Clinical Characteristics and Prognosis of HER2-0 and HER2-Low-Positive Breast Cancer Patients: Real-World Data from Patients Treated with Neoadjuvant Chemotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
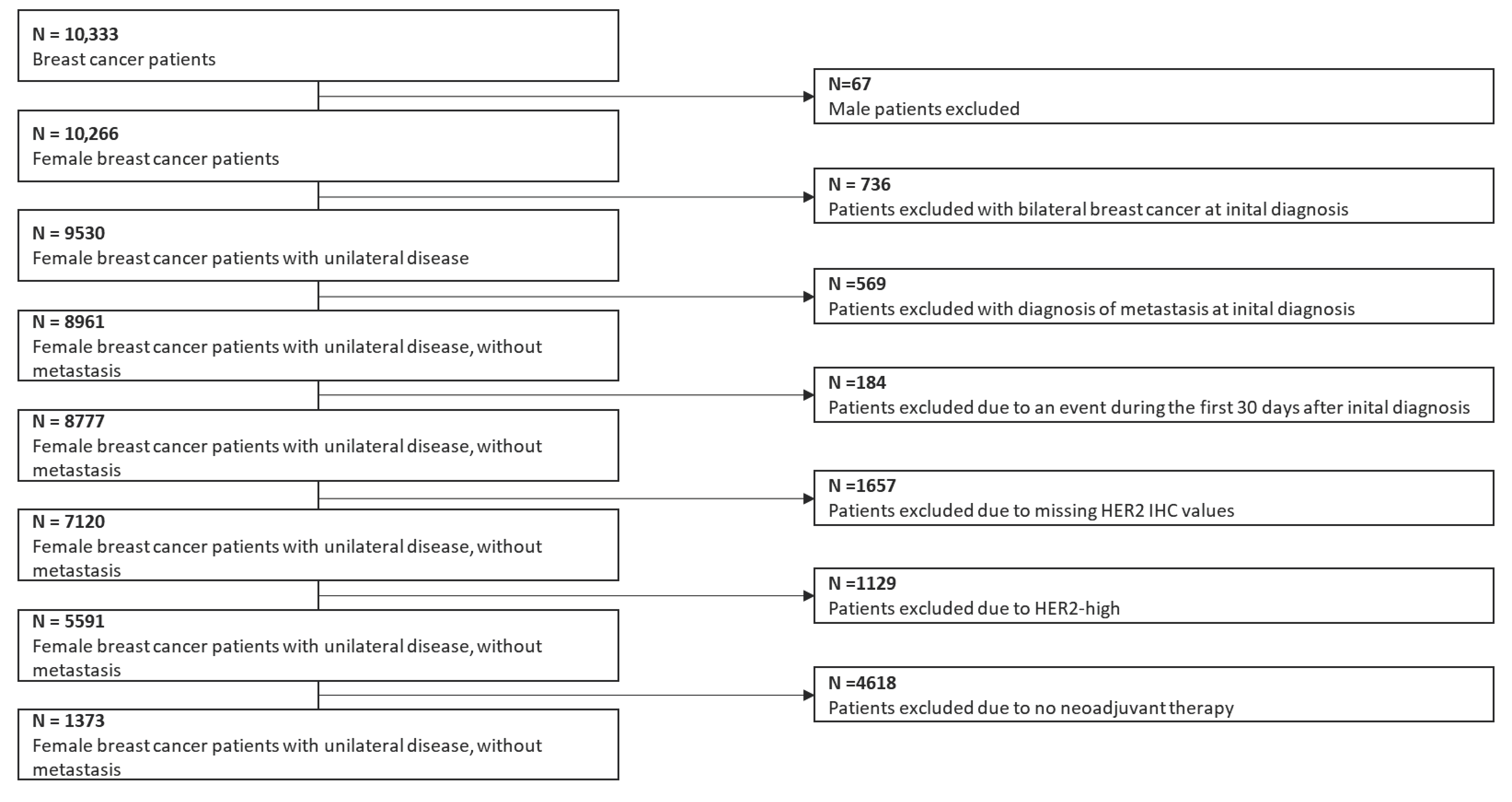
2.1. Patient Selection
2.2. Histopathological Data
2.3. Clinical Data
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pauletti, G.; Godolphin, W.; Press, M.F.; Slamon, D.J. Detection and quantitation of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene 1996, 13, 63–72. [Google Scholar]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Giuliani, S.; Ciniselli, C.M.; Leonardi, E.; Polla, E.; Decarli, N.; Luchini, C.; Cantaloni, C.; Gasperetti, F.; Cazzolli, D.; Berlanda, G.; et al. In a cohort of breast cancer screened patients the proportion of HER2 positive cases is lower than that earlier reported and pathological characteristics differ between HER2 3+ and HER2 2+/HER2 amplified cases. Virchows Arch. 2016, 469, 45–50. [Google Scholar] [CrossRef]
- Lal, P.; Salazar, P.A.; Hudis, C.A.; Ladanyi, M.; Chen, B. HER-2 testing in breast cancer using immunohistochemical analysis and fluorescence in situ hybridization: A single-institution experience of 2279 cases and comparison of dual-color and single-color scoring. Am. J. Clin. Pathol. 2004, 121, 631–636. [Google Scholar] [CrossRef]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef]
- Tarantino, P.; Viale, G.; Press, M.; Hu, X.; Penault-Llorca, F.; Bardia, A.; Batistatou, A.; Burstein, H.; Carey, L.; Cortes, J.; et al. ESMO expert consensus statements (ECS) on the definition, diagnosis, and management of HER2-low breast cancer. Ann. Oncol. 2023, 34, 645–659. [Google Scholar] [CrossRef]
- Müller, V.; Welslau, M.; Lüftner, D.; Schütz, F.; Stickeler, E.; Fasching, P.A.; Janni, W.; Thomssen, C.; Witzel, I.; Fehm, T.N.; et al. Update Breast Cancer 2022 Part 2—Advanced Stage Breast Cancer. Geburtshilfe Frauenheilkd. 2022, 82, 590–600. [Google Scholar] [CrossRef]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients with HER2-Low-Expressing Advanced Breast Cancer: Results from a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer, C.E., Jr.; Rastogi, P.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.-F.; Provencher, L.; et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy with or Without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and with IHC 1+ or 2. J. Clin. Oncol. 2020, 38, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Seither, F.; Schneeweiss, A.; Link, T.; Blohmer, J.-U.; Just, M.; Wimberger, P.; Forberger, A.; Tesch, H.; Jackisch, C.; et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: Pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021, 22, 1151–1161. [Google Scholar] [CrossRef]
- Almstedt, K.; Heimes, A.-S.; Kappenberg, F.; Battista, M.J.; Lehr, H.-A.; Krajnak, S.; Lebrecht, A.; Gehrmann, M.; Stewen, K.; Brenner, W.; et al. Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur. J. Cancer 2022, 173, 10–19. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Sauter, G.; Lee, J.; Bartlett, J.M.; Slamon, D.J.; Press, M.F. Guidelines for human epidermal growth factor receptor 2 testing: Biologic and methodologic considerations. J. Clin. Oncol. 2009, 27, 1323–1333. [Google Scholar] [CrossRef]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Park-Simon, T.-W.; Albanell, J.; Lassen, U.; Cortés, J.; Dieras, V.; May, M.; Schindler, C.; Marmé, F.; Cejalvo, J.M.; et al. Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Investig. New Drugs 2018, 36, 848–859. [Google Scholar] [CrossRef]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; McShane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO-College of American Pathologists Guideline Update. J. Clin. Oncol. 2023, 41, 3867–3872. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, M.W.; Brucker, C.; Hanf, V.; Rauh, C.; Bani, M.R.; Knob, S.; Petsch, S.; Schick, S.; Fasching, P.A.; Hartmann, A.; et al. Quality assured health care in certified breast centers and improvement of the prognosis of breast cancer patients. Onkologie 2011, 34, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, C.; Ferencz, J.; Brucker, S.Y.; Kreienberg, R.; Wesselmann, S. Quality of care in breast cancer centers: Results of benchmarking by the German Cancer Society and German Society for Breast Diseases. Breast 2015, 24, 118–123. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 31 May 2023).
- Kang, S.; Lee, S.H.; Lee, H.J.; Jeong, H.; Jeong, J.H.; Kim, J.E.; Ahn, J.-H.; Jung, K.H.; Gong, G.; Kim, H.H.; et al. Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy. Eur. J. Cancer 2022, 176, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Jin, Q.; Tayob, N.; Jeselsohn, R.M.; Schnitt, S.J.; Vincuilla, J.; Parker, T.; Tyekucheva, S.; Li, T.; Lin, N.U.; et al. Prognostic and Biologic Significance of ERBB2-Low Expression in Early-Stage Breast Cancer. JAMA Oncol. 2022, 8, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, D.S.; Zhao, F.; Chen, N.; Hahn, O.M.; Nanda, R.; Olopade, O.I.; Huo, D.; Howard, F.M. Clinicopathologic Characteristics and Prognosis of ERBB2-Low Breast Cancer Among Patients in the National Cancer Database. JAMA Oncol. 2023, 9, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- AGO Breast Committee. Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer. Recommendations 2023. Available online: www.agoonline.de (accessed on 31 May 2023).
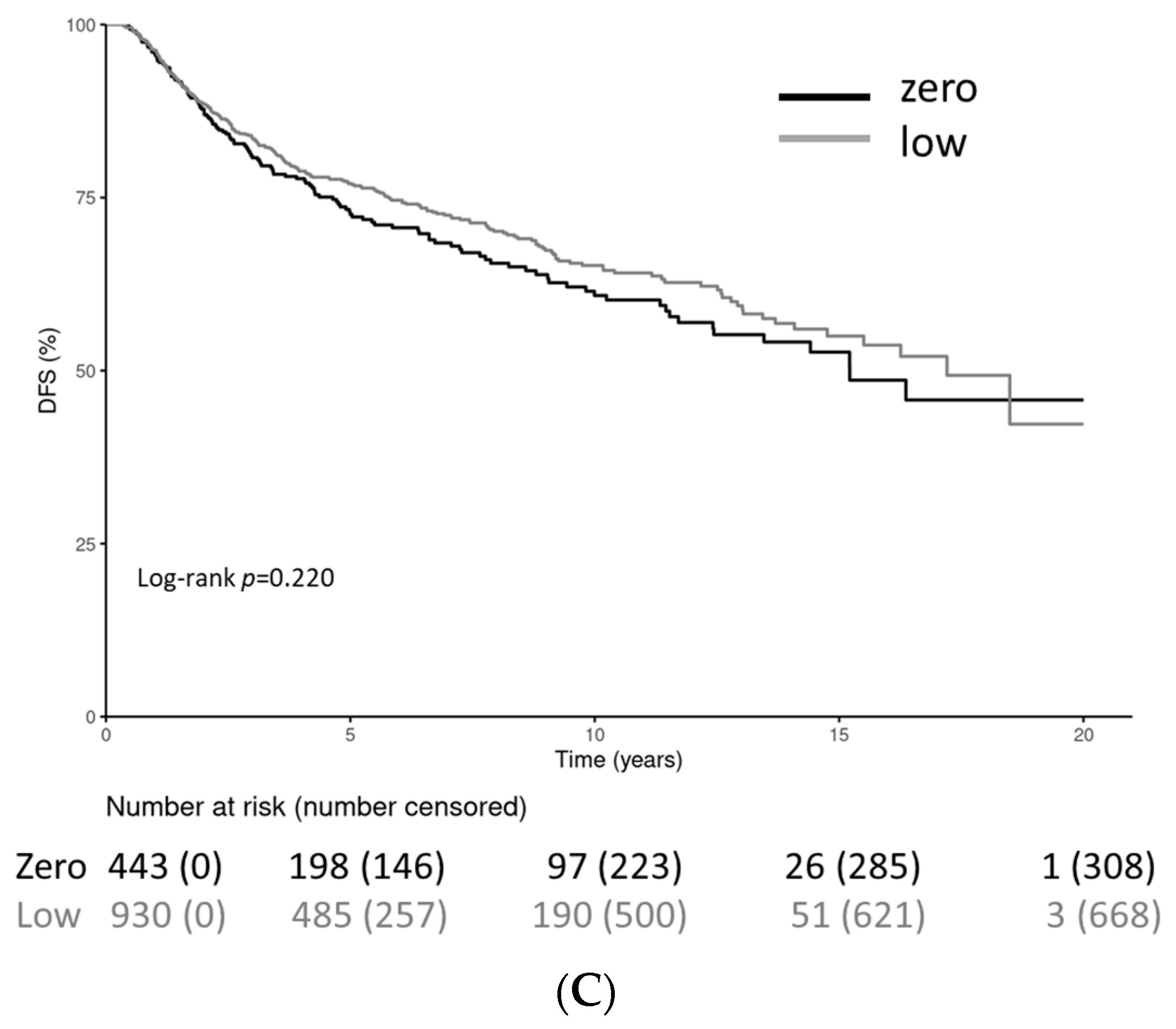
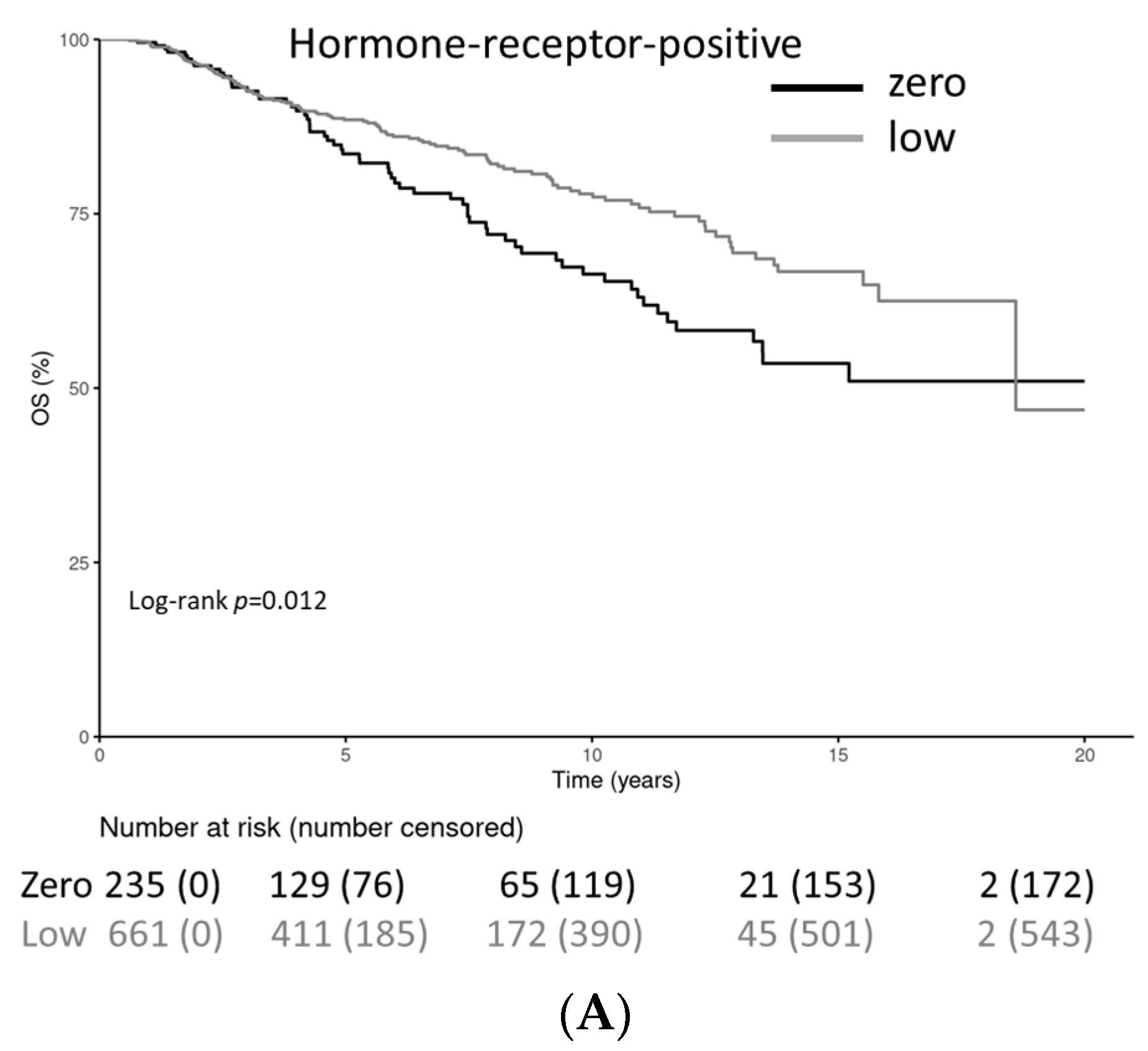
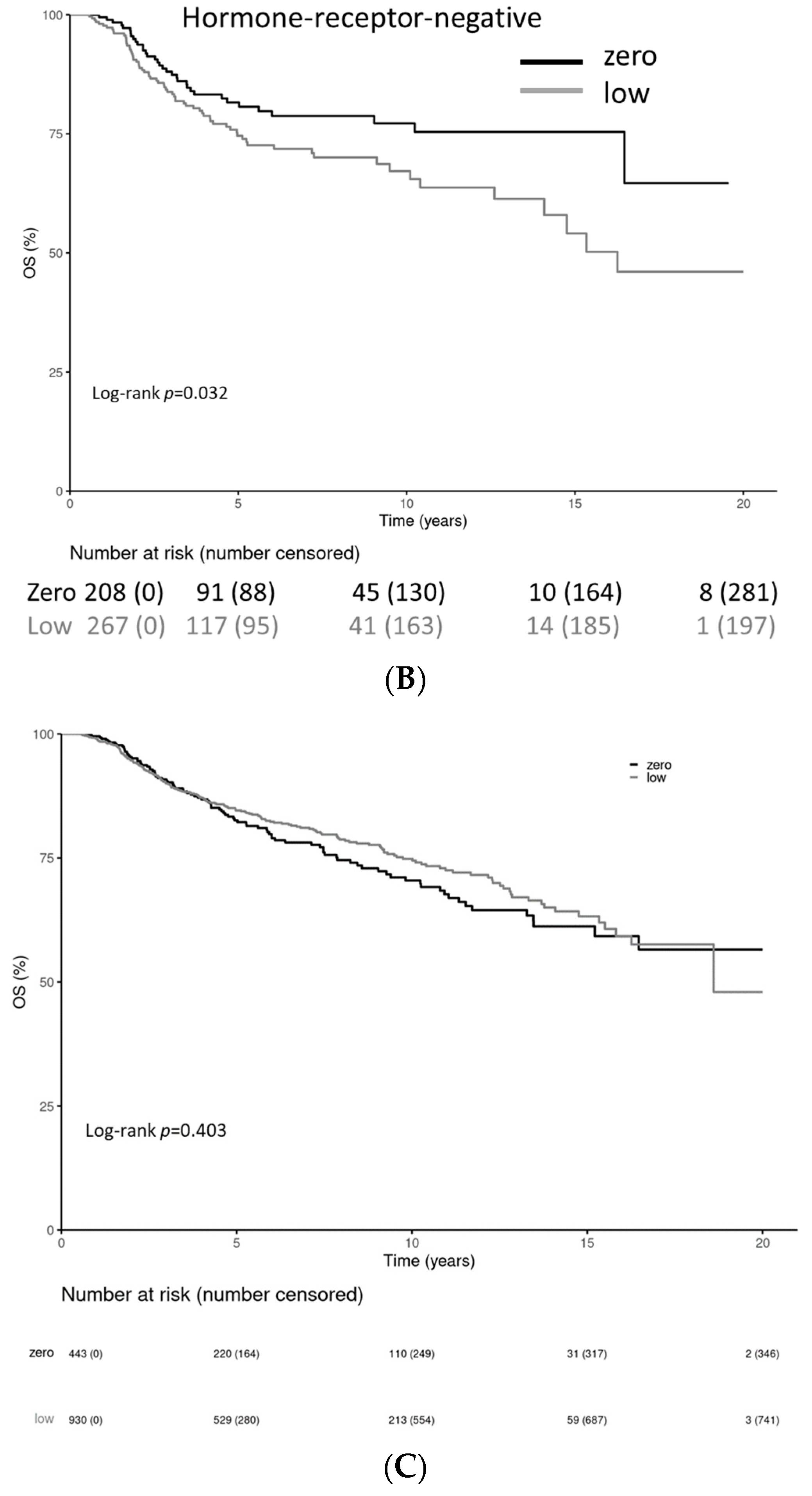
| Characteristic | HER2-0 (n = 443) | HER2-Low (n = 930) |
|---|---|---|
| Age | ||
| <50 | 203 (45.8) | 375 (40.3) |
| ≥50 | 240 (54.1) | 555 (59.7) |
| BMI | ||
| Low | 14 (3.3) | 12 (1.4) |
| Medium | 190 (45.0) | 426 (47.8) |
| High | 218 (51.7) | 453 (50.8) |
| Tumor size | ||
| T1 | 108 (24.6) | 226 (24.5) |
| T2–4 | 331 (75.4) | 698 (75.5) |
| Nodal status | ||
| N+ | 209 (47.8) | 486 (52.8) |
| N0 | 228 (52.2) | 435 (47.2) |
| Histology | ||
| Ductal | 302 (68.6) | 666 (71.7) |
| Lobular | 46 (10.5) | 78 (8.4) |
| Other | 92 (20.9) | 185 (19.9) |
| Grading | ||
| G1 | 20 (4.6) | 32 (3.5) |
| G2 | 156 (35.78) | 351 (37.9) |
| G3 | 260 (59.6) | 544 (58.7) |
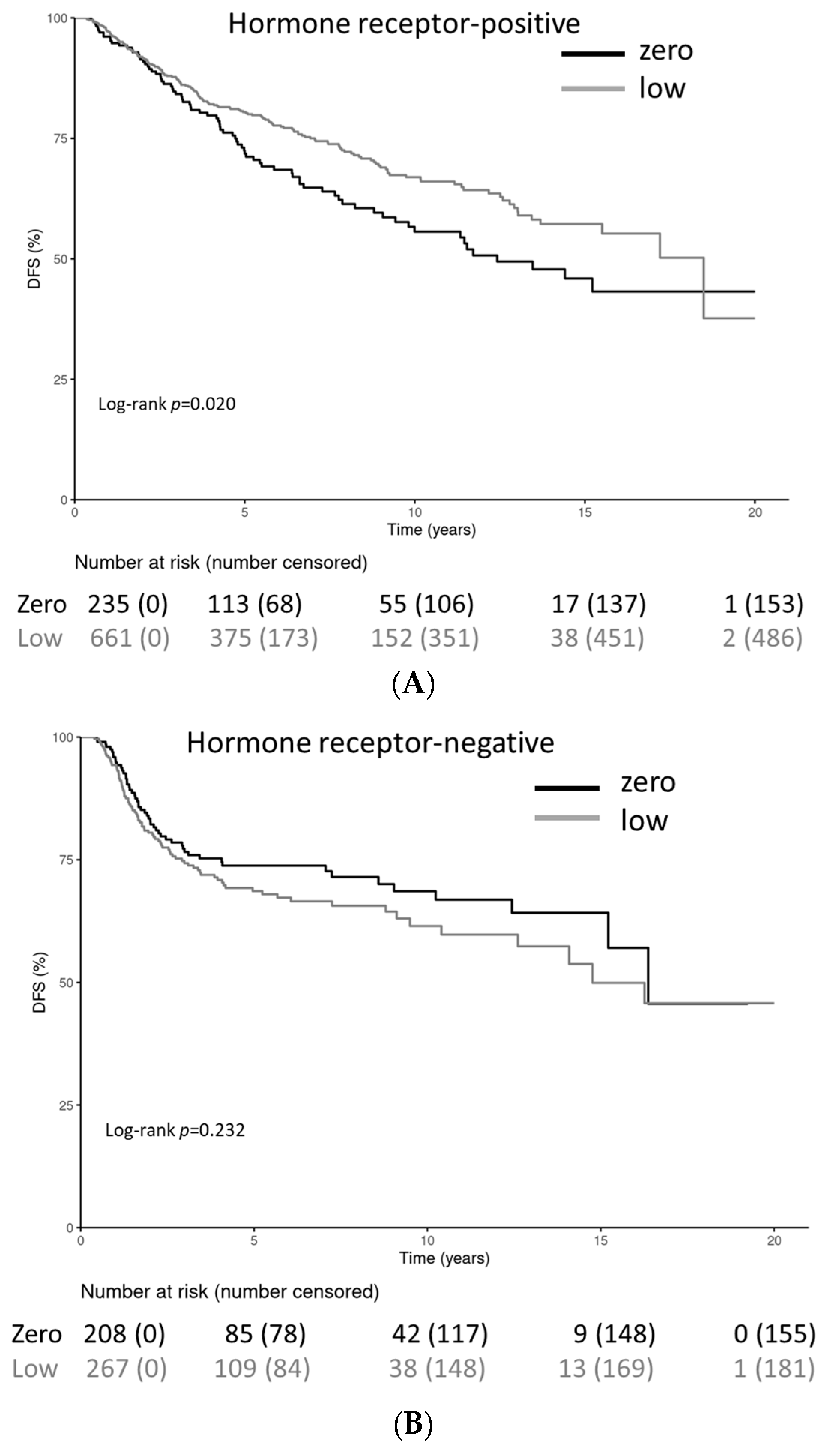
| HR | ||
| HR-negative | 208 (47.0) | 267 (28.8) |
| HR-positive | 235 (53.0) | 661 (71.2) |
| Ki-67 | ||
| ≤15% | 87 (20.4) | 196 (21.4) |
| >15–25% | 87 (20.4) | 252 (27.5) |
| >35% | 253 (59.3) | 469 (51.2) |
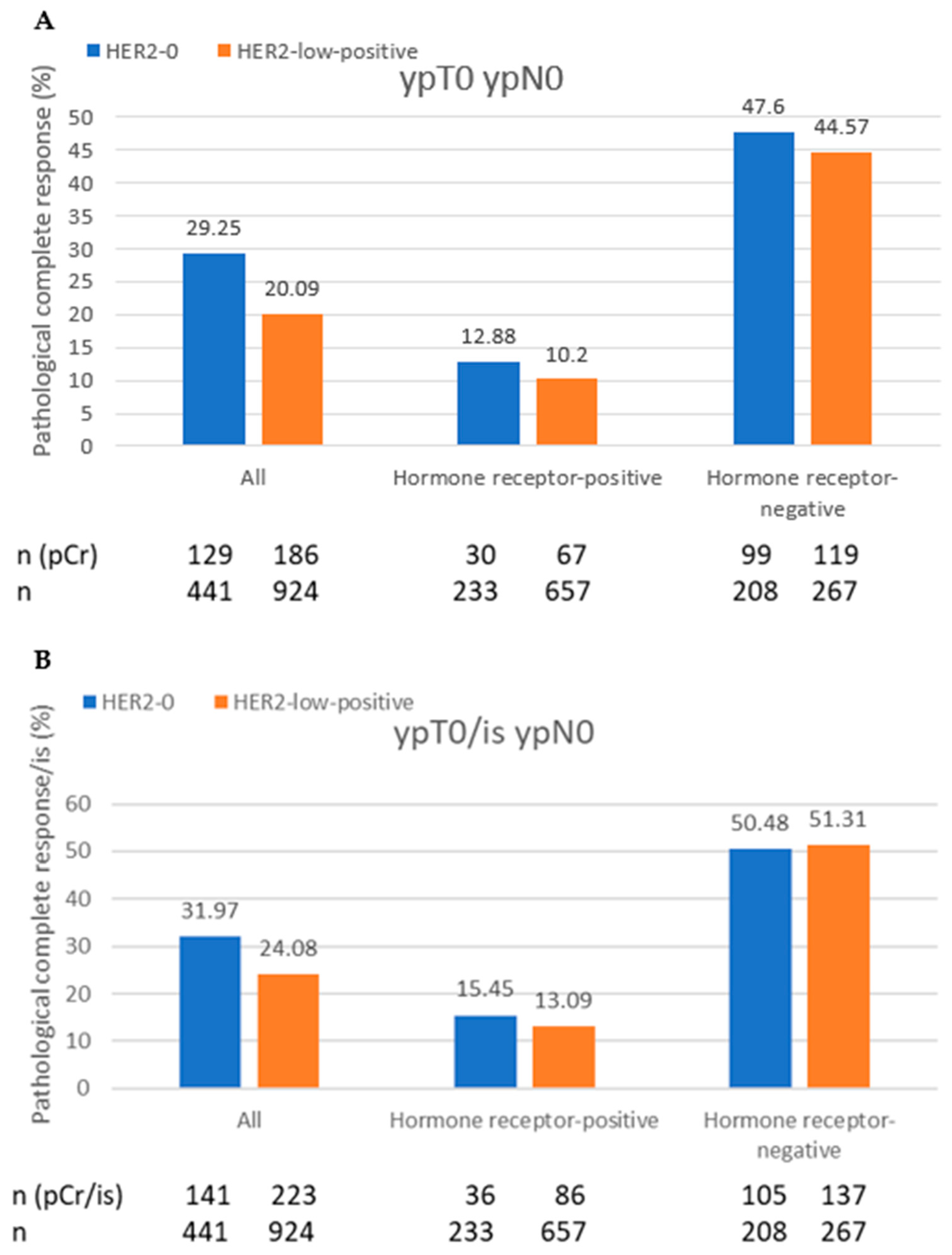
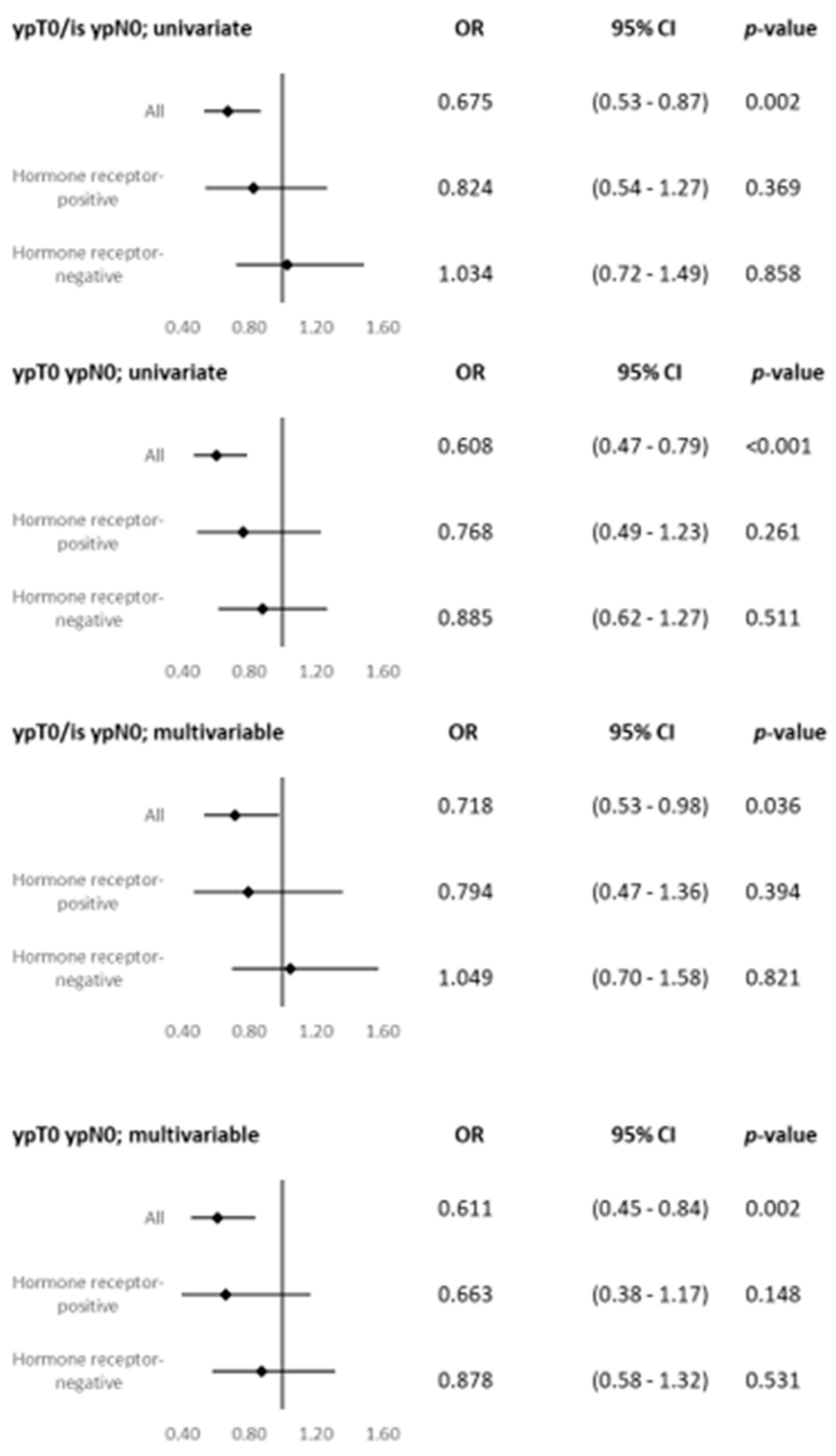
| pCR (ypT0 ypN0) | ||
| Yes | 129 (29.3) | 186 (20.1) |
| No | 312 (70.8) | 740 (79.9) |
| pCR/is (ypT0/is ypN0) | ||
| Yes | 141 (32.0) | 223 (24.1) |
| No | 300 (68.0) | 703 (76.0) |
| Surgery Kind | ||
| Breast-conserving surgery | 282 (63.6) | 599 (64.5) |
| Mastectomy | 161 (36.3) | 331 (35.6) |
| Chemotherapy regimes | ||
| Anthracylcine and taxane | 236 (53.3) | 542 (58.3) |
| (+/−platinum) | ||
| Platinum-based and taxane | 83 (19.3) | 152 (16.3) |
| (Anthracycline-free) | ||
| CMF | 4 (0.01) | 25 (0.03) |
| Platinum monotherapy | 0 | 2 (0.02) |
| Taxane monotherapy | 17 (3.9) | 52 (5.6) |
| Anthracycline monotherapy | 56 (13.0) | 98 (10.5) |
| Other | 27 (6.3) | 35 (3.7) |
| Missing values | 17 (3.9) | 24 (2.6) |
| Adjuvant therapy | ||
| Radiotherapy | 278 (73.7) | 576 (74.4) |
| Missing values (radiotherapy) | 53 (12.3) | 156 (16.8) |
| Endocrine therapy | 165 (44.9) | 469 (61.2) |
| Missing values (endocrine therapy) | 54 (12.3) | 170 (18.3) |
| Chemotherapy | HER2-0 HR-Positive pCr; pCr/is (n = 235) | HER2-0 HR-Negative pCr; pCr/is (n = 208) | HER2-Low HR-Positive pCr; pCr/is (n = 663) | HER2-Low HR-Negative pCr; pCr/is (n = 267) |
|---|---|---|---|---|
| Anthracylcine and taxane | 15.9; 19.3 (145) | 46.2; 50.5 (91) | 40.7; 53.8 (451) | 40.7; 22.2 (91) |
| Platinum and taxane | 60.0; 60.0 (10) | 58.9; 60.3 (73) | 54.5; 57.6 (33) | 53.9; 62.2 (119) |
| CMF | 0.0; 0.0 (4) | 0 | 9.5; 9.5 (21) | 50.0; 50.0 (4) |
| Platinum monotherapy | 0 | 0 | 0 | 50.0; 50.0 (2) |
| Taxane monotherapy | 0 | 23.5; 23.5 (17) | 11.8; 14.7 (34) | 50.0; 50.0 (18) |
| Anthracycline monotherapy | 0.0; 0.0 (40) | 18.8; 25.0 (16) | 6.9; 9.7 (72) | 19.2; 23.1 (26) |
| Predictor | Multivariate Hazard Ratio for HR-Positive Patients | p Value | Multivariate Hazard Ratio for HR-Negative Patients |
|---|---|---|---|
| Age | |||
| <50 | |||
| ≥50 | 1.075 (0.815; 1.417) | 0.609 | 0.513 (0.344; 0.765) |
| BMI | |||
| Low | |||
| Medium | 0.407 (0.126; 1.316) | 0.133 | 1.254 (0.448; 3.51) |
| High | 0.443 (0.136; 1.439) | 0.175 | 1.574 (0.558; 4.439) |
| Tumor size | |||
| T1 | |||
| T2 | 1.333 (0.862; 2.06) | 0.196 | 3.851 (2.135; 6.944) |
| T3 | 1.848 (0.968; 3.528) | 0.063 | 6.06 (2.519; 14.576) |
| T4 | 2.408 (1.43; 4.057) | 0.001 | 5.101 (2.816; 13.216) |
| Nodal status | |||
| N0 | |||
| N1 | 1.576 (1.187; 2.093) | 0.002 | 2.139 (1.452; 3.151) |
| Grading | |||
| G1 | |||
| G2 | 1.013 (0.568; 1.808) | 0.964 | 1.544 (0.319; 7.474) |
| G3 | 1.06 (0.563; 1.995) | 0.856 | 1.635 (0.325; 8.217) |
| HER2 status | |||
| Zero | |||
| Low | 0.704 (0.53; 0.934) | 0.015 | 1.554 (1.064; 2.271) |
| Ki-67 | |||
| ≤15% | |||
| >15–35% | 1.23 (0.881; 1.717) | 0.223 | 0.435 (0.158; 1.202) |
| >35% | 2.04 (1.395; 2.984) | < 0.001 | 0.407 (0.167; 0.993) |
| pCR (ypT0 ypN0) | |||
| Yes | |||
| No | 4.242 (1.96; 9.184) | < 0.001 | 3.68 (2.342; 5.783) |
| Predictor | Multivariate Hazard Ratio for HR-Positive Patients | p Value | Multivariate Hazard Ratio for HR-Negative Patients |
|---|---|---|---|
| Age | |||
| <50 | |||
| ≥50 | 1.513 (1.075; 2.129) | 0.018 | 0.615 (0.386; 0.978) |
| BMI | |||
| Low | |||
| Medium | 1.897 (0.254; 14.168) | 0.533 | 1.374 (0.416; 4.535) |
| High | 1.68 (0.224; 12.597) | 0.614 | 1.371 (0.411; 4.57) |
| Tumor size | |||
| T1 | |||
| T2 | 1.209 (0.703; 2.08) | 0.493 | 4.23 (2.011; 8.897) |
| T3 | 2.499 (1.208; 5.171) | 0.014 | 6.468 (2.345; 17.843) |
| T4 | 2.614 (1.4; 4.878) | 0.003 | 8.926 (3.604; 22.106) |
| Nodal status | |||
| N0 | |||
| N1 | 1.454 (1.037; 2.04) | 0.03 | 2.414 (1.516; 3.843) |
| Grading | |||
| G1 | |||
| G2 | 0.665 (0.367; 1.202) | 0.177 | 0.848 (0.159; 4.515) |
| G3 | 0.671 (0.343; 1.315) | 0.246 | 0.866 (0.155; 4.844) |
| HER2 status | |||
| Zero | |||
| Low | 0.643 (0.46; 0.898) | 0.01 | 1.906 (1.214; 2.991) |
| Ki-67 | |||
| ≤15% | |||
| >15–35% | 1.086 (0.726; 1.623) | 0.689 | 0.58 (0.166; 2.021) |
| >35% | 2.339 (1.492; 3.666) | <0.001 | 0.675 (0.233; 1.952) |
| pCR (ypT0/ypN0) | |||
| Yes | |||
| No | 4.296 (1.716; 10.755) | 0.002 | 3.368 (1.996; 5.682) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pöschke, P.; Fasching, P.A.; Adler, W.; Rübner, M.; Beckmann, M.W.; Hack, C.C.; Heindl, F.; Hartmann, A.; Erber, R.; Gass, P. Clinical Characteristics and Prognosis of HER2-0 and HER2-Low-Positive Breast Cancer Patients: Real-World Data from Patients Treated with Neoadjuvant Chemotherapy. Cancers 2023, 15, 4678. https://doi.org/10.3390/cancers15194678
Pöschke P, Fasching PA, Adler W, Rübner M, Beckmann MW, Hack CC, Heindl F, Hartmann A, Erber R, Gass P. Clinical Characteristics and Prognosis of HER2-0 and HER2-Low-Positive Breast Cancer Patients: Real-World Data from Patients Treated with Neoadjuvant Chemotherapy. Cancers. 2023; 15(19):4678. https://doi.org/10.3390/cancers15194678
Chicago/Turabian StylePöschke, Patrik, Peter A. Fasching, Werner Adler, Matthias Rübner, Matthias W. Beckmann, Carolin C. Hack, Felix Heindl, Arndt Hartmann, Ramona Erber, and Paul Gass. 2023. "Clinical Characteristics and Prognosis of HER2-0 and HER2-Low-Positive Breast Cancer Patients: Real-World Data from Patients Treated with Neoadjuvant Chemotherapy" Cancers 15, no. 19: 4678. https://doi.org/10.3390/cancers15194678
APA StylePöschke, P., Fasching, P. A., Adler, W., Rübner, M., Beckmann, M. W., Hack, C. C., Heindl, F., Hartmann, A., Erber, R., & Gass, P. (2023). Clinical Characteristics and Prognosis of HER2-0 and HER2-Low-Positive Breast Cancer Patients: Real-World Data from Patients Treated with Neoadjuvant Chemotherapy. Cancers, 15(19), 4678. https://doi.org/10.3390/cancers15194678







