Assessment of Narrow Band Imaging Algorithm for Video Capsule Endoscopy Based on Decorrelated Color Space for Esophageal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. NBI
2.3. Parameters for Comparision
2.3.1. SSIM
2.3.2. Entropy
2.3.3. PSNR
3. Results
3.1. SSIM
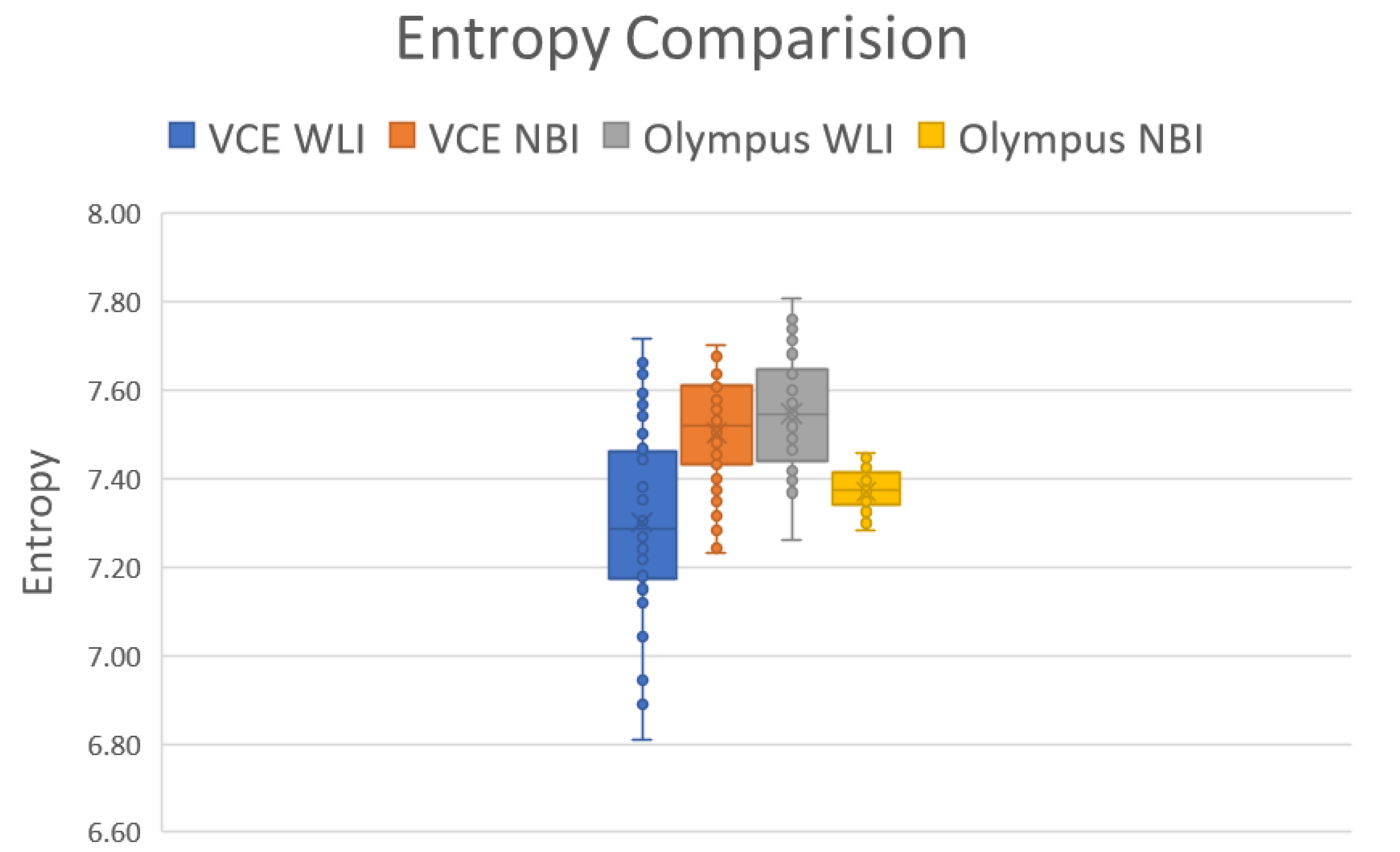
3.2. Entropy
3.3. PSNR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahafi, A.; Wang, Y.; Rasmussen, C.; Bollen, P.; Baatrup, G.; Blanes-Vidal, V.; Herp, J.; Nadimi, E. Edge artificial intelligence wireless video capsule endoscopy. Sci. Rep. 2022, 12, 13723. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, W.; Zhang, K.; Ming, F.; Yangdai, T.; Xu, T.; Shi, H.; Bao, Y.; Yao, H.; Peng, H. Novel scheme for non-invasive gut bioinformation acquisition with a magnetically controlled sampling capsule endoscope. Gut 2021, 70, 2297–2306. [Google Scholar] [CrossRef]
- Hosoe, N.; Takabayashi, K.; Ogata, H.; Kanai, T. Capsule endoscopy for small-intestinal disorders: Current status. Dig. Endosc. 2019, 31, 498–507. [Google Scholar] [CrossRef]
- Vasilakakis, M.; Koulaouzidis, A.; Marlicz, W.; Iakovidis, D. The future of capsule endoscopy in clinical practice: From diagnostic to therapeutic experimental prototype capsules. Gastroenterol. Rev. Przegląd Gastroenterol. 2020, 15, 179–193. [Google Scholar] [CrossRef]
- Noormohammadi, R.; Khaleghi, A.; Balasingham, I. Battery-free wireless communication for video capsule endoscopy. In Proceedings of the 2019 13th International Symposium on Medical Information and Communication Technology (ISMICT), Oslo, Norway, 8–10 May 2019; pp. 1–5. [Google Scholar]
- Vedaei, S.S.; Wahid, K.A. A localization method for wireless capsule endoscopy using side wall cameras and IMU sensor. Sci. Rep. 2021, 11, 11204. [Google Scholar] [CrossRef]
- Duan, Z.; Xu, L.-J.; Gao, S.; Geyi, W. Integrated design of wideband omnidirectional antenna and electronic components for wireless capsule endoscopy systems. IEEE Access 2018, 6, 29626–29636. [Google Scholar] [CrossRef]
- Jang, J.; Lee, J.; Lee, K.-R.; Lee, J.; Kim, M.; Lee, Y.; Bae, J.; Yoo, H.-J. 4-Camera VGA-resolution capsule endoscope with 80Mb/s body-channel communication transceiver and Sub-cm range capsule localization. In Proceedings of the 2018 IEEE International Solid-State Circuits Conference-(ISSCC), San Francisco, CA, USA, 11–15 February 2018; pp. 282–284. [Google Scholar]
- Almalioglu, Y.; Ozyoruk, K.B.; Gokce, A.; Incetan, K.; Gokceler, G.I.; Simsek, M.A.; Ararat, K.; Chen, R.J.; Durr, N.J.; Mahmood, F. EndoL2H: Deep super-resolution for capsule endoscopy. IEEE Trans. Med. Imaging 2020, 39, 4297–4309. [Google Scholar] [CrossRef]
- Yen, C.-T.; Lai, Z.-W.; Lin, Y.-T.; Cheng, H.-C. Optical design with narrow-band imaging for a capsule endoscope. J. Healthc. Eng. 2018, 2018, 5830759. [Google Scholar] [CrossRef]
- Marmo, R.; Rotondano, G.; Piscopo, R.; Bianco, M.A.; Siani, A.; Catalano, O.; Cipolletta, L. Capsule endoscopy versus enteroclysis in the detection of small-bowel involvement in Crohn’s disease: A prospective trial. Clin. Gastroenterol. Hepatol. 2005, 3, 772–776. [Google Scholar] [CrossRef]
- Sano, Y.; Tanaka, S.; Kudo, S.e.; Saito, S.; Matsuda, T.; Wada, Y.; Fujii, T.; Ikematsu, H.; Uraoka, T.; Kobayashi, N. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig. Endosc. 2016, 28, 526–533. [Google Scholar] [CrossRef]
- Sun, C.; Han, X.; Li, X.; Zhang, Y.; Du, X. Diagnostic performance of narrow band imaging for laryngeal cancer: A systematic review and meta-analysis. Otolaryngol. –Head Neck Surg. 2017, 156, 589–597. [Google Scholar] [CrossRef]
- Vu, A.; Farah, C.S. Narrow band imaging: Clinical applications in oral and oropharyngeal cancer. Oral Dis. 2016, 22, 383–390. [Google Scholar] [CrossRef]
- Aloisi, A.; Sonoda, Y.; Gardner, G.J.; Park, K.J.; Elliott, S.L.; Zhou, Q.C.; Iasonos, A.; Abu-Rustum, N.R. Prospective comparative study of laparoscopic narrow band imaging (NBI) versus standard imaging in gynecologic oncology. Ann. Surg. Oncol. 2018, 25, 984–990. [Google Scholar] [CrossRef]
- Silva, M.F.; Rodrigues, J.A.; Ghaderi, M.; Goncalves, L.M.; De Graaf, G.; Wolffenbuttel, R.F.; Correia, J.H. NBI optical filters in minimally invasive medical devices. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 165–171. [Google Scholar] [CrossRef]
- Kimza, H.; Jackowska, J.; Wierzbicka, M. The usefulness of the NBI–narrow band imaging for the larynx assessment. Pol. J. Otolaryngol. 2018, 72, 1–3. [Google Scholar] [CrossRef]
- White, J.R.; Sami, S.S.; Reddiar, D.; Mannath, J.; Ortiz-Fernández-Sordo, J.; Beg, S.; Scott, R.; Thiagarajan, P.; Ahmad, S.; Parra-Blanco, A. Narrow band imaging and serology in the assessment of premalignant gastric pathology. Scand. J. Gastroenterol. 2018, 53, 1611–1618. [Google Scholar] [CrossRef]
- Wisotzky, E.L.; Uecker, F.C.; Arens, P.; Dommerich, S.; Hilsmann, A.; Eisert, P. Intraoperative hyperspectral determination of human tissue properties. J. Biomed. Opt. 2018, 23, 091409. [Google Scholar] [CrossRef]
- Zhou, F.; Wu, L.; Huang, M.; Jin, Q.; Qin, Y.; Chen, J. The accuracy of magnifying narrow band imaging (ME-NBI) in distinguishing between cancerous and noncancerous gastric lesions: A meta-analysis. Medicine 2018, 97, 5851730. [Google Scholar] [CrossRef]
- Tsai, T.-J.; Mukundan, A.; Chi, Y.-S.; Tsao, Y.-M.; Wang, Y.-K.; Chen, T.-H.; Wu, I.-C.; Huang, C.-W.; Wang, H.-C. Intelligent Identification of Early Esophageal Cancer by Band-Selective Hyperspectral Imaging. Cancers 2022, 14, 4292. [Google Scholar] [CrossRef]
- Gono, K.; Obi, T.; Yamaguchi, M.; Ohyama, N.; Machida, H.; Sano, Y.; Yoshida, S.; Hamamoto, Y.; Endo, T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J. Biomed. Opt. 2004, 9, 568–577. [Google Scholar] [CrossRef]
- Piazza, C.; Dessouky, O.; Peretti, G.; Cocco, D.; De Benedetto, L.; Nicolai, P. Narrow-band imaging: A new tool for evaluation of head and neck squamous cell carcinomas. Review of the literature. Acta Otorhinolaryngol. Ital. 2008, 28, 49. [Google Scholar] [PubMed]
- Pasha, S.F.; Leighton, J.A.; Das, A.; Gurudu, S.; Sharma, V.K. Narrow band imaging (NBI) and white light endoscopy (WLE) have a comparable yield for detection of colon polyps in patients undergoing screening or surveillance colonoscopy: A meta-analysis. Gastrointest. Endosc. 2009, 69, AB363. [Google Scholar] [CrossRef]
- Fang, Y.-J.; Mukundan, A.; Tsao, Y.-M.; Huang, C.-W.; Wang, H.-C. Identification of Early Esophageal Cancer by Semantic Segmentation. J. Pers. Med. 2022, 12, 1204. [Google Scholar] [CrossRef] [PubMed]
- Puvanakrishnan, P.; Diagaradjane, P.; Kazmi, S.S.; Dunn, A.K.; Krishnan, S.; Tunnell, J.W. Narrow band imaging of squamous cell carcinoma tumors using topically delivered anti-EGFR antibody conjugated gold nanorods. Lasers Surg. Med. 2012, 44, 310–317. [Google Scholar] [CrossRef]
- Tan, N.C.-W.; Herd, M.K.; Brennan, P.A.; Puxeddu, R. The role of narrow band imaging in early detection of head and neck cancer. Br. J. Oral Maxillofac. Surg. 2012, 50, 132–136. [Google Scholar] [CrossRef]
- Watanabe, A.; Taniguchi, M.; Tsujie, H.; Hosokawa, M.; Fujita, M.; Sasaki, S. The value of narrow band imaging for early detection of laryngeal cancer. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 1017–1023. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Dong, Z.; Du, S.; Ji, G.; Yan, J.; Yang, J.; Wang, Q.; Feng, C.; Phillips, P. Feed-forward neural network optimized by hybridization of PSO and ABC for abnormal brain detection. Int. J. Imaging Syst. Technol. 2015, 25, 153–164. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Sui, Y.; Yang, M.; Liu, B.; Cheng, H.; Sun, J.; Jia, W.; Phillips, P.; Gorriz, J.M. Multivariate approach for Alzheimer’s disease detection using stationary wavelet entropy and predator-prey particle swarm optimization. J. Alzheimer’s Dis. 2018, 65, 855–869. [Google Scholar] [CrossRef]
- Zhang, Y.-D.; Zhao, G.; Sun, J.; Wu, X.; Wang, Z.-H.; Liu, H.-M.; Govindaraj, V.V.; Zhan, T.; Li, J. Smart pathological brain detection by synthetic minority oversampling technique, extreme learning machine, and Jaya algorithm. Multimed. Tools Appl. 2018, 77, 22629–22648. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Mukundan, A.; Chung, C.-S.; Chen, Y.-H.; Wang, Y.-K.; Chen, T.-H.; Tseng, Y.-S.; Huang, C.-W.; Wu, I.-C.; Wang, H.-C. Hyperspectral Imaging Combined with Artificial Intelligence in the Early Detection of Esophageal Cancer. Cancers 2021, 13, 4593. [Google Scholar] [CrossRef]
- Lee, C.; Chang, C.; Lee, Y.; Tai, C.; Wang, W.; Tseng, P.-H.; Hwang, J.; Hwang, T.; Wang, C.; Lin, J. Narrow-band imaging with magnifying endoscopy for the screening of esophageal cancer in patients with primary head and neck cancers. Endoscopy 2010, 42, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Inoue, H.; Usui, S.; Satodate, H.; Fukami, N.; Kudo, S.-e. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest. Endosc. 2004, 59, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Thamir, N.N.; Mohammed, F.G. Early Esophageal Cancer detection using Deep learning Techniques. J. Phys. Conf. Ser. 2021, 1963, 012066. [Google Scholar] [CrossRef]
- Reinhard, E.; Adhikhmin, M.; Gooch, B.; Shirley, P. Color transfer between images. IEEE Comput. Graph. Appl. 2001, 21, 34–41. [Google Scholar] [CrossRef]
- Ruderman, D.L.; Cronin, T.W.; Chiao, C.C. Statisticsof Cone Responses to Natural Images: Implications forVisual Coding. J. Opt. Soc. Am. 1998, 15, 2036–2045. [Google Scholar] [CrossRef]
- Brunet, D.; Vrscay, E.R.; Wang, Z. On the mathematical properties of the structural similarity index. IEEE Trans. Image Process. 2011, 21, 1488–1499. [Google Scholar] [CrossRef]
- Wang, Z.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P. Image quality assessment: From error visibility to structural similarity. IEEE Trans. Image Process. 2004, 13, 600–612. [Google Scholar] [CrossRef]
- Hore, A.; Ziou, D. Image quality metrics: PSNR vs. SSIM. In Proceedings of the 2010 20th International Conference on Pattern Recognition, Istanbul, Turkey, 23–26 August 2010; pp. 2366–2369. [Google Scholar]
- Dosselmann, R.; Yang, X.D. A comprehensive assessment of the structural similarity index. Signal Image Video Process. 2011, 5, 81–91. [Google Scholar] [CrossRef]
- Sara, U.; Akter, M.; Uddin, M.S. Image quality assessment through FSIM, SSIM, MSE and PSNR—A comparative study. J. Comput. Commun. 2019, 7, 8–18. [Google Scholar] [CrossRef]
- Setiadi, D.R.I.M. PSNR vs SSIM: Imperceptibility quality assessment for image steganography. Multimed. Tools Appl. 2021, 80, 8423–8444. [Google Scholar] [CrossRef]
- Ndajah, P.; Kikuchi, H.; Yukawa, M.; Watanabe, H.; Muramatsu, S. SSIM image quality metric for denoised images. In Proceedings of the 3rd WSEAS International Conference on Visualization, Imaging and Simulation, Faro, Portugal, 3–5 November 2010; pp. 53–58. [Google Scholar]
- Nilsson, J.; Akenine-Möller, T. Understanding ssim. arXiv 2020, arXiv:2006.13846. [Google Scholar]
- Tsai, D.-Y.; Lee, Y.; Matsuyama, E. Information Entropy Measure for Evaluation of Image Quality. J. Digit. Imaging 2008, 21, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Pal, N.R.; Pal, S.K. Entropy: A new definition and its applications. IEEE Trans. Syst. Man Cybern. 1991, 21, 1260–1270. [Google Scholar] [CrossRef]
- Winkler, S.; Mohandas, P. The Evolution of Video Quality Measurement: From PSNR to Hybrid Metrics. IEEE Trans. Broadcast. 2008, 54, 660–668. [Google Scholar] [CrossRef]
- Huynh-Thu, Q.; Ghanbari, M. The accuracy of PSNR in predicting video quality for different video scenes and frame rates. Telecommun. Syst. 2012, 49, 35–48. [Google Scholar] [CrossRef]
- Tanchenko, A. Visual-PSNR measure of image quality. J. Vis. Commun. Image Represent. 2014, 25, 874–878. [Google Scholar] [CrossRef]
- Deshpande, R.G.; Ragha, L.L.; Sharma, S.K. Video quality assessment through psnr estimation for different compression standards. Indones. J. Electr. Eng. Comput. Sci. 2018, 11, 918–924. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.-Y.; Fang, Y.-J.; Karmakar, R.; Mukundan, A.; Tsao, Y.-M.; Huang, C.-W.; Wang, H.-C. Assessment of Narrow Band Imaging Algorithm for Video Capsule Endoscopy Based on Decorrelated Color Space for Esophageal Cancer. Cancers 2023, 15, 4715. https://doi.org/10.3390/cancers15194715
Yang K-Y, Fang Y-J, Karmakar R, Mukundan A, Tsao Y-M, Huang C-W, Wang H-C. Assessment of Narrow Band Imaging Algorithm for Video Capsule Endoscopy Based on Decorrelated Color Space for Esophageal Cancer. Cancers. 2023; 15(19):4715. https://doi.org/10.3390/cancers15194715
Chicago/Turabian StyleYang, Kai-Yao, Yu-Jen Fang, Riya Karmakar, Arvind Mukundan, Yu-Ming Tsao, Chien-Wei Huang, and Hsiang-Chen Wang. 2023. "Assessment of Narrow Band Imaging Algorithm for Video Capsule Endoscopy Based on Decorrelated Color Space for Esophageal Cancer" Cancers 15, no. 19: 4715. https://doi.org/10.3390/cancers15194715
APA StyleYang, K.-Y., Fang, Y.-J., Karmakar, R., Mukundan, A., Tsao, Y.-M., Huang, C.-W., & Wang, H.-C. (2023). Assessment of Narrow Band Imaging Algorithm for Video Capsule Endoscopy Based on Decorrelated Color Space for Esophageal Cancer. Cancers, 15(19), 4715. https://doi.org/10.3390/cancers15194715









