes. Bull. Inst. Chem. Res. Kyoto Univ. 1991, 69, 459–475. [Google Scholar]
- Goldberger, A.L.; Amaral, L.A.N.; Hausdorff, J.M.; Ivanov, P.C.; Peng, C.-K. Fractal dynamics in physiology: Altera-tions with disease and aging. Proc. Natl. Acad. Sci. USA 2001, 99 (Suppl. S1), 2466–2472. [Google Scholar] [CrossRef]
- Garg, A.D.; Nowis, D.; Golab, J.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. Immunogen-ic cell death, DAMPs and anticancer therapeutics: An emerging amalgamation. Biochim. Biophys. Acta 2010, 1805, 53–71. [Google Scholar] [PubMed]
- Galluzzi, L.; Buqué, A.; Keep, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.-L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef]
- Multhoff, G.; Botzler, C.; Wiesnet, M.; Müller, E.; Meier, T.; Wilmanns, W.; Issels, R.D. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int. J. Cancer 1995, 61, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Botzler, C.; Jennen, L.; Schmidt, J.; Ellwart, J.; Issels, R. Heat shock protein 72 on tumor cells: A recognition structure for natural killer cells. J. Immunol. 1997, 158, 4341–4350. [Google Scholar] [CrossRef] [PubMed]
- Vancsik, T.; Mathe, D.; Horvath, I.; Várallyaly, A.A.; Benedek, A.; Bergmann, R.; Krenács, T.; Benyó, Z.; Balogh, A. Modulated electro-hyperthermia facilitates NK-cell infiltration and growth arrest of human A2058 melanoma in a xenograft model. Front. Oncol. 2021, 11, 590764. [Google Scholar] [CrossRef]
- Klune, J.R.; Dhuper, R.; Cardinal, J.; Billiar, T.R.; Tsung, A. HMGB1: Endogenous Danger Signaling. Mol. Med. 2008, 14, 476–484. [Google Scholar] [CrossRef]
- Asadzadeh, Z.; Safarzadeh, E.; Safaei, S.; Baradaran, A.; Mohammadi, A.; Hajiasgharzadeh, K.; Derakhshani, A.; Argentiero, A.; Silvestris, N.; Baradaran, B. Current Approaches for Combination Therapy of Cancer: The Role of Immunogenic Cell Death. Cancers 2020, 12, 1047. [Google Scholar] [CrossRef]
- Medina, C.B.; Ravichandran, K.S. Do not let death do us part: ‘find-me’ signals in communication between dying cells and the phagocytes. Cell Death Differ. 2016, 23, 979–989. [Google Scholar] [CrossRef]
- Szasz, O. Local Treatment with Systemic Effect: Abscopal Outcome. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; Volume 11, pp. 192–205. Available online: https://www.cambridgescholars.com/challenges-and-solutions-of-oncological-hyperthermia (accessed on 9 October 2020).
- Krenacs, T.; Meggyeshazi, N.; Forika, G.; Kiss, E.; Hamar, P.; Szekely, T.; Vancsik, T. Modulated electro-hyperthermia-induced tumor damage mechanisms revealed in cancer models. Int. J. Mol. Sci. 2020, 21, 6270. [Google Scholar] [CrossRef]
- Szasz, A.M.; Minnaar, C.A.; Szentmartoni, G.; Szigeti, G.P.; Dank, M. Review of the clinical evidences of modulated electro-hyperthermia (mEHT) method: An update for the practicing oncologist. Front. Oncol. 2019, 9, 1012. [Google Scholar] [CrossRef]
- Herold, Z.; Szasz, A.M.; Dank, M. Evidence based tools to improve efficiency of currently administered oncotherapies for tumors of the hepatopancreatobiliary system. World J. Gastrointest. Oncol. 2021, 15, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, J.-H.; Han, Y.-H.; Cho, D.H. The effect of modulated electro-hyperthermia on temperature and blood flow in human cervical carcinoma. Int. J. Hyperth. 2018, 34, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, M.-G. The effect of modulated electro-hyperthermia on the pharmacokinetic properties of nefopam in healthy volunteers: A andomized, single-dose, crossover open-label study. Int. J. Hyperth. 2015, 31, 869–874. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, S.Y.; Kim, M.-G. Effect of modulated electrohyperthermia on the pharmacokinetics of oral transmucosal fentanyl citrate in healthy volunteers. Clin. Ther. 2016, 38, 2548–2554. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Technological Innovation in Medicine; Gelijns, A. Comparing the Development of Drugs, Devices, and Clinical Procedures; National Academies Press (US): Washington, DC, USA, 1990. [Google Scholar]
- Bru, A.; Albertos, S.; Subiza, J.L.; García-Asenjo, J.L.; Bru, I. The universal dynamics of tumor growth. Biophys. J. 2003, 85, 2948–2961. [Google Scholar] [CrossRef]
- Guiot, C.; Degiorgis, P.G.; Delsanto, P.P.; Gabriele, P.; Deisboeck, T.S. Does tumor growth follow a ‘universal law’? J. Theor. Biol. 2003, 225, 147–151. [Google Scholar] [CrossRef]
- Szasz, O.; Szasz, A.M.; Szigeti, G.P.; Szasz, A. Data mining and evaluation of single arm clinical studies, Chapter 2. In Recent Developments in Engineering Research; Yong, X., Ed.; GAN Publishing: London, UK, 2020; Volume 3, pp. 15–74. [Google Scholar]
- Etikan, I.; Abubakar, S.; Alkassim, R. The Kaplan Meier Estimate in Survival Analysis. Biom. Biostat. Int. J. 2017, 5, 00128. [Google Scholar] [CrossRef]
- Szasz, O.; Szigeti, G.P.; Szasz, A. On the Self-Similarity in Biological Processes. Open J. Biophys. 2017, 7, 183–196. [Google Scholar] [CrossRef]
- González, M.M.; Joa, J.A.G.; Cabrales, L.E.B.; Pupo, A.E.B.; Schneider, B.; Kondakci, S.; Ciria, H.M.C. Is cancer a pure growth curve or does it follow a kinetics of dynamical structural transformation? BMC Cancer 2017, 17, 174. [Google Scholar] [CrossRef]
- Kurakin, A. The self-organizing fractal theory as a universal discovery method: The phenomenon of life. Theor. Biol. Med. Model. 2011, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.R.; Rubin, D.B. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. Reducing Bias in Observational Studies Using Subclassification on the Propensity Score. J. Am. Stat. Assoc. 1984, 79, 516–524. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. Constructing a Control Group Using Multivariate Matched Sampling Methods That Incorporate the Propensity Score. Am. Stat. 1985, 39, 33–38. [Google Scholar]
- Cochran, W.G. The Effectiveness of Adjustment by Sub-Classification in Removing Bias in Observational Studies. Biometrics 1968, 24, 295–313. [Google Scholar] [CrossRef]
- Szasz, O.; Szasz, A. Parametrization of survival measures, Part I: Consequences of self-organizing. Int. J. Clin. Med. 2020, 11, 316–347. [Google Scholar] [CrossRef]
- Szasz, A.; Szigeti, G.P.; Szasz, A.M. Parametrization of survival measures, Part III: Clinical evidences in single arm studies with endpoint of overall survival. Int. J. Clin. Med. 2020, 11, 389–419. [Google Scholar] [CrossRef]
- Wismeth, C.; Dudel, C.; Pascher, C.; Ramm, P.; Pietsch, T.; Hirschmann, B.; Reinert, C.; Proescholdt, M.; Rümmele, P.; Schuierer, G.; et al. Transcranial electro-hyperthermia combined with alkylating chemotherapy in patients with relapsed high-grade gliomas—Phase I clinical results. J. Neurooncol. 2010, 98, 395–405. [Google Scholar] [CrossRef]
- Seo, Y.J.; Cho, W.H.; Kang, D.W.; Cha, S.H. Extraneural metastasis of glioblastoma multiforme presenting as an unusual neck mass. J. Korean Neurosurg. Soc. 2012, 51, 147–150. [Google Scholar] [CrossRef]
- Singh, M.; Singh, T.; Soni, S. Pre-operative Assessment of Ablation Margins for Variable Blood Perfusion Metrics in a Magnetic Resonance Imaging Based Complex Breast Tumour Anatomy: Simulation Paradigms in Thermal Therapies. Comput. Methods Programs Biomed. 2021, 198, 105781. [Google Scholar] [CrossRef]
- Yoo, H.J.; Lim, M.C.; Seo, S.-S.; Kang, S.; Joo, J.; Park, S.Y. Phase I/II clinical trial of modulated electro-hyperthermia treatment in patients with relapsed, refractory or progressive heavily treated ovarian cancer. Jpn. J. Clincal Oncol. 2019, 49, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Zhu, X.; Lu, Y.; Zhao, C.; Zhang, H.; Wang, X.; Gui, X.; Wang, J.; Zhang, X.; Zhang, T.; et al. The safety and pharmacokinetics of high dose intravenous ascorbic acid synergy with modulated electrohyperthermia in Chinese patients with stage III-IV non-small cell lung cancer. Eur. J Pharm. Sci. 2017, 109, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Iyikesici, M.S. Long-term survival outcomes of metabolically supported chemotherapy with Gemcitabine-based or FOLFIRINOX regimen combined with Ketogenic diet, hyperthermia, and hyperbaric oxygen therapy in metastatic pancreatic cancer. Complement. Med. Res. 2020, 27, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Iyikesici, M.S. Survival outcomes of metabolically supported chemotherapy combined with Ketogenic diet, hyperthermia, and hyperbaric oxygen therapy in advanced gastric cancer. Niger. J. Clin. Pract. 2020, 23, 734–740. [Google Scholar] [CrossRef]
- Iyikesici, M.S. Feasibility study of metabolically supported chemotherapy with weekly carboplatin/paclitaxel combined with ketogenic diet, hyperthermia and hyperbaric oxygen therapy in metastatic non-small cell lung cancer. Int. J. Hyperth. 2019, 36, 445–454. [Google Scholar] [CrossRef]
- Iyikesici, M.S.; Slocum, A.; Turkmen, E.; Akdemir, O.; Slocum, A.K.; Berkarda, F.B. Complete response of locally advanced (stage III) rectal cancer to metabolically supported chemoradiotherapy with hyperthermia. Int. J. Cancer Res. Mech. 2016, 2, 1–4. [Google Scholar]
- Jeung, T.S.; Ma, S.Y.; Yu, J.; Lim, S. Cases that respond to oncothermia monotherapy. Conf. Pap. Med. 2013, 2013, 392480. [Google Scholar] [CrossRef]
- Minnaar, C.A.; Szigeti, G.P.; Szasz, A.M.; Kotzen, J.A. Review on the use of modulated electro-hyperthermia as a stand-alone therapy in a palliative setting: Potential for further research? J. Cancer Ther. 2022, 13, 362–377. [Google Scholar] [CrossRef]
- Minnaar, C.; Baeyens, A.; Kotzen, J.; Vangu, M.D. Survival of cervical cancer patients with or without associated HIV infection and treated with modulated electro-hyperthermia combined with chemo-radiotherapy, 32nd Annual Meeting of the European Hyperthermia Society, OP 13. Strahlenther. Onkol. 2018, 194, 476. [Google Scholar]
- Minnaar, C.A.; Kotzen, J.A.; Ayeni, O.A.; Naidoo, T.; Tunmer, M.; Sharma, V.; Baeyens, A. The effect of modulated electro-hyperthermia on local disease control in HIV-positive and -negative cervical cancer women in South Africa: Early results from a phase III randomized controlled trial. PLoS ONE 2019, 14, e0217894. [Google Scholar] [CrossRef]
- Minnaar, C.A.; Baeyens, A.; Ayeni, O.A.; Kotzen, J.A.; Vangu, M.-D. Defining characteristics of nodal disease on PET/CT scans in patients with HIV-positive and -negative locally advanced cervical cancer in South Africa. Tomography 2019, 5, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Minnaar, C.A.; Maposa, I.; Kotzen, J.A.; Baeyens, A. Effects of modulated electro-hyperthermia (mEHT) on two and three year survival of locally advanced cervical cancer patients. Cancers 2022, 14, 656. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Lee, N.-R.; Cho, D.-H.; Kim, J.S. Treatment outcome analysis of chemotherapy combined with modulated electro-hyperthermia compared with chemotherapy alone for recurrent cervical cancer, following irradiation. Oncol. Lett. 2017, 14, 73–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, S.-Y. Concurrent Chemo-Hyperthermia for Recurrent Cervical Cancer after Previous CCRT. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; Volume 9, pp. 163–186. [Google Scholar]
- Lee, S.Y.; Lee, D.H.; Cho, D.-H. Modulated electrohyperthermia in locally advanced cervical cancer: Results of an observa-tional study of 95 patients. Medicine 2023, 102, e32727. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, G.; Sarti, D.; Casadei, V.; Milandri, C.; Dentico, P.; Mambrini, A.; Guadagni, S. Modulated Electro-Hyperthermia for the Treatment of Relapsed Brain Gliomas. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; Volume 6, pp. 110–125. [Google Scholar]
- Fiorentini, G.; Sarti, D.; Casadei, V.; Milandri, C.; Dentico, P.; Mambrini, A.; Nani, R.; Fiorentini, C.; Guadagni, S. Modulated electro-hyperthermia as palliative treatment for pancreas cancer: A retrospective observational study on 106 patients. Integr. Cancer Ther. 2019, 18, 1534735419878505. [Google Scholar] [CrossRef]
- Petenyi, F.G.; Garay, T.; Muhl, D.; Izso, B.; Karaszi, A.; Borbenyi, E.; Herold, M.; Herold, Z.; Szasz, A.M.; Dank, M. Modulated electro-hyperthermic (mEHT) treatment in the therapy of inoperable pancreatic cancer patients—A single-center case-control study. Diseases 2021, 9, 81. [Google Scholar] [CrossRef]
- You, S.H.; Kim, S. Feasibility of modulated electro-hyperthermia in preoperative treatment for locally-advanced rectal cancer: Early phase 2 clinical results. Neoplasma 2020, 67, 677–683. [Google Scholar] [CrossRef]
- Pang, C.L.; Zhang, X.; Wang, Z.; Ou, J.; Lu, Y.; Chen, P.; Zhao, C.; Wang, X.; Zhang, H.; Roussakow, S.V. Local modulated electro-hyperthermia in combination with traditional Chinese medicine vs. intraperitoneal chemoinfusion for the treatment of peritoneal carcinomatosis with malignant ascites: A phase II randomized trial. Mol. Clin. Oncol. 2017, 6, 723–732. [Google Scholar] [CrossRef][Green Version]
- Ou, J.; Zhu, X.; Chen, P.; Du, Y.; Lu, Y.; Peng, X.; Bao, S.; Wang, J.; Zhang, X.; Zhang, T.; et al. A randomized phase II trial of best supportive care with or without hyperthermia and vitamin C for heavily pretreated, advanced, refractory non-small-cell lung cancer. J. Adv. Res. 2020, 24, 175–182. [Google Scholar] [CrossRef]
- Lee, D.Y.; Haam, S.J.; Kim, T.H.; Lim, J.Y.; Kim, E.J.; Kim, N.Y. Oncothermia with chemotherapy in the patients with Small Cell Lung Cancer. Hindawi Publishing Corporation. Conf. Pap. Med. 2013, 2013, 910363. [Google Scholar]
- Garay, T.; Kiss, E.; Szentmartoni, G.; Borbényi, E.; Mühl, D.; Karászi, Á.; Désfalvi, J.; Mohácsi, R.; Kvasnika, M.; Szasz, A.M.; et al. Gastrointestinal Cancer Series Treated with Modulated Electro-Hyperthermia (mEHT)—A Single Centre Experience. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; Volume 8, pp. 159–162. [Google Scholar]
- Ranieri, G.; Laface, C.; Laforgia, M.; De Summa, S.; Porcelli, M.; Macina, F.; Ammendola, M.; Molinari, P.; Lauletta, G.; Di Palo, A.; et al. Bevacizumab plus FOLFOX-4 combined with deep electro-hyperthermia as first-line therapy in metastatic colon cancer: A pilot study. Front. Oncol. 2020, 10, 590707. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.H.; Cha, J.; You, S.H. Beneficial effects of modulated electro-hyperthermia during neoadjuvant treatment for locally advanced rectal cancer. Int. J. Hyperth. 2021, 38, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Marangos, M.; Danilidis, L.; Vakalis, I.; Kouridakis, P.; Papastavrou, A.; Barich, A. Inoperable multifocal intrahepatic cholangiocarcinoma treated with Hyperthermia, IV Vitamin C and ozonated blood autotransfusion Oncothermia. Oncothermia J. 2017, 20, 236–248. [Google Scholar]
- Reimnitz, U. Cholangiocellular carcinomas: Survival without symptoms with hyperthermia—A case study. Oncothermia J. 2010, 1, 20–22. [Google Scholar]
- Gadaleta-Caldarola, G.; Infusino, S.; Galise, I.; Ranieri, G.; Vinciarelli, G.; Fazio, V.; Divella, R.; Daniele, A.; Filippelli, G.; Gadaleta, C.D. Sorafenib and locoregional deep electro-hyperthermia in advanced hepatocellular carcinoma. A phase II study. Oncol. Lett. 2014, 8, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, V.D.; De Ponti, S.; Valcamonico, F.; Amoroso, V.; Grisanti, S.; Rangoni, G.; Marpicati, P.; Vassalli, L.; Simoncini, E.; Marini, G. Deep electro-hyperthermia (EHY) with or without thermo-active agents in patients with advanced hepatic cell carcinoma: Phase II study. J. Clin. Oncol. 2007, 25, 15168. [Google Scholar] [CrossRef]
- Nagata, T.; Kanamori, M.; Sekine, S.; Arai, M.; Moriyama, M.; Fujii, T. Clinical study of modulated electro-hyperthermia for advanced metastatic breast cancer. Mol. Clin. Oncol. 2021, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Garay, T.; Borbényi, E.; Szasz, A.M.; Kulka, J.; Madaras, L.; Somorácz, A.; Lóránt, G.; Molnar, B.A.; Gyorke, T.; Galgoczy, H.; et al. Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; Volume 10, pp. 187–191. [Google Scholar]
- Iyikesici, M.S.; Slocum, A.K.; Slocum, A.; Berkarda, F.B.; Kalamian, M.; Seyfried, T.N. Efficacy of metabolically supported chemotherapy combined with ketogenic diet, hyperthermia, and hyperbaric oxygen therapy for stage IV triple-negative breast cancer. Cureus 2017, 9, e1445. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, N.-R. Positive response of a primary leiomyosarcoma of the breast following salvage hyperthermia and pazopanib. Korean J. Intern. Med. 2018, 33, 442–445. [Google Scholar] [CrossRef]
- Szasz, A.M.; Szentmartoni, G.; Garay, T.; Borbényi, E.; Mohacsi, R.; Kulka, J.; Madaras, L.; Kovács, K.A.; Lóránt, G.; Molnár, B.Á.; et al. Breast Cancer Series Treated with Modulated Electro-Hyperthermia (mEHT)—A Single Centre Experience. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; Volume 5, pp. 105–109. [Google Scholar]
- Kim, K.; Kim, J.-H.; Kim, S.C.; Kim, Y.B.; Nam, B.-H.; No, J.H.; Cho, H.; Ju, W.; Suh, D.H.; Kim, Y.H. Modulated electro-hyperthermia with weekly paclitaxel or cisplatin in patients with recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal carcinoma: The KGOG 3030 trial. Exp. Ther. Med. 2021, 22, 787. [Google Scholar] [CrossRef]
- Deniz, G.I.; Can, A.; Tansan, S. Chemotherapy and radiofrequency hyperthermia in advanced ovarian cancer. J. Clin. Oncol. 2022, 40 (Suppl. S16), e17550. [Google Scholar] [CrossRef]
- Sahinbas, H.; Groenemeyer, D.H.W.; Boecher, E.; Szasz, A. Retrospective clinical study of adjuvant electro-hyperthermia treatment for advanced brain-gliomas. Dtsch. Z. Fuer Onkol. 2007, 39, 154–160. [Google Scholar] [CrossRef]
- Fiorentini, G.; Sarti, D.; Milandri, C.; Dentico, P.; Mambrini, A.; Fiorentini, C.; Mattioli, G.; Casadei, V.; Guadagni, S. Modulated electrohyperthermia in integrative cancer treatment for relapsed malignant glioblastoma and astrocytoma: Retrospective multicenter controlled study. Integr. Cancer Ther. 2018, 18, 1534735418812691. [Google Scholar] [CrossRef] [PubMed]
- Douwes, F.; Douwes, O.; Migeod, F.; Grote, C.; Bogovic, J. Hyperthermia in Combination with ACNU Chemotherapy in the Treatment of Recurrent Glioblastoma; St. Georg Klinik: Hamburg, Germany, 2006. [Google Scholar]
- Fiorentini, G.; Sarti, D.; Milandri, C.; Dentico, P.; Mambrini, A.; Guadagni, S. Retrospective observational clinical study on relapsed malignant gliomas treated with electro-hyperthermia. Int. J. Neurooncol. Brain Tumors 2017, 1, 9–13. [Google Scholar]
- Parmar, G.; Rurak, E.; Elderfield, M.; Li, K.; Soles, S.; Rinas, A. 8-Year Observational Study on Naturopathic Treatment with Modulated Electro-Hyperthermia (mEHT): A Single-Centre Experience. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; Volume 13, pp. 227–266. [Google Scholar]
- Solodkiy, V.A.; Panshin, G.A.; Izmailov, T.R.; Shevchenko, T.A. The first experience of application of remote radiotherapy in combination with hyperthermia (oncothermia) in the treatment of patients with primary gliomas of the brain of a high degree of malignancy. Boпрoсы Онкoлoгии (Probl. Oncol.) 2021, 67, 2. (In Russian) [Google Scholar] [CrossRef]
- Hager, E.D.; Sahinbas, H.; Groenemeyer, D.H.; Migeod, F. Prospective phase II trial for recurrent high-grade malignant gliomas with capacitive coupled low radiofrequency (LRF) deep hyperthermia. ASCO. J. Clin. Oncol. 2008, 26, 2047. [Google Scholar] [CrossRef]
- Hager, E.D.; Dziambor, H.; App, E.M.; Popa, C.; Popa, O.; Hertlein, M. The treatment of patients with high-grade malignant gliomas with RF-hyperthermia. Proc. Am. Soc. Clin. Oncol. 2003, 22, 1–5. [Google Scholar]
- Szasz, A.; Dani, A.; Varkonyi, A.; Magyar, T. Retrospective Analysis of 1180 Oncological Patients Treated by Electro-Hyperthermia in Hungary. In Proceedings of the Jahreskongress der Deutschen Gesellschaft für Radioonkologie, Karlsruhe, Germany, 26–29 May 2005. DEGRO 11. [Google Scholar]
- Roussakow, S. Clinical and economic evaluation of modulated electrohyperthermia concurrent to dose-dense temozolomide 21/28 days regimen in the treatment of recurrent glioblastoma: A retrospective analysis of a two-centre German cohort trial with systematic comparison and effect-to-treatment analysis. BMJ Open 2017, 7, e017387. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group; Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Sneed, P.K.; Stauffer, P.R.; McDermott, M.W.; Lamborn, K.R.; Weaver, K.A.; Prados, M.D.; Chang, S.; Malec, M.K.; Spry, L.; Malec, M.K.; et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/− hyperthermia for glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 287–295. [Google Scholar] [CrossRef]
- Wong, E.T.; Lok, E.; Swanson, K.D. An evidence-based review of alternating electric fields therapy for malignant gliomas. Curr. Treat. Options Oncol. 2015, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of Cancer Cell Replication by Alternating Electric Fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef]
- Kirson, E.D.; Dbaly, V.; Tovarys, F.; Vymazal, J.; Soustiel, J.F.; Itzhaki, A.; Mordechovich, D.; Steinberg-Shapira, S.; Gurvich, Z.; Schneiderman, R.; et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumor. Proc. Natl. Acad. Sci. USA 2007, 104, 10152–10157. [Google Scholar] [CrossRef]
- Szasz, A.M.; Arrojo, E.E.; Fiorentini, G.; Herold, M.; Herold, Z.; Sarti, D.; Dank, M. Meta-Analysis of Modulated Elec-tro-Hyperthermia and Tumor Treating Fields in the Treatment of Glioblastomas. Cancers 2023, 15, 880. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma, A randomized clinical trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, S.W.; Makalowski, J.; Fiore, S.; Sprenger, T.; Prix, L.; Schirrmacher, V.; Stuecker, W. Randomized controlled immunotherapy clinical trials for GBM challenged. Cancers 2021, 13, 32. [Google Scholar] [CrossRef]
- Pastore, C.; Fioranelli, M.; Roccia, M.G. Rescue therapy in patient with glioblastoma multiforme combining chemotherapy, hyperthermia, phytotherapy. J. Integr. Oncol. 2017, 6, 199. [Google Scholar]
- Hager, E.D.; Birkenmeier, J. Glioblastoma multiforme Grad IV: Regionale Tiefenhyperthermie, Antiangiogenese mit Thalidomid, Hochdosis-Ascorbinsäureinfusionen und komplementäre Therapie. Dtsch. Z. für Onkol. 2006, 38, 133–135. [Google Scholar] [CrossRef]
- Mohme, M.; Maire, C.L.; Schliffke, S.; Joosse, S.A.; Alawi, M.; Matschke, J.; Schüller, U.; Dierlamm, J.; Martens, T.; Pantel, K.; et al. Molecular Profiling of an Osseous Metastasis in Glioblastoma During Checkpoint Inhibition: Potential Mechanisms of Immune Escape. Acta Neuropathol. Commun. 2020, 8, 28. [Google Scholar] [CrossRef]
- Fiorentini, G.; Sarti, D.; Casadei, V. Modulated electro-hyperthermia (mEHT) [oncothermia®] protocols as complementary treatment. Oncothermia J. 2019, 25, 85–115. [Google Scholar]
- Szasz, A.M.; Arkosy, P.; Arrojo, E.E.; Bakacs, T.; Balogh, A.; Barich, A.; Borbenyi, E.; Chi, K.H.; Csoszi, T.; Daniilidis, L.; et al. Guidelines for Local Hyperthermia Treatment in Oncology. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; Volume 2, pp. 32–71. Available online: https://www.cambridgescholars.com/challenges-and-solutions-of-oncological-hyperthermia (accessed on 9 October 2020).
- Fiorentini, G.; Sarti, D.; Ranieri, G.; Gadaleta, C.D.; Fiorentini, C.; Milandri, C.; Mambrini, A.; Guadagni, S. Modulated electro-hyperthermia in stage III and IV pancreatic cancer: Results of an observational study on 158 patients. World J. Clin. Oncol. 2021, 12, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Volovat, C.; Volovat, S.R.; Scripcaru, V.; Miron, L. Second-line chemotherapy with gemcitabine and oxaliplatin in combination with loco-regional hyperthermia (EHY-2000) in patients with refractory metastatic pancreatic cancer—Preliminary results of a prospective trial. Rom. Rep. Phys. 2014, 66, 166–174. [Google Scholar]
- Dani, A.; Varkonyi, A.; Magyar, T.; Szasz, A. Clinical study for advanced pancreas cancer treated by oncothermia. Forum Hyperthermie 2008, 1, 13–20. [Google Scholar]
- Douwes, F.R. Thermochemotherapy of the advanced pancreas carcinoma. Biol. Med. 2006, 35, 126–130. [Google Scholar]
- Son, B.; Jeon, J.; Lee, S.; Kim, H.; Kang, H.; Youn, H.; Jo, S.; Youn, B. Radiotherapy in combination with hyperthermia suppresses lung cancer progression via increased NR4A3 and KLF11 expression. Int. J. Radiat Biol. 2019, 95, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.-G. Definitive radiotherapy with concurrent oncothermia for stage IIIB non-small-cell lung cancer: A case report. Exp. Ther. Med. 2015, 10, 769–772. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, Y.-P.; Choi, Y.; Kim, S.; Park, Y.-S.; Oh, I.-J.; Kim, K.-S.; Kim, Y.-C. Conventional cancer treatment alone or with regional hyperthermia for pain relief in lung cancer: A case-control study. Complement. Ther. Med. 2015, 23, 381–387. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Park, S.-J.; Jung, H.-C.; Byun, E.S.; Haam, S.J.; Lee, S.S. The Outcome of the Chemotherapy and Oncothermia for Far Advanced Adenocarcinoma of the Lung: Case reports of four patients. Adv. Lung Cancer 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Lee, D.Y. Complete Remission of SCLC with Chemotherapy and Oncothermia (Case report). Oncothermia J. 2012, 5, 43–51. [Google Scholar]
- Szasz, A. Current status of oncothermia therapy for lung cancer. Korean J. Thorac. Cardiovasc. Surg. 2014, 47, 77–93. [Google Scholar] [CrossRef]
- Roussakow, S.V. Systematic Review of Brain Glioma and Lung Cancer Trials with Modulated Electro-Hyperthermia, with Meta-Analysis and Economic Evaluation (Level II Evidence). Oncothermia J. 2017, 20, 170–216. [Google Scholar]
- Dani, A.; Varkonyi, A.; Magyar, T.; Szasz, A. Clinical study for advanced non-small-cell lung-cancer treated by oncothermia. Oncothermia J. 2009, 3, 40–49. [Google Scholar]
- Roussakow, S.V. Pharmacoeconomic study of oncothermia (modulated electro-hyperthermia) in the treatment of lung cancer. Oncothermia J. 2016, 18, 116–138. [Google Scholar]
- Dani, A.; Varkonyi, A.; Magyar, T.; Kalden, M.; Szasz, A. Clinical study for advanced pancreas cancer treated by oncothermia. Oncothermia J. 2012, 6, 11–25. [Google Scholar]
- Hager, E.D.; Dziambor, H.; Höhmann, D.; Gallenbeck, D.; Stephan, M.; Popa, C. Deep hyperthermia with radiofrequencies in patients with liver metastases from colorectal cancer. Anticancer Res. 1999, 19, 3403–3408. [Google Scholar] [PubMed]
- Szasz, A.; Szasz, N.; Szasz, O. Oncothermia—Principles and Practices; Springer Science: Heidelberg, Germany, 2010. [Google Scholar]
- Minnaar, C.A.; Kotzen, J.A.; Naidoo, T.; Tunmer, M.; Sharma, V.; Vangu, M.-D.; Baeyens, A. Analysis of the effects of mEHT on the treatment-related toxicity and quality of life of HIV-positive cervical cancer patients. Int. J. Hyperth. 2020, 37, 263–272. [Google Scholar] [CrossRef]
- Falk, R.E.; Moffat, F.L.; Lawler, M.; Heine, J.; Makowka, L.; Falk, J.A. Combination therapy for resectable and unresectable adenocarcinoma of Pancreas. Cancer 1986, 57, 685–688. [Google Scholar] [CrossRef]
- Andocs, G.; Szasz, A.; Szasz, I.; Szasz, N. Tumor Vaccination Patent, 6 October 2020, A1, USA. Available online: http://www.freepatentsonline.com/20150217099.pdf (accessed on 15 April 2021).
- Kleef, R. Hyperthermic Oncology. Oncothermia J. 2018, 24, 270–302. [Google Scholar]
- Chi, K.-H. Tumour-Directed Immunotherapy: Clinical Results of Radiotherapy with Modulated Electro-Hyperthermia. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; Volume 12, pp. 206–226. [Google Scholar]
- Pang, C.L.K. The Immune Regulating Effect of Hyperthermia in Combination with TCM on Cancer Patients. Oncothermia J. 2016, 18, 170–179. [Google Scholar]
- Minnaar, C.A.; Kotzen, J.A.; Ayeni, O.A.; Vangu, M.-D.-T.; Baeyens, A. Potentiation of the abscopal effect by modulated electro-hyperthermia in locally advanced cervical cancer patients. Front. Oncol. 2020, 10, 376. [Google Scholar] [CrossRef]
- Kleef, R.; Moss, R.; Szasz, M.; Bohdjalian, A.; Bojar, H.; Bakacs, T. Complete Clinical Remission of Stage IV Triple-Negative Breast Cancer Lung Metastasis Administering Low-Dose Immune Checkpoint Blockade in Combination With Hyperthermia and Interleukin-2. Integr. Cancer Ther. 2018, 17, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Kleef, R.; Kekic, S.; Ludwig, N. Successful treatment of advanced ovarian cancer with thermochemotherapy and adjuvant immune therapy. Case Rep. Oncol. 2012, 5, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.-S.; Wu, J.-H.; Shaw, S.; Wu, C.-J.; Chen, L.-K.; Hsu, H.-C.; Chi, K.-H. Marked local and distant response of heavily treated breast cancer with cardiac metastases treated by combined low dose radiotherapy, low dose immunotherapy and hyperthermia: A case report. Ther. Radiol. Oncol. 2021, 5, 17. [Google Scholar] [CrossRef]
- Chi, M.-S.; Mehta, M.P.; Yang, K.-L.; Lai, H.-C.; Lin, Y.-C.; Ko, H.-L.; Wang, Y.-S.; Liao, K.-W.; Chi, K.-H. Putative abscopal effect in three patients treated by combined radiotherapy and modulated electrohyperthermia. Front. Oncol. 2020, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V.; Lorenzen, D.; Van Gool, S.W.; Stuecker, W. A new strategy of cancer immunotherapy combining hyperthermia/oncolytic virus pretreatment with specific autologous anti-tumor vaccination—A review. Austin Oncol. Case Rep. 2017, 2, 1006. [Google Scholar]
- Schirrmacher, V.; Stücker, W.; Lulei, M.; Bihari, A.S.; Sprenger, T. Long-term survival of a breast cancer patient with extensive liver metastases upon immune and virotherapy: A case report. Immunotherapy 2015, 7, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, S.; Makalowski, J.; Marko, M. Multimodal immunotherapy for patients with ovarian cancer. Oncothermia J. 2019, 27, 138–152. [Google Scholar]
- Van Gool, S.; Makalowski, J.; Feyen, O. Can we monitor immunogenic cell death (ICD) induced with modulated electrohyperthermia and oncolytivc virus injections? Oncothermia J. 2019, 26, 120–125. [Google Scholar]
- Van Gool, S.; Makalowski, J.; Marko, M. Hyperthermia as part of multimodal immunotherapy for patients with GBM. Oncothermia J. 2019, 27, 122–137. [Google Scholar]
- Van Gool, S.W.; Makalowski, J.; Domogalla, M.P.; Marko, M.; Feyen, O.; Sprenger, K.; Schirrmacher, V.; Stuecker, W. Personalised Medicine in Glioblastoma Multiforme. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; Volume 7, pp. 126–158. [Google Scholar]
- Van Gool, S.W.; Makalowski, J.; Feyen, O.; Prix, L.; Schirrmacher, V.; Stuecker, W. The induction of immunogenic cell death (ICD) during maintenance chemotherapy and subsequent multimodal immunotherapy for glioblastoma (GBM). Austin Oncol. Case Rep. 2018, 3, 1010. [Google Scholar]
- Van Gool, S.W.; Makalowski, J.; Bonner, E.R.; Feyen, O.; Domogalla, M.P.; Prix, L.; Schirrmacher, V.; Nazarian, J.; Stuecker, W. Addition of multimodal immunotherapy to combination treatment strategies for children with DIPG: A single institution experience. Medicines 2020, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Szasz, A. The capacitive coupling modalities for oncological hyperthermia. Open J. Biophys. 2021, 11, 252–313. [Google Scholar] [CrossRef]
- Szasz, A. Towards the immunogenic hyperthermic action: Modulated electro-hyperthermia. Clin. Oncol. Res. 2020, 3, 5–6. [Google Scholar]
- Vancsik, T.; Kovago, C.; Kiss, E.; Papp, E.; Forika, G.; Benyo, Z.; Meggyeshazi, N.; Krenacs, T. Modulated electro-hyperthermia induced loco-regional and systemic tumor destruction in colorectal cancer allografts. J. Cancer 2018, 9, 41–53. [Google Scholar] [CrossRef]
- Tsang, Y.-W.; Huang, C.-C.; Yang, K.-L.; Chi, M.-S.; Chiang, H.-C.; Wang, Y.-S.; Andocs, G.; Szasz, A.; Li, W.-T.; Chi, K.-H. Improving immunological tumor microenvironment using electro-hyperthermia followed by dendritic cell immunotherapy. BMC Cancer 2015, 15, 708. [Google Scholar] [CrossRef][Green Version]
- Qin, W.; Akutsu, Y.; Andocs, G.; Suganami, A.; Hu, X.; Yusup, G.; Komatsu-Akimoto, A.; Hoshino, I.; Hanari, N.; Mori, M.; et al. Modulated electro-hyperthermia enhances dendritic cell therapy through an abscopal effect in mice. Oncol. Rep. 2014, 32, 2373–2379. [Google Scholar] [CrossRef]
- Minnaar, C. Challenges Associated with Hyperthermia. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; Volume 1, pp. 1–31. [Google Scholar]
- Datta, K. Application of SWOT-TOWS matrix and analytical hierarchy process (AHP) in the Formulation of geoconservation and geotourism development strategies for Mama Bhagne Pahar: An important geomorphosite in West Bengal, India. Geoheritage 2020, 12, 45. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef]
- Singh, M.; Ma, R.; Zhu, L. Quantitative evaluation of effects of coupled temperature elevation, thermal damage, and enlarged porosity on nanoparticle migration in tumors during magnetic nanoparticle hyperthermia. Int. Commun. Heat Mass Transf. 2021, 126, 105393. [Google Scholar] [CrossRef]
- Szasz, O.; Szigeti, G.P.; Szasz, A.M. Electrokinetics of temperature for development and treatment of effusions. Adv. Biosci. Biotechnol. 2017, 8, 434–449. [Google Scholar] [CrossRef][Green Version]
- Szasz, O.; Szigeti, G.P.; Szasz, A.; Benyo, Z. Role of electrical forces in angiogenesis. Op. J. Biophys. 2018, 8, 49–67. [Google Scholar] [CrossRef][Green Version]
- Chen, C.-C.; Chen, C.-L.; Li, J.-J.; Chen, Y.-Y.; Wang, C.-Y.; Wang, Y.-S.; Chi, K.-H.; Wang, H.-E. The presence of gold nanoparticles in cells associated with the cell-killing effect of modulated electro-hyperthermia. ACS Appl. Bio Mater. 2019, 2, 3573–3581. [Google Scholar] [CrossRef]
- Singh, M. Biological heat and mass transport mechanisms behind nanoparticles migration revealed under microCT image guidance. Int. J. Therm. Sci. 2023, 184, 107996. [Google Scholar] [CrossRef]
- Zotin, A.I. Thermodynamic Bases of Biological Processes; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar] [PubMed]
- Dudar, T.E.; Jain, R.K. Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res. 1984, 44, 605–612. [Google Scholar]
- Song, C.W.; Lokshina, A.; Rhee, J.G.; Patten, M.; Levitt, S.H. Implication of blood-flow in hyperthermic treatment of tumors. IEEE Trans. Biomed. Eng. 1984, 31, 9–16. [Google Scholar] [CrossRef]
- Qwaider, Y.Z.; Sell, N.M.; Stafford, C.E.; Kunitake, H.; Cusack, J.C.; Ricciardi, R.; Bordeianou, L.G.; Deshpande, V.; Goldstone, R.N.; Cauley, C.E.; et al. Infiltrating Tumor Border Configuration is a Poor Prognostic Factor in Stage II and III Colon Adenocarcinoma. Ann. Surg. Oncol. 2021, 28, 3408–3414. [Google Scholar] [CrossRef]
- Sing, M. Incorporating vascular-stasis based blood perfusion to evaluate the thermal signatures of cell-death using modified Arrhenius equation with regeneration of living tissues during nanoparticle-assisted thermal therapy. Int. Commun. Heat Mass Transf. 2022, 135, 106046. [Google Scholar] [CrossRef]
- Vaupel, P.W.; Kelleher, D.K. Metabolic Status and Reaction to Heat of Normal and Tumor Tissue. In Thermo-Radiotherapy and Thermo-Chemotherapy. Biology, Physiology and Physics; Seegenschmiedt, M.-H., Fessenden, P., Vernon, C.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 1, pp. 157–176. [Google Scholar]
- Nomura, D.K.; Long, J.Z.; Niessen, S.; Hoover, H.S.; Ng, S.-W.; Cravatt, B.F. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell 2010, 140, 49–61. [Google Scholar] [CrossRef]
- Warburg, O. Oxygen, The Creator of Differentiation, Biochemical Energetics. In The Prime Cause and Prevention of Cancer: Revised Lecture at the Meeting of the Nobel-Laureates on 30 June 1966, Lindau, Lake Constance, Germany; Academic Press: New York, NY, USA, 1996. [Google Scholar]
- Peppicelli, S.; Andreucci, E.; Ruzzolini, J.; Bianchini, F.; Calorini, L. FDG uptake in cancer: A counting debate. Theranostics 2020, 10, 2944–2948. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Gabai, V.L. Heat Shock Proteins and Cytoprotection: ATP-Deprived Mammalian Cells. (Molecular Biology Intelligence Unit); Springer: Berlin, Heidelberg, Germany, 1997. [Google Scholar]
- Dikomey, E.; Franzke, J. Effect of heat on induction and repair of DNA strand breaks in X-irradiated CHO cells. Int. J. Radiat. Biol. 1992, 61, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Oei, A.L.; Vriend, L.E.M.; Crezee, J. Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat. Oncol. 2015, 10, 165. [Google Scholar] [CrossRef]
- Okumura, Y.; Ihara, M.; Shimasaki, T.; Takeshita, S.; Okaichi, K. Heat Inactivation of DNA-Dependent Protein Kinase: Possible Mechanism of Hyperthermic Radio-Sensitization. In Thermotherapy for Neoplasia, Inflammation, and Pain; Kosaka, M., Sugahara, T., Schmidt, K.L., Simon, E., Eds.; Springer: Tokyo, Japan, 2001; pp. 420–423. [Google Scholar]
- Hayashi, S.; Kano, E.; Hatashita, M.; Ohtsubo, T.; Katayama, K.; Matsumoto, H. Fundamental Aspects of Hyperthermia on Cellular and Molecular Levels; Kosaka, M., Sugahara, T., Schmidt, K.L., Simon, E., Eds.; Springer: Tokyo, Japan, 2001; pp. 335–345. [Google Scholar]
- Kühl, N.M.; Rensing, L. Heat shock effects on cell cycle progression. Cell. Mol. Life Sci. CMLS 2000, 57, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Gascoyne, P.R.C.; Pethig, R.; Szent-Gyorgyi, A. Water structure-dependent charge transport in proteins. Proc. Natl. Acad. Sci. USA 1981, 78, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, T.; Miklavcic, D. Theoretical evaluation of the distributed power dissipation in biological cells exposed to electric fields. Bioelectromagnetics 2000, 21, 385–394. [Google Scholar] [CrossRef]
- Gombos, I.; Crul, T.; Piotto, S.; Güngör, B.; Török, Z.; Balogh, G.; Péter, M.; Slotte, J.P.; Campana, F.; Pilbat, A.-M.; et al. Membrane-Lipid therapy in operation: The HSP co-inducer GBP-15 activates stress signal transduction pathways by remodeling plasma membrane rafts. PLoS ONE 2011, 6, e28818. [Google Scholar] [CrossRef]
- Beachy, S.H.; Repasky, E.A. Toward establishment of temperature thresholds for immunological impact of heat exposure in humans. Int. J. Hyperth. 2011, 27, 344–352. [Google Scholar] [CrossRef]
- Tell, R.A.; Tell, C.A. Perspectives on setting limits for RF contact currents: A commentary. Tell Tell Biomed. Eng. OnLine 2018, 17, 2. [Google Scholar] [CrossRef]











| Group | Number of Patients | mEHT/Week (6 Cycles) |
|---|---|---|
| 1 | 4 | 2 |
| 2 | 4 | 3 |
| 3 | 4 | 4 |
| 4 | 3 | 5 |
| Groups | Number of Patients | % | Average Age (y) | 3 y Overall Survival (%) | p-Value | |
|---|---|---|---|---|---|---|
| All | 210 | 100 | ||||
| RT + ChT alone | HIV positive | 55 | 52.9 | 50.6 | 33.7 | 0.04 |
| HiV negative | 49 | 47.1 | ||||
| RT + ChT + mEHT | HIV positive | 52 | 49.1 | 49.2 | 44 | |
| HiV negative | 54 | 50.9 | ||||
| Groups | Number of Patients | % | OS after 5 y | CR with Lymph Node Mets. | CR (NED) | DFS after 5 y | DFS with Lymph Node Mets. after 5 y | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | 95 | 100 | % | p-value | % | p-value | % | p-value | % | p-value | % | p-value |
| RT + ChT alone | 50 | 53 | 79.5 | 0.079 | 45 | 0.0377 | 58 | 0.0315 | 73 | 0.166 | 73 | 0.166 |
| RT + ChT + mEHT | 40 | 42 | 81 | 71 | 82 | 80 | 80 | |||||
| Response | Astrocytoma | Glioblastoma | ||||||
|---|---|---|---|---|---|---|---|---|
| without mEHT (n, %) | with mEHT (n, %) | without mEHT (n, %) | with mEHT (n, %) | |||||
| n | % | n | % | n | % | n | % | |
| CR | 6 | 28.6 | 2 | 6.9 | 2 | 2.4 | 1 | 3.4 |
| PR | 1 | 4.8 | 10 | 34.5 | 2 | 2.4 | 6 | 20.7 |
| SD | 5 | 23.8 | 9 | 31.0 | 13 | 15.3 | 11 | 37.9 |
| PD | 8 | 38.1 | 6 | 20.7 | 63 | 74.1 | 11 | 37.9 |
| No data | 1 | 4.8 | 2 | 6.9 | 5 | 5.9 | 0 | 0.0 |
| OS median (months) | 17 | 72 | 12 | 15 | ||||
| OS range | 3–120 | 3–156 | 2–84 | 2–108 | ||||
| p-value | 0.0006 | 0.026 | ||||||
| Response | Fiorentini et al. | Petenyi et al. | ||||||
|---|---|---|---|---|---|---|---|---|
| without mEHT (n, %) | with mEHT (n, %) | without mEHT (n, %) | with mEHT (n, %) | |||||
| n | % | n | % | n | % | n | % | |
| Patients no. | 67 | 39 | 39 | 39 | ||||
| Males | 38 | 56.7 | 24 | 61.5 | 19 | 46.2 | 18 | 46.2 |
| Females | 29 | 43.3 | 15 | 38.5 | 20 | 53.8 | 21 | 55.8 |
| Age (mean, y) | 66 | 61.8 | 66.02 | 65.9 | ||||
| Distant metastasis * | 37 | 55.2 | 25 | 64.1 | 24 | 61.5 | 20 | 51.3 |
| Non metastatic ** | 30 | 44.8 | 14 | 35.9 | 15 | 38.5 | 19 | 48.7 |
| Gemcitabine combination | 64 | 95.5 | 27 | 69.2 | 31 | 79.5 | 31 | 79.5 |
| Other complementary | 3 | 4.5 | 12 | 30.8 | 8 | 20.5 | 8 | 20.5 |
| OS median (months) | 10.9 | 18 | 10.58 | 17.02 | ||||
| OS range | 0.4–55.4 | 1.5–68 | 2.4–48.8 | 4.4–47.1 | ||||
| p | 0.00165 | 0.0301 | ||||||
| No. | Number of Patients | Treatments | OS Median (Months) | Reference |
|---|---|---|---|---|
| 1 | 35 | mEHT + RT + ChT + BST | 26.4 | Parmar, et al. 2020 [99] |
| 2 | 28 | mEHT + RT + ChT + BSC, (palliative) | 14 | Fiorentini, et al. 2018 [96] |
| 3 | 92 | mEHT + RT + ChT | 20.4 | Sahinbas, et al. 2007 [95] |
| 4 | 126 | mEHT + RT + ChT | 20.3 | Hager, et al. 2008 [101] |
| 5 | 29 | mEHT + RT + ChT | 14 | Szasz, et al. 2010 [103] |
| 6 | 19 | ChT (ACNU) | 21.8 | Douwes, et al. 2006 [97] |
| No. | Number of Patients | Treatments | OS Median (Months) | Reference |
|---|---|---|---|---|
| 1 | 39 | GMZ combination with mEHT | 18 | Fiorentini et al. 2019, [75] |
| 2 | 27 | GMZ combination with mEHT | 13.2 | Parmar et al. 2020 [99] |
| 3 | 99 | GMZ combination with mEHT | 12 | Dani et al. 2012 [120] |
| 4 | 39 | GMZ combination with mEHT | 17 | Petenyi et al. 2021 [76] |
| 5 | 34 | GMZ combination without mEHT | 6.5 | Dani et al. 2012 [120] |
| No. | Number of Patients | Treatments | OS Median (Months) | Reference |
|---|---|---|---|---|
| 1 | 75 | RT + ChT + OP with mEHT | 16.4 | Szasz, 2014 [127] |
| 2 | 197 | RT + ChT + OP with mEHT | 15.6 | Dani, et al. 2012 [129] |
| 3 | 61 | RT + ChT + OP with mEHT | 16.4 | Dani, et al. 2009 [131] |
| 4 | 54 | RT + ChT + BSC with mEHT | 18 | Parmar, et al. 2020 [99] |
| No. | Number of Patients | Treatments | OS Median (Months) | Reference |
|---|---|---|---|---|
| 1 | 79 | ChT with mEHT | 48 | Parmar, et al. 2020 [99] |
| 2 | 218 | OP + ChT with mEHT | 28.5 | Szasz, et al. 2010 [133] |
| 3 | 50 | BSC with mEHT | 25 | Hager, et al. 2020 [132] |
| 4 | 30 | ChT + BSC with mEHT | 23 | Hager, et al. 2020 [132] |
| 5 | 40 | ChT with mEHT | 21.4 | Ranieri, et al. 2020 [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-Y.; Lorant, G.; Grand, L.; Szasz, A.M. The Clinical Validation of Modulated Electro-Hyperthermia (mEHT). Cancers 2023, 15, 4569. https://doi.org/10.3390/cancers15184569
Lee S-Y, Lorant G, Grand L, Szasz AM. The Clinical Validation of Modulated Electro-Hyperthermia (mEHT). Cancers. 2023; 15(18):4569. https://doi.org/10.3390/cancers15184569
Chicago/Turabian StyleLee, Sun-Young, Gergo Lorant, Laszlo Grand, and Attila Marcell Szasz. 2023. "The Clinical Validation of Modulated Electro-Hyperthermia (mEHT)" Cancers 15, no. 18: 4569. https://doi.org/10.3390/cancers15184569
APA StyleLee, S.-Y., Lorant, G., Grand, L., & Szasz, A. M. (2023). The Clinical Validation of Modulated Electro-Hyperthermia (mEHT). Cancers, 15(18), 4569. https://doi.org/10.3390/cancers15184569