Radiomic Assessment of Radiation-Induced Alterations of Skeletal Muscle Composition in Head and Neck Squamous Cell Carcinoma within the Currently Clinically Defined Optimal Time Window for Salvage Surgery—A Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
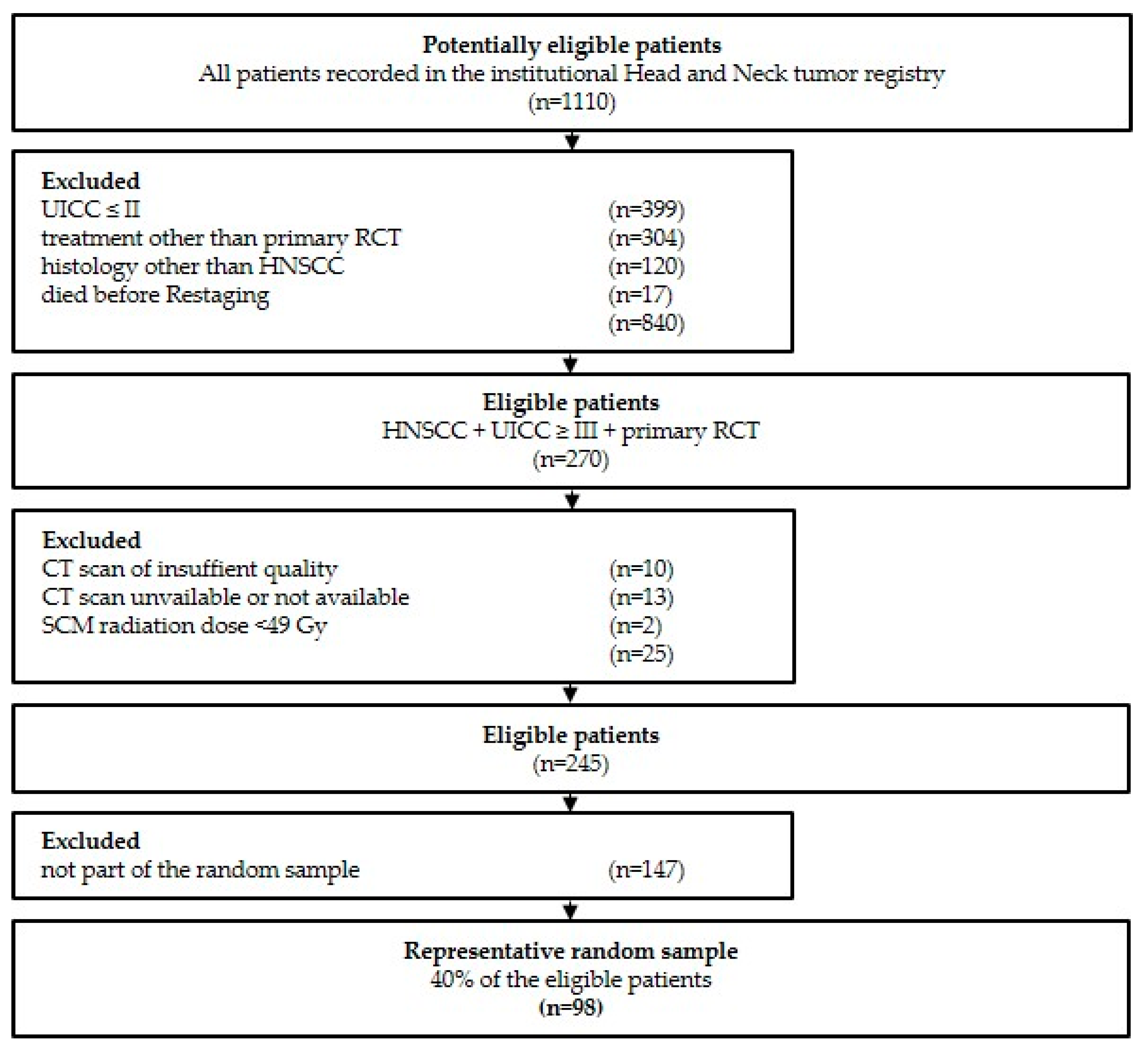
2.1. Study Population and Additional Clinical Data
2.2. Clinical Data
2.3. CT Imaging Acquisition
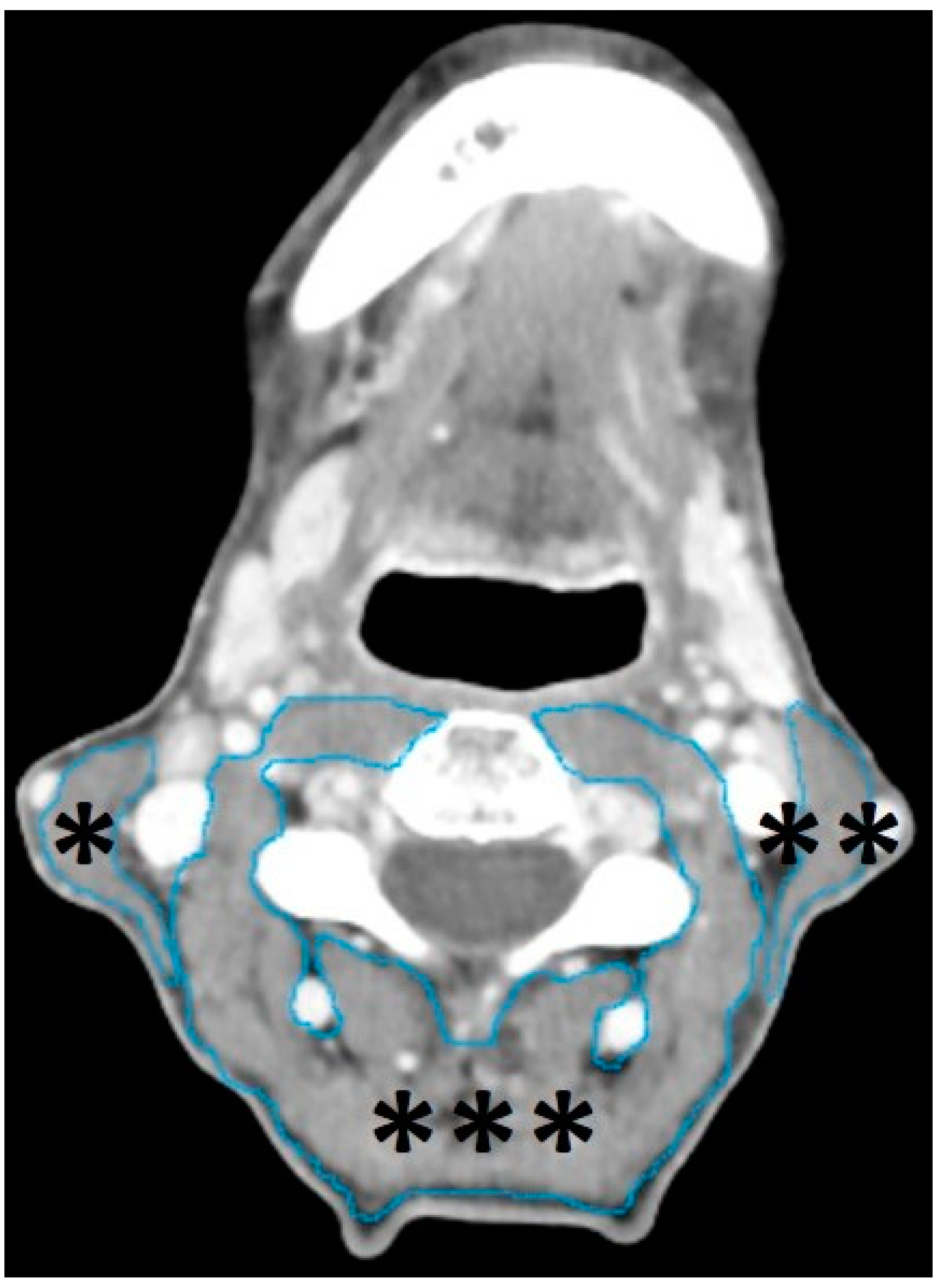
2.4. Segmentation of Head and Neck Musculature
2.5. Data Analysis
2.6. Ethical Considerations
3. Results
3.1. Patient Population
3.2. Primary Radiochemotherapy and Time Intervals
3.3. Variable Reduction (Principal Component Analysis)
3.4. Factors Influencing Pretherapeutic Volume, Uniformity, and MPP
3.5. Volume, Uniformity, and MPP before and after Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| N | Minimum | Maximum | Mean | Standard Deviation | |
|---|---|---|---|---|---|
| SCMright_Short_Axis | 98 | 4.7 | 24.3 | 14.251 | 3.4045 |
| SCMright_Long_Axis | 98 | 19.5 | 60.9 | 42.026 | 7.5338 |
| SCMright_Volume | 98 | 3.3 | 17.8 | 8.971 | 3.1417 |
| SCMright_Entropy | 98 | 5.5 | 7.3 | 6.489 | 0.3232 |
| SCMright_Kurtosis | 98 | 3.1 | 24.4 | 6.557 | 2.7009 |
| SCMright_MPP | 98 | 31.6 | 79.2 | 59.880 | 8.3491 |
| SCMright_Density_Max. | 98 | 72 | 234 | 137.21 | 36.768 |
| SCMright_Density_Min. | 98 | −155 | −66 | −105.32 | 17.386 |
| SCMright_Density_Mean | 98 | 23.8 | 77.5 | 51.176 | 10.9033 |
| SCMright_Density_Skewness | 98 | −3.0 | −1.0 | −1.75 | 0.373 |
| SCMright_Density_SD | 98 | 13.9 | 45.3 | 32.183 | 5.9952 |
| SCMright_UPP | 98 | 0.0076 | 0.0298 | 0.016484 | 0.0041614 |
| SCMright_Uniformity | 98 | 0.0078 | 0.0298 | 0.016621 | 0.0040749 |
| SCMleft_Short_Axis | 98 | 5.0 | 27.7 | 14.547 | 3.7930 |
| SCMleft_Long_Axis | 98 | 23.8 | 57.5 | 42.623 | 7.6126 |
| SCMleft_Volume | 98 | 2.9 | 15.7 | 8.930 | 3.0336 |
| SCMleft_Entropy | 98 | 5.5 | 7.3 | 6.494 | 0.3395 |
| SCMleft_Kurtosis | 98 | 2.0 | 14.6 | 6.262 | 2.0012 |
| SCMleft_MPP | 98 | 37.1 | 86.0 | 59.344 | 8.4566 |
| SCMleft_Density M_Axis | 98 | 73 | 200 | 138.57 | 28.363 |
| SCMleft_Density_Min. | 98 | −178 | −48 | −105.65 | 18.427 |
| SCMleft_Density_Mean | 98 | 23.6 | 78.6 | 50.546 | 10.9604 |
| SCMleft_Density_Skewness | 98 | −3.4 | −0.8 | −1.717 | 0.4310 |
| SCMleft_Density SD | 98 | 14.1 | 47.9 | 32.052 | 6.5302 |
| SCMleft_UPP | 98 | 0.0075 | 0.0291 | 0.016344 | 0.0043101 |
| SCMleft_Uniformity | 98 | 0.0078 | 0.0291 | 0.016514 | 0.0042213 |
| PVM_Short_Axig | 98 | 43.7 | 88.9 | 66.209 | 10.0206 |
| PVM_Long _Axis | 98 | 63.3 | 176.3 | 100.281 | 18.8547 |
| PVM_Volume | 98 | 34.4 | 183.9 | 96.452 | 30.1608 |
| PVM_Entropy | 98 | 5.9675 | 7.5142 | 6.959589 | 0.2929190 |
| PVM_Kurtosis | 98 | 1.20 | 32.40 | 7.1407 | 4.72660 |
| PVM_MPP | 98 | 34.9 | 77.2 | 56.324 | 8.6289 |
| PVM_Density_Max. | 98 | 220.0 | 1278.0 | 520.678 | 211.4958 |
| PVM_Density Min. | 98 | −198 | −86 | −127.35 | 19.423 |
| PVM_Density_Mean | 98 | 11.9 | 70.0 | 43.765 | 12.7727 |
| PVM_Density_Skewness | 98 | −2.1 | 3.0 | −0.735 | 0.8544 |
| PVM_Density_SD | 98 | 20.1 | 61.8 | 39.053 | 7.0062 |
| PVM_UPP | 98 | 0.0057 | 0.0235 | 0.011340 | 0.0032782 |
| PVM_Uniformity | 98 | 0.0067 | 0.0235 | 0.011584 | 0.0030456 |
| SCM-rad_Volume | 98 | 3.30 | 17.80 | 8.9992 | 3.15632 |
| SCM-rad_Entropyopy | 98 | 5.45 | 7.28 | 6.4731 | 0.33946 |
| SCM-rad_MPP | 98 | 31.60 | 86.00 | 60.1269 | 8.70200 |
| SCM-rad_Density Mean | 98 | 23.60 | 78.60 | 51.9051 | 11.08669 |
| SCM-rad_Uniformity | 98 | 0.01 | 0.03 | 0.0168 | 0.00425 |
Appendix B
| Short _Axis | Long _Axis | Volume | Entropy | Kurtosis | MPP | Density_Max. | Density_Min. | Density_Mean | Density_Skewness | Density_SD | UPP | Uniformity | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Short_Axis | 1.00 | 0.11 | 0.45 | −0.12 | 0.12 | 0.06 | 0.00 | 0.04 | 0.13 | −0.12 | −0.16 | 0.10 | 0.09 |
| Long_Axis | 0.11 | 1.00 | 0.72 | −0.09 | 0.12 | −0.03 | 0.04 | −0.04 | 0.01 | −0.13 | −0.07 | 0.10 | 0.10 |
| Volume | 0.45 | 0.72 | 1.00 | −0.12 | 0.13 | −0.06 | −0.03 | −0.07 | −0.01 | −0.16 | −0.12 | 0.12 | 0.11 |
| Entropy | −0.12 | −0.09 | −0.12 | 1.00 | −0.72 | −0.04 | 0.28 | −0.58 | −0.37 | 0.70 | 0.87 | −0.96 | −0.96 |
| Kurtosis | 0.12 | 0.12 | 0.13 | −0.72 | 1.00 | 0.27 | 0.02 | 0.18 | 0.46 | −0.76 | −0.57 | 0.75 | 0.75 |
| MPP | 0.06 | −0.03 | −0.06 | −0.04 | 0.27 | 1.00 | 0.35 | 0.22 | 0.90 | −0.11 | 0.06 | 0.12 | 0.11 |
| Density M_Axis | 0.00 | 0.04 | −0.03 | 0.28 | 0.02 | 0.35 | 1.00 | −0.13 | 0.27 | 0.24 | 0.18 | −0.25 | −0.25 |
| Density Min | 0.04 | −0.04 | −0.07 | −0.58 | 0.18 | 0.22 | −0.13 | 1.00 | 0.47 | −0.01 | −0.68 | 0.48 | 0.47 |
| Density Mean | 0.13 | 0.01 | −0.01 | −0.37 | 0.46 | 0.90 | 0.27 | 0.47 | 1.00 | −0.25 | −0.35 | 0.38 | 0.36 |
| Density Skew | −0.12 | −0.13 | −0.16 | 0.70 | −0.76 | −0.11 | 0.24 | −0.01 | −0.25 | 1.00 | 0.40 | −0.76 | −0.76 |
| Density STD | −0.16 | −0.07 | −0.12 | 0.87 | −0.57 | 0.06 | 0.18 | −0.68 | −0.35 | 0.40 | 1.00 | −0.73 | −0.72 |
| UPP | 0.10 | 0.10 | 0.12 | −0.96 | 0.75 | 0.12 | −0.25 | 0.48 | 0.38 | −0.76 | −0.73 | 1.00 | 1.00 |
| Uniformity | 0.09 | 0.10 | 0.11 | −0.96 | 0.75 | 0.11 | −0.25 | 0.47 | 0.36 | −0.76 | −0.72 | 1.00 | 1.00 |
Appendix C
| Component | Sums of Squared Loadings | % of Variance | Cumulative % |
|---|---|---|---|
| Uniformity | 5.3 | 40.8 | 40.8 |
| Intensity | 2.2 | 16.6 | 57.4 |
| Dimension | 2.0 | 15.2 | 72.6 |
Appendix D
| Factor Loadings | Uniformity | Intensity | Dimension |
|---|---|---|---|
| Entropy | −0.99 | ||
| UPP | 0.97 | ||
| Uniformity | 0.97 | ||
| Density SD | −0.84 | ||
| Kurtosis | 0.77 | ||
| Density Skewness | −0.74 | ||
| Density Min. | 0.57 | ||
| MPP | 0.94 | ||
| Density Mean | 0.41 | 0.88 | |
| Density Max. | −0.31 | 0.62 | |
| Volume | 0.93 | ||
| Long_Axis | 0.82 | ||
| Short_Axis | 0.54 |
Appendix E
| Dimension-Factor | Uniformity-Factor | Intensity-Factor | Original Volume | Original Uniformity | Original Intensity (MPP) | ||
|---|---|---|---|---|---|---|---|
| Dimension-Factor | r | 1 | 0.08 | 0.017 | 0.937 | 0.165 | 0.018 |
| p | 0.435 | 0.865 | 0.001 | 0.104 | 0.858 | ||
| Uniformity-Factor | r | 1 | 0.268 | 0.075 | 0.966 | 0.317 | |
| p | 0.008 | 0.462 | 0.001 | 0.001 | |||
| Intensity-Factor | r | 1 | −0.074 | 0.228 | 0.963 | ||
| p | 0.468 | 0.024 | 0.001 | ||||
| Original Volume | r | 1 | 0.155 | −0.057 | |||
| p | 0.127 | 0.575 | |||||
| Original Uniformity | r | 1 | 0.284 | ||||
| p | 0.005 | ||||||
| Original Intensity | r | 1 | |||||
| (MPP) | p |
References
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.P.; Leemans, C.R.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Reprint of “Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up”. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Chen, C.-Y.; Lee, H.-L.; Chiou, J.-F.; Chen, Y.-J. Molecular Mechanisms of Chemotherapy Resistance in Head and Neck Cancers. Front. Oncol. 2021, 11, 640392. [Google Scholar] [CrossRef] [PubMed]
- Maloney, S.M.; Hoover, C.A.; Morejon-Lasso, L.V.; Prosperi, J.R. Mechanisms of Taxane Resistance. Cancers 2020, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Alterio, D.; Marvaso, G.; Ferrari, A.; Volpe, S.; Orecchia, R.; Jereczek-Fossa, B.A. Modern radiotherapy for head and neck cancer. Semin. Oncol. 2019, 46, 233–245. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef]
- Gujral, D.M.; Nutting, C.M. Patterns of failure, treatment outcomes and late toxicities of head and neck cancer in the current era of IMRT. Oral Oncol. 2018, 86, 225–233. [Google Scholar] [CrossRef]
- Coppes, R.P.; van der Goot, A.; Lombaert, I.M. Stem cell therapy to reduce radiation-induced normal tissue damage. Semin. Radiat. Oncol. 2009, 19, 112–121. [Google Scholar] [CrossRef]
- Kuhmann, C.; Weichenhan, D.; Rehli, M.; Plass, C.; Schmezer, P.; Popanda, O. DNA methylation changes in cells regrowing after fractioned ionizing radiation. Radiother. Oncol. 2011, 101, 116–121. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C. Are epigenetic mechanisms involved in radiation-induced bystander effects? Front. Genet. 2012, 3, 74. [Google Scholar] [CrossRef]
- Stone, H.B.; Coleman, C.N.; Anscher, M.S.; McBride, W.H. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 2003, 4, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Yarnold, J.; Brotons, M.C. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 2010, 97, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sheng, X.; Deng, F.; Wang, H.; Shen, L.; Zeng, Y.; Ni, Q.; Zhan, S.; Zhou, X. Radiation-induced muscle fibrosis rat model: Establishment and valuation. Radiat. Oncol. 2018, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Baudelet, M.; Steen, L.V.D.; Tomassen, P.; Bonte, K.; Deron, P.; Huvenne, W.; Rottey, S.; De Neve, W.; Sundahl, N.; Van Nuffelen, G.; et al. Very late xerostomia, dysphagia, and neck fibrosis after head and neck radiotherapy. Head Neck 2019, 41, 3594–3603. [Google Scholar] [CrossRef]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Offiah, C.; Hall, E. Post-treatment imaging appearances in head and neck cancer patients. Clin. Radiol. 2011, 66, 13–24. [Google Scholar] [CrossRef]
- Saito, N.; Nadgir, R.N.; Nakahira, M.; Takahashi, M.; Uchino, A.; Kimura, F.; Truong, M.T.; Sakai, O. Posttreatment CT and MR Imaging in Head and Neck Cancer: What the Radiologist Needs to Know. RadioGraphics 2012, 32, 1261–1284; discussion 1282–1284. [Google Scholar] [CrossRef]
- Wen, X.; Yu, X.; Cheng, W.; Li, Y.; Tian, J. Quantitative Evaluation of Shear Wave Elastography on Radiation-Induced Neck Fibrosis in Patients With Nasopharyngeal Carcinoma. Ultrasound Q. 2019, 37, 178–182. [Google Scholar] [CrossRef]
- Bardosi, Z.R.; Dejaco, D.; Santer, M.; Kloppenburg, M.; Mangesius, S.; Widmann, G.; Ganswindt, U.; Rumpold, G.; Riechelmann, H.; Freysinger, W. Benchmarking Eliminative Radiomic Feature Selection for Head and Neck Lymph Node Classification. Cancers 2022, 14, 477. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Ridner, S.H.; Dietrich, M.S.; Niermann, K.; Cmelak, A.; Mannion, K.; Murphy, B. A Prospective Study of the Lymphedema and Fibrosis Continuum in Patients with Head and Neck Cancer. Lymphat. Res. Biol. 2016, 14, 198–205. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Golm, L.; Dejaco, D.; Riedl, D.; Kofler, B.; Url, C.; Wolfram, D.; Riechelmann, H. Surgical rescue for persistent head and neck cancer after first-line treatment. Eur. Arch. Otorhinolaryngol. 2020, 277, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Steinbichler, T.B.; Lichtenecker, M.; Anegg, M.; Dejaco, D.; Kofler, B.; Schartinger, V.H.; Kasseroler, M.-T.; Forthuber, B.; Posch, A.; Riechelmann, H. Persistent Head and Neck Cancer Following First-Line Treatment. Cancers 2018, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Boyd, T.S.; Harari, P.M.; Tannehill, S.P.; Voytovich, M.C.; Hartig, G.K.; Ford, C.N.; Foote, R.L.; Campbell, B.H.; Schultz, C.J. Planned postradiotherapy neck dissection in patients with advanced head and neck cancer. Head Neck 1998, 20, 132–137. [Google Scholar] [CrossRef]
- Denaro, N.; Russi, E.G.; Numico, G.; Pazzaia, T.; Vitiello, R.; Merlano, M.C. The role of neck dissection after radical chemoradiation for locally advanced head and neck cancer: Should we move back? Oncology 2013, 84, 174–185. [Google Scholar] [CrossRef]
- Stenson, K.M.; Haraf, D.J.; Pelzer, H.; Recant, W.; Kies, M.S.; Weichselbaum, R.R.; Vokes, E.E. The role of cervical lymphadenectomy after aggressive concomitant chemoradiotherapy: The feasibility of selective neck dissection. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 950–956. [Google Scholar] [CrossRef]
- Lango, M.N.; Andrews, G.A.; Ahmad, S.; Feigenberg, S.; Tuluc, M.; Gaughan, J.; Ridge, J.A. Postradiotherapy neck dissection for head and neck squamous cell carcinoma: Pattern of pathologic residual carcinoma and prognosis. Head Neck 2009, 31, 328–337. [Google Scholar] [CrossRef]
- Moloney, E.C.; Brunner, M.; Alexander, A.J.; Clark, J. Quantifying fibrosis in head and neck cancer treatment: An overview. Head Neck 2015, 37, 1225–1231. [Google Scholar] [CrossRef]
- Bourgier, C.; Auperin, A.; Rivera, S.; Boisselier, P.; Petit, B.; Lang, P.; Lassau, N.; Taourel, P.; Tetreau, R.; Azria, D.; et al. Pravastatin Reverses Established Radiation-Induced Cutaneous and Subcutaneous Fibrosis in Patients With Head and Neck Cancer: Results of the Biology-Driven Phase 2 Clinical Trial Pravacur. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 365–373. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.E.; Pothen, A.J.; Wegner, I.; Smid, E.J.; Swart, K.M.; de Bree, R.; Leenen, L.P.; Grolman, W. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. 2016, 62, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Zigon, G.; Berrino, F.; Gatta, G.; Sánchez, M.-J.; van Dijk, B.; Van Eycken, E.; Francisci, S. Prognoses for head and neck cancers in Europe diagnosed in 1995–1999: A population-based study. Ann. Oncol. 2011, 22, 165–174. [Google Scholar] [CrossRef]
- Dejaco, D.; Steinbichler, T.; Schartinger, V.H.; Fischer, N.; Anegg, M.; Dudas, J.; Posch, A.; Widmann, G.; Riechelmann, H. Prognostic value of tumor volume in patients with head and neck squamous cell carcinoma treated with primary surgery. Head Neck 2018, 40, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Dejaco, D.; Steinbichler, T.; Schartinger, V.H.; Fischer, N.; Anegg, M.; Dudas, J.; Posch, A.; Widmann, G.; Riechelmann, H. Specific growth rates calculated from CTs in patients with head and neck squamous cell carcinoma: A retrospective study performed in Austria. BMJ Open 2019, 9, e025359. [Google Scholar] [CrossRef] [PubMed]
- Dejaco, D.; Url, C.; Schartinger, V.H.; Haug, A.K.; Fischer, N.; Riedl, D.; Posch, A.; Riechelmann, H.; Widmann, G. Approximation of head and neck cancer volumes in contrast enhanced CT. Cancer Imaging 2015, 15, 16. [Google Scholar] [CrossRef]
- Chamchod, S.; Fuller, C.D.; Mohamed, A.S.; Grossberg, A.; Messer, J.A.; Heukelom, J.; Gunn, G.B.; Kantor, M.E.; Eichelberger, H.; Garden, A.S.; et al. Quantitative body mass characterization before and after head and neck cancer radiotherapy: A challenge of height-weight formulae using computed tomography measurement. Oral Oncol. 2016, 61, 62–69. [Google Scholar] [CrossRef]
- Choi, Y.; Ahn, K.; Jang, J.; Shin, N.; Jung, S.; Kim, B.; Kim, M.; Kim, Y. Prognostic value of computed tomography-based volumetric body composition analysis in patients with head and neck cancer: Feasibility study. Head Neck 2020, 42, 2614–2625. [Google Scholar] [CrossRef]
- Findlay, M.; White, K.; Stapleton, N.; Bauer, J. Is sarcopenia a predictor of prognosis for patients undergoing radiotherapy for head and neck cancer? A meta-analysis. Clin. Nutr. 2021, 40, 1711–1718. [Google Scholar] [CrossRef]
- Grossberg, A.J.; Chamchod, S.; Fuller, C.D.; Mohamed, A.S.R.; Heukelom, J.; Eichelberger, H.; Kantor, M.E.; Hutcheson, K.A.; Gunn, G.B.; Garden, A.S.; et al. Association of Body Composition With Survival and Locoregional Control of Radiotherapy-Treated Head and Neck Squamous Cell Carcinoma. JAMA Oncol. 2016, 2, 782–789. [Google Scholar] [CrossRef]
- Platek, M.E.; Myrick, E.; McCloskey, S.A.; Gupta, V.; Reid, M.E.; Wilding, G.E.; Cohan, D.; Arshad, H.; Rigual, N.R.; Hicks, W.L.; et al. Pretreatment weight status and weight loss among head and neck cancer patients receiving definitive concurrent chemoradiation therapy: Implications for nutrition integrated treatment pathways. Support. Care Cancer 2013, 21, 2825–2833. [Google Scholar] [CrossRef] [PubMed]
- Microvascular Committee of the American Academy of Otolaryngology-Head & Neck Surgery; Oxford, L.E.; Urken, M.; Lazarus, C.; Cheney, J.; Scharpf, J.; Diaz, J. Salvage laryngectomy and laryngopharyngectomy: Multicenter review of outcomes associated with a reconstructive approach. Head Neck 2019, 41, 16–29. [Google Scholar]
- Danan, D.; Shonka, D.C., Jr.; Selman, Y.; Chow, Z.; Smolkin, M.E.; Jameson, M.J. Prognostic value of albumin in patients with head and neck cancer. Laryngoscope 2016, 126, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Santer, M.; Kloppenburg, M.; Gottfried, T.M.; Runge, A.; Schmutzhard, J.; Vorbach, S.M.; Mangesius, J.; Riedl, D.; Mangesius, S.; Widmann, G.; et al. Current Applications of Artificial Intelligence to Classify Cervical Lymph Nodes in Patients with Head and Neck Squamous Cell Carcinoma—A Systematic Review. Cancers 2022, 14, 5397. [Google Scholar] [CrossRef] [PubMed]
| Radiomic Key Features | Individual Radiomic Features |
|---|---|
| Shape features | Short axis diameter 1 Long axis diameter 1 Volume 2 |
| Texture features 3 | Entropy Uniformity |
| Intensity features 3 | Maximal density Minimal density Mean density Skewness of density Standard deviation of density MPP Uniformity of distribution of positive pixels (UPP) Kurtosis |
| Number | Percentages | ||
|---|---|---|---|
| Sex | Male | 73 | 74.5% |
| Female | 25 | 25.5% | |
| Age | ≤50 | 11 | 11.2% |
| 51–60 | 35 | 35.7% | |
| 61–70 | 33 | 33.7% | |
| ≥71 | 19 | 19.4% | |
| Tumor site | Oral cavity | 13 | 13.3% |
| Oropharynx | 46 | 46.9% | |
| Hypopharynx | 20 | 20.4% | |
| Larynx | 15 | 15.3% | |
| CUP 1 | 4 | 4.1% | |
| UICC 1 truncated | Stage III | 19 | 19.4% |
| Stage IV | 79 | 80.6% | |
| ASA | ASA I/II | 45 | 45.9% |
| ASA III/IV | 53 | 54.1% | |
| Alcohol consumption | <Daily | 52 | 53.1% |
| Daily | 46 | 46.9% | |
| Smoking habits | <10 PY | 24 | 24.5% |
| ≥10 PY | 74 | 75.5% | |
| BMI classified | Underweight | 7 | 8.9% |
| Normal weight | 41 | 51.9% | |
| Overweight | 25 | 31.6% | |
| Adipose | 6 | 7.6% | |
| Radiation dose 2 | ≤60 Gy | 26 | 26.5% |
| >60 Gy | 72 | 73.5% |
| Mean (Days) | Minimum (Days) | Maximum (Days) | Standard Deviation (Days) | |
|---|---|---|---|---|
| Imaging interval 1 | 148 | 108 | 315 | ±33 |
| Restaging interval 2 | 55 | 29 | 229 | ±28 |
| Radiomic Key Features | Staging CT (SD) | Restaging CT (SD) | p-Value 1 | Cohen’s d 2 |
|---|---|---|---|---|
| SCM Volume (mL) | 9.00 (±3.2) | 8.4 (±2.7) | 0.007 | 0.28 |
| SCM MPP (HU) | 60.1 (±8.7) | 59.7 (±8.1) | 0.664 | 0.04 |
| SCM Uniformity * | 16.8 (±4.3) | 16.4 (±4.1) | 0.342 | 0.10 |
| PVM Volume (mL) | 96.5 (±30.2) | 91.9 (±25.8) | 0.007 | 0.28 |
| PVM MPP (HU) | 56.3 (±8.6) | 58.0 (±8.6) | 0.061 | −0.19 |
| PVM Uniformity * | 11.6 (±3.1) | 12.0 (±2.8) | 0.058 | −0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santer, M.; Riechelmann, H.; Hofauer, B.; Schmutzhard, J.; Freysinger, W.; Runge, A.; Gottfried, T.M.; Zelger, P.; Widmann, G.; Kranebitter, H.; et al. Radiomic Assessment of Radiation-Induced Alterations of Skeletal Muscle Composition in Head and Neck Squamous Cell Carcinoma within the Currently Clinically Defined Optimal Time Window for Salvage Surgery—A Pilot Study. Cancers 2023, 15, 4650. https://doi.org/10.3390/cancers15184650
Santer M, Riechelmann H, Hofauer B, Schmutzhard J, Freysinger W, Runge A, Gottfried TM, Zelger P, Widmann G, Kranebitter H, et al. Radiomic Assessment of Radiation-Induced Alterations of Skeletal Muscle Composition in Head and Neck Squamous Cell Carcinoma within the Currently Clinically Defined Optimal Time Window for Salvage Surgery—A Pilot Study. Cancers. 2023; 15(18):4650. https://doi.org/10.3390/cancers15184650
Chicago/Turabian StyleSanter, Matthias, Herbert Riechelmann, Benedikt Hofauer, Joachim Schmutzhard, Wolfgang Freysinger, Annette Runge, Timo Maria Gottfried, Philipp Zelger, Gerlig Widmann, Hanna Kranebitter, and et al. 2023. "Radiomic Assessment of Radiation-Induced Alterations of Skeletal Muscle Composition in Head and Neck Squamous Cell Carcinoma within the Currently Clinically Defined Optimal Time Window for Salvage Surgery—A Pilot Study" Cancers 15, no. 18: 4650. https://doi.org/10.3390/cancers15184650
APA StyleSanter, M., Riechelmann, H., Hofauer, B., Schmutzhard, J., Freysinger, W., Runge, A., Gottfried, T. M., Zelger, P., Widmann, G., Kranebitter, H., Mangesius, S., Mangesius, J., Kocher, F., & Dejaco, D. (2023). Radiomic Assessment of Radiation-Induced Alterations of Skeletal Muscle Composition in Head and Neck Squamous Cell Carcinoma within the Currently Clinically Defined Optimal Time Window for Salvage Surgery—A Pilot Study. Cancers, 15(18), 4650. https://doi.org/10.3390/cancers15184650






