Predictors of Non-Sentinel Lymph Node Metastasis in Patients with Positive Sentinel Lymph Node in Early-Stage Cervical Cancer: A SENTICOL GROUP Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
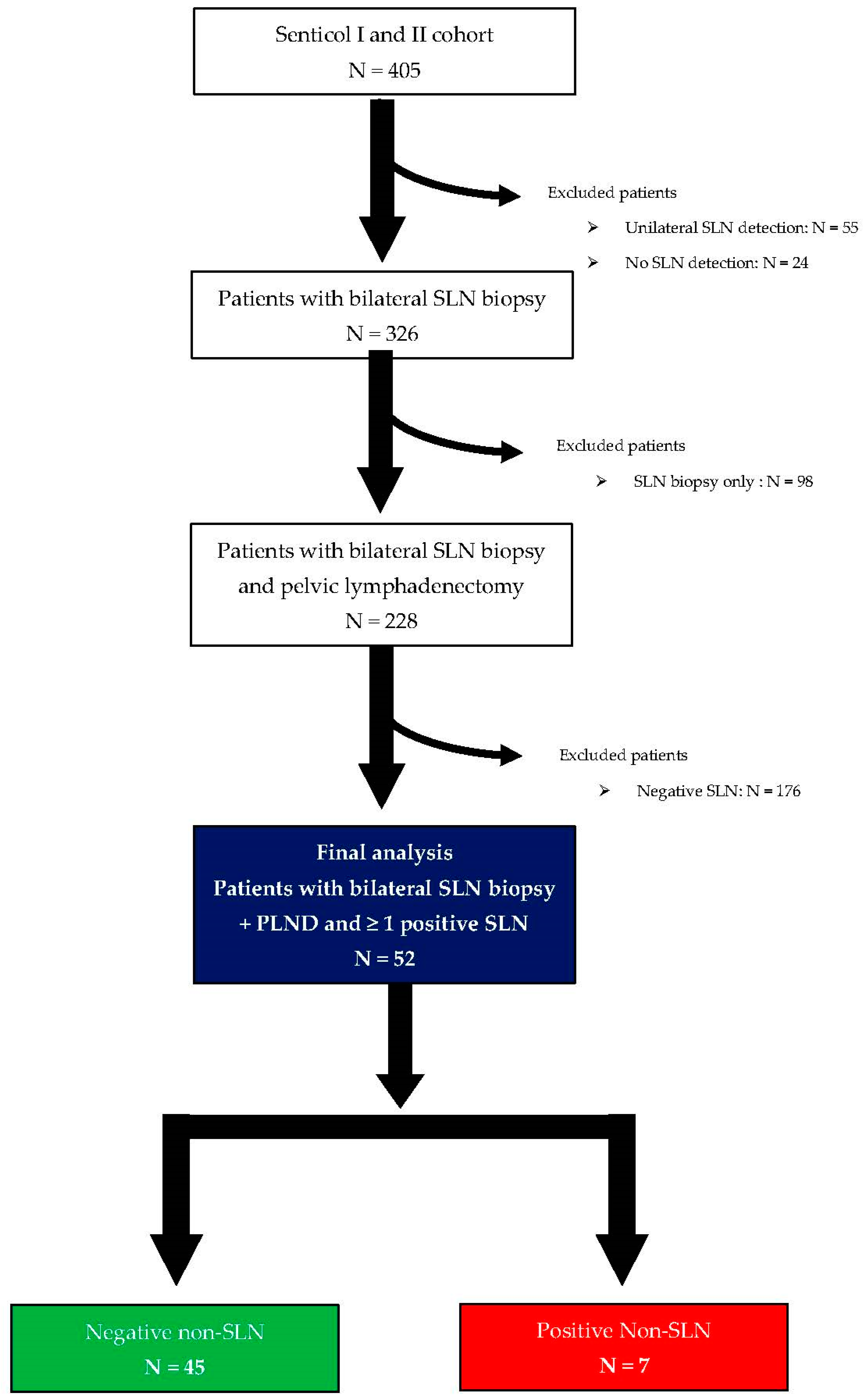
2.1. Cohort Description
2.2. Data Analysis
2.3. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjose, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [PubMed]
- Margolis, B.; Cagle-Colon, K.; Chen, L.; Tergas, A.I.; Boyd, L.; Wright, J.D. Prognostic significance of lymphovascular space invasion for stage IA1 and IA2 cervical cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 735–743. [Google Scholar] [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Matsuo, K.; Huang, Y.; Tergas, A.I.; Hou, J.Y.; Khoury-Collado, F.; Clair, C.M.S.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L. Prognostic Performance of the 2018 International Federation of Gynecology and Obstetrics Cervical Cancer Staging Guidelines. Obstet. Gynecol. 2019, 134, 49–57. [Google Scholar] [CrossRef]
- Cibula, D.; Potter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie Meder, C.; Kohler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients With Cervical Cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2018, 28, 641–655. [Google Scholar] [CrossRef]
- Tax, C.; Rovers, M.M.; de Graaf, C.; Zusterzeel, P.L.; Bekkers, R.L. The sentinel node procedure in early stage cervical cancer, taking the next step; a diagnostic review. Gynecol. Oncol. 2015, 139, 559–567. [Google Scholar] [CrossRef]
- Cibula, D.; McCluggage, W.G. Sentinel lymph node (SLN) concept in cervical cancer: Current limitations and unanswered questions. Gynecol. Oncol 2019, 152, 202–207. [Google Scholar] [CrossRef]
- Tantari, M.; Bogliolo, S.; Morotti, M.; Balaya, V.; Bouttitie, F.; Buenerd, A.; Magaud, L.; Lecuru, F.; Guani, B.; Mathevet, P.; et al. Lymph Node Involvement in Early-Stage Cervical Cancer: Is Lymphangiogenesis a Risk Factor? Results from the MICROCOL Study. Cancers 2022, 14, 212. [Google Scholar] [CrossRef]
- Lecuru, F.; Mathevet, P.; Querleu, D.; Leblanc, E.; Morice, P.; Darai, E.; Marret, H.; Magaud, L.; Gillaizeau, F.; Chatellier, G.; et al. Bilateral negative sentinel nodes accurately predict absence of lymph node metastasis in early cervical cancer: Results of the SENTICOL study. J. Clin. Oncol. 2011, 29, 1686–1691. [Google Scholar] [CrossRef]
- Mathevet, P.; Lecuru, F.; Uzan, C.; Boutitie, F.; Magaud, L.; Guyon, F.; Querleu, D.; Fourchotte, V.; Baron, M.; Bats, A.S.; et al. Sentinel lymph node biopsy and morbidity outcomes in early cervical cancer: Results of a multicentre randomised trial (SENTICOL-2). Eur. J. Cancer 2021, 148, 307–315. [Google Scholar] [CrossRef]
- Mathevet, P.; Guani, B.; Ciobanu, A.; Lamarche, E.M.; Boutitie, F.; Balaya, V.; Lecuru, F. Histopathologic Validation of the Sentinel Node Technique for Early-Stage Cervical Cancer Patients. Ann. Surg. Oncol. 2021, 28, 3629–3635. [Google Scholar] [CrossRef]
- Balaya, V.; Mathevet, P.; Magaud, L.; Bonsang-Kitzis, H.; Delomenie, M.; Montero Macias, R.; Ngô, C.; Bats, A.S.; Lécuru, F. Predictive factors of unexpected lymphatic drainage pathways in early-stage cervical cancer. Gynecol. Oncol. 2019, 154, 102–109. [Google Scholar] [CrossRef]
- Gianoni, M.; Mathevet, P.; Uzan, C.; Bats, A.S.; Magaud, L.; Boutitie, F.; Lécuru, F. Does the Sentinel Lymph Node Sampling Alone Improve Quality of Life in Early Cervical Cancer Management? Front. Surg. 2020, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Balaya, V.; Bresset, A.; Guani, B.; Magaud, L.; Montero Macias, R.; Delomenie, M.; Bonsang-Kitzis, H.; Ngô, C.; Bats, A.S.; Mathevet, P.; et al. Risk factors for failure of bilateral sentinel lymph node mapping in early-stage cervical cancer. Gynecol. Oncol. 2020, 156, 93–99. [Google Scholar] [CrossRef]
- Favre, G.; Guani, B.; Balaya, V.; Magaud, L.; Lecuru, F.; Mathevet, P. Sentinel Lymph-Node Biopsy in Early-Stage Cervical Cancer: The 4-Year Follow-Up Results of the Senticol 2 Trial. Front. Oncol. 2020, 10, 621518. [Google Scholar] [CrossRef]
- Balaya, V.; Guani, B.; Morice, P.; Querleu, D.; Fourchotte, V.; Leblanc, E.; Daraï, E.; Baron, M.; Marret, H.; Levêque, J.; et al. Long-term oncological safety of sentinel lymph node biopsy in early-stage cervical cancer: A post-hoc analysis of SENTICOL I and SENTICOL II cohorts. Gynecol. Oncol. 2022, 164, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 64–84. [Google Scholar] [CrossRef] [PubMed]
- Guani, B.; Mathevet, P. ASO Author Reflections: Validation Based on Low-Volume Metastasis of the Sentinel Lymph Node Biopsy in Early-Stage Cervical Cancer. Ann. Surg. Oncol. 2020, 28, 3636. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Dostalek, L.; Hillemanns, P.; Scambia, G.; Jarkovsky, J.; Persson, J.; Raspagliesi, F.; Novak, Z.; Jaeger, A.; Capilna, M.E.; et al. Completion of radical hysterectomy does not improve survival of patients with cervical cancer and intraoperatively detected lymph node involvement: ABRAX international retrospective cohort study. Eur. J. Cancer 2021, 143, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Morita, K. The prognostic significance of number of positive nodes in cervical carcinoma stages IB, IIA, and IIB. Cancer 1990, 65, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Benedetti-Panici, P.; Maneschi, F.; Scambia, G.; Greggi, S.; Cutillo, G.; D’Andrea, G.; Rabitti, C.; Coronetta, F.; Capelli, A.; Mancuso, S. Lymphatic spread of cervical cancer: An anatomical and pathological study based on 225 radical hysterectomies with systematic pelvic and aortic lymphadenectomy. Gynecol. Oncol. 1996, 62, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lee, K.B.; Lee, S.K.; Park, C.Y. Pattern of lymph node metastasis and the optimal extent of pelvic lymphadenectomy in FIGO stage IB cervical cancer. J. Obstet. Gynaecol. Res. 2007, 33, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Kocian, R.; Plaikner, A.; Jarkovsky, J.; Klat, J.; Zapardiel, I.; Pilka, R.; Torne, A.; Sehnal, B.; Ostojich, M.; et al. Sentinel lymph node mapping and intraoperative assessment in a prospective, international, multicentre, observational trial of patients with cervical cancer: The SENTIX trial. Eur. J. Cancer 2020, 137, 69–80. [Google Scholar] [CrossRef]
- Bader, A.A.; Winter, R.; Haas, J.; Tamussino, K.F. Where to look for the sentinel lymph node in cervical cancer. Am. J. Obstet. Gynecol. 2007, 197, 678.e1–678.e7. [Google Scholar] [CrossRef]
- Cibula, D.; Zikan, M.; Slama, J.; Fischerova, D.; Kocian, R.; Germanova, A.; Burgetova, A.; Dusek, L.; Dundr, P.; Gregova, M.; et al. Risk of micrometastases in non-sentinel pelvic lymph nodes in cervical cancer. Gynecol. Oncol. 2016, 143, 83–86. [Google Scholar] [CrossRef]
- Altgassen, C.; Hertel, H.; Brandstädt, A.; Köhler, C.; Dürst, M.; Schneider, A.; Group, A.G.O.S. Multicenter validation study of the sentinel lymph node concept in cervical cancer: AGO Study Group. J. Clin. Oncol. 2008, 26, 2943–2951. [Google Scholar] [CrossRef]
- Diaz, J.P.; Gemignani, M.L.; Pandit-Taskar, N.; Park, K.J.; Murray, M.P.; Chi, D.S.; Sonoda, Y.; Barakat, R.R.; Abu-Rustum, N.R. Sentinel lymph node biopsy in the management of early-stage cervical carcinoma. Gynecol. Oncol. 2011, 120, 347–352. [Google Scholar] [CrossRef]
- Roy, M.; Bouchard-Fortier, G.; Popa, I.; Grégoire, J.; Renaud, M.-C.; Têtu, B.; Plante, M. Value of sentinel node mapping in cancer of the cervix. Gynecol. Oncol. 2011, 122, 269–274. [Google Scholar] [CrossRef]
- Vercellino, G.F.; Erdemoglu, E.; Lichtenberg, P.; Muallem, M.Z.; Richter, R.; Abu-Rustum, N.R.; Plante, M.; Lécuru, F.; Greggi, S.; Monk, B.J.; et al. A GCIG international survey: Clinical practice patterns of sentinel lymph node biopsies in cervical cancer. Arch. Gynecol. Obstet. 2019, 300, 191–199. [Google Scholar] [CrossRef]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2023, 33, 649–666. [Google Scholar] [CrossRef]
- Balaya, V.; Guani, B.; Mereaux, J.; Magaud, L.; Pache, B.; Bonsang-Kitzis, H.; Ngô, C.; Desseauve, D.; Mathevet, P.; Lécuru, F.; et al. Can Conization Specimens Predict Sentinel Lymph Node Status in Early-Stage Cervical Cancer? A SENTICOL Group Study. Cancers 2021, 13, 5423. [Google Scholar] [CrossRef] [PubMed]
- Barranger, E.; Cortez, A.; Commo, F.; Marpeau, O.; Uzan, S.; Darai, E.; Callard, P. Histopathological validation of the sentinel node concept in cervical cancer. Ann. Oncol. 2004, 15, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Niikura, H.; Yoshinaga, K.; Nagase, S.; Takano, T.; Ito, K.; Yaegashi, N. Detection of micrometastases in cervical cancer with a system that evaluates both sentinel and nonsentinel lymph nodes. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2009, 19, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Guani, B.; Mahiou, K.; Crestani, A.; Cibula, D.; Buda, A.; Gaillard, T.; Mathevet, P.; Kocian, R.; Sniadecki, M.; Wydra, D.G.; et al. Clinical impact of low-volume lymph node metastases in early-stage cervical cancer: A comprehensive meta-analysis. Gynecol. Oncol. 2022, 164, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Kocian, R.; Slama, J.; Fischerova, D.; Germanova, A.; Burgetova, A.; Dusek, L.; Dundr, P.; Nemejcova, K.; Jarkovsky, J.; Sebestova, S.; et al. Micrometastases in Sentinel Lymph Nodes Represent a Significant Negative Prognostic Factor in Early-Stage Cervical Cancer: A Single-Institutional Retrospective Cohort Study. Cancers 2020, 12, 1438. [Google Scholar] [CrossRef]
- Popa, I.; Plante, M.; Renaud, M.-C.; Roy, M.; Têtu, B. Negative sentinel lymph node accurately predicts negative status of pelvic lymph nodes in uterine cervix carcinoma. Gynecol. Oncol. 2006, 103, 649–653. [Google Scholar] [CrossRef]
- Parpinel, G.; Laas-Faron, E.; Balaya, V.; Guani, B.; Zola, P.; Mathevet, P.; Paoletti, X.; Lecuru, F.R. Survival after sentinel lymph node biopsy for early cervical cancers: A systematic review and meta-analysis. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2023. [Google Scholar] [CrossRef]
- Chiyoda, T.; Yoshihara, K.; Kagabu, M.; Nagase, S.; Katabuchi, H.; Mikami, M.; Tabata, T.; Hirashima, Y.; Kobayashi, Y.; Kaneuchi, M.; et al. Sentinel node navigation surgery in cervical cancer: A systematic review and metaanalysis. Int. J. Clin. Oncol. 2022, 27, 1247–1255. [Google Scholar] [CrossRef]
- Rychlik, A.; Angeles, M.A.; Migliorelli, F.; Croce, S.; Mery, E.; Martinez, A.; Ferron, G.; Guyon, F.; Querleu, D. Frozen section examination of sentinel lymph nodes can be used as a decisional tool in the surgical management of early cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 358–363. [Google Scholar] [CrossRef]
- Balaya, V.; Guani, B.; Benoit, L.; Magaud, L.; Bonsang-Kitzis, H.; Ngô, C.; Le Frère-Belda, M.A.; Mathevet, P.; Lécuru, F. Diagnostic value of frozen section examination of sentinel lymph nodes in early-stage cervical cancer at the time of ultrastaging. Gynecol. Oncol. 2020, 158, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, N.; Pedone Anchora, L.; Zannoni, G.F.; Santoro, A.; Valente, M.; Inzani, F.; Gallotta, V.; Conte, C.; Chiantera, V.; Fanfani, F.; et al. Role of one-step nucleic acid amplification (OSNA) to detect sentinel lymph node low-volume metastasis in early-stage cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Abu-Rustum, N.R.; Dusek, L.; Zikan, M.; Zaal, A.; Sevcik, L.; Kenter, G.G.; Querleu, D.; Jach, R.; Bats, A.S.; et al. Prognostic significance of low volume sentinel lymph node disease in early-stage cervical cancer. Gynecol. Oncol. 2012, 124, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Touhami, O.; Trinh, X.B.; Gregoire, J.; Sebastianelli, A.; Renaud, M.C.; Grondin, K.; Plante, M. Predictors of non-sentinel lymph node (non-SLN) metastasis in patients with sentinel lymph node (SLN) metastasis in endometrial cancer. Gynecol. Oncol. 2015, 138, 41–45. [Google Scholar] [CrossRef]
- Baiocchi, G.; Mantoan, H.; Gonçalves, B.T.; Faloppa, C.C.; Kumagai, L.Y.; Badiglian-Filho, L.; da Costa, A.; De Brot, L. Size of Sentinel Node Metastasis Predicts Non-sentinel Node Involvement in Endometrial Cancer. Ann. Surg. Oncol. 2020, 27, 1589–1594. [Google Scholar] [CrossRef]
- Dostálek, L.; Benešová, K.; Klát, J.; Kim, S.H.; Falconer, H.; Kostun, J.; Dos Reis, R.; Zapardiel, I.; Landoni, F.; Ortiz, D.I.; et al. Stratification of lymph node metastases as macrometastases, micrometastases, or isolated tumor cells has no clinical implication in patients with cervical cancer: Subgroup analysis of the SCCAN project. Gynecol. Oncol. 2023, 168, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Diniz, T.P.; Faloppa, C.C.; Mantoan, H.; Goncalves, B.T.; Kumagai, L.Y.; Menezes, A.N.O.; Badiglian-Filho, L.; Guimaraes, A.P.G.; da Costa, A.; De Brot, L.; et al. Pathological factors associated with non-sentinel lymph node metastasis in early stage cervical cancer. J. Surg. Oncol. 2021, 123, 1115–1120. [Google Scholar] [CrossRef]
- Strnad, P.; Robova, H.; Skapa, P.; Pluta, M.; Hrehorcak, M.; Halaska, M.; Rob, L. A prospective study of sentinel lymph node status and parametrial involvement in patients with small tumour volume cervical cancer. Gynecol. Oncol. 2008, 109, 280–284. [Google Scholar] [CrossRef]
- Balaya, V.; Bresset, A.; Guani, B.; Benoit, L.; Magaud, L.; Bonsang-Kitzis, H.; Ngo, C.; Mathevet, P.; Lécuru, F.; SENTICOL Group. Pre-operative surgical algorithm: Sentinel lymph node biopsy as predictor of parametrial involvement in early-stage cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 1317–1325. [Google Scholar] [CrossRef]
- Bizzarri, N.; Arciuolo, D.; Certelli, C.; Pedone Anchora, L.; Gallotta, V.; Teodorico, E.; Carbone, M.V.; Piermattei, A.; Fanfani, F.; Fagotti, A.; et al. Ultrastaging of the Parametrium in Cervical Cancer: A Clinicopathological Study. Cancers 2023, 15, 1099. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Xu, T.; Yuan, L.; Yang, X. Sentinel lymph node mapping in early-stage cervical cancer: Meta-analysis. Medicine 2021, 100, e27035. [Google Scholar] [CrossRef]
- Cao, L.; Kong, W.; Li, J.; Song, D.; Jin, B.; Liu, T.; Han, C. Analysis of Lymph Node Metastasis and Risk Factors in 975 Patients with FIGO 2009 Stage IA–IIA Cervical Cancer. Gynecol. Obstet. Investig. 2023, 88, 30–36. [Google Scholar] [CrossRef]
- Zhao, J.; Cai, J.; Wang, H.; Dong, W.; Zhang, Y.; Wang, S.; He, X.; Sun, S.; Huang, Y.; Huang, B.; et al. Region-specific Risk Factors for Pelvic Lymph Node Metastasis in Patients with Stage IB1 Cervical Cancer. J. Cancer 2021, 12, 2624–2632. [Google Scholar] [CrossRef] [PubMed]
- Balaya, V.; Guani, B.; Magaud, L.; Bonsang-Kitzis, H.; Ngô, C.; Mathevet, P.; Lécuru, F.; on behalf of the SENTICOL Group. Validation of the 2018 FIGO Classification for Cervical Cancer: Lymphovascular Space Invasion Should Be Considered in IB1 Stage. Cancers 2020, 12, 3554. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, N.A.; Kamaluddin, M.A.; Adon, M.Y.; Noh, M.A.; Bakhtiar, M.F.; Ibrahim Tamim, N.S.; Mahmud, S.H.; Aris, T. Survival rates of cervical cancer patients in Malaysia. Asian Pac. J. Cancer Prev. 2015, 16, 3067–3072. [Google Scholar] [CrossRef]
- Brun, J.L.; Stoven-Camou, D.; Trouette, R.; Lopez, M.; Chene, G.; Hocké, C. Survival and prognosis of women with invasive cervical cancer according to age. Gynecol. Oncol. 2003, 91, 395–401. [Google Scholar] [CrossRef]
- Guani, B.; Dorez, M.; Magaud, L.; Buenerd, A.; Lecuru, F.; Mathevet, P. Impact of micrometastasis or isolated tumor cells on recurrence and survival in patients with early cervical cancer: SENTICOL Trial. Int. J. Gynecol. Cancer 2019, 29, 447–452. [Google Scholar] [CrossRef]
- Quinn, B.A.; Deng, X.; Colton, A.; Bandyopadhyay, D.; Carter, J.S.; Fields, E.C. Increasing age predicts poor cervical cancer prognosis with subsequent effect on treatment and overall survival. Brachytherapy 2019, 18, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.J.; Park, H.; Yang, K.M.; Kim, T.J.; Lim, K.T.; Shim, J.U.; Park, C.T.; Lee, K.H. Detection of sentinel lymph nodes in patients with early stage cervical cancer. J. Korean Med. Sci. 2007, 22, 105–109. [Google Scholar] [CrossRef]
- González-Loyola, A.; Petrova, T.V. Development and aging of the lymphatic vascular system. Adv. Drug Deliv. Rev. 2021, 169, 63–78. [Google Scholar] [CrossRef]
- Lecuru, F.R.; McCormack, M.; Hillemanns, P.; Anota, A.; Leitao, M.; Mathevet, P.; Zweemer, R.; Fujiwara, K.; Zanagnolo, V.; Zahl Eriksson, A.G.; et al. SENTICOL III: An international validation study of sentinel node biopsy in early cervical cancer. A GINECO, ENGOT, GCIG and multicenter study. Int. J. Gynecol. Cancer 2019, 29, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Huang, H.; Xian, B.; Li, J.; Wang, P.; Zhao, W.; Chen, X.; Xie, X.; Wang, C.; Kong, B.; et al. Sentinel lymph node biopsy versus pelvic lymphadenectomy in early-stage cervical cancer: A multi-center randomized trial (PHENIX/CSEM 010). Int. J. Gynecol. Cancer 2020, 30, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
| Predictive Variable | Total Population N = 52 | |
|---|---|---|
| n Mean ± SD | [%] [Range] | |
| Age [years] | ||
| Mean | 42.2 ± 10.4 | [25–77] |
| BMI [kg/m2] | ||
| Mean | 23.1 ± 4.77 | [17.0–38.8] |
| Parity status | ||
| 0 | 14 | 26.9 |
| ≥1 | 38 | 73.1 |
| Histology | ||
| Squamous cell carcinoma | 42 | 80.8 |
| Adenocarcinoma | 10 | 19.2 |
| Preoperative brachytherapy | ||
| Yes | 10 | 23.3 |
| No | 33 | 76.7 |
| Not specified | 9 | |
| Preoperative conization | ||
| Yes | 22 | 42.3 |
| No | 30 | 57.7 |
| Clinical 2018 FIGO stage | ||
| IA | 3 | 5.8 |
| IB1 | 35 | 67.3 |
| IB2 | 13 | 25.0 |
| IIA | 1 | 1.9 |
| SLN status | ||
| Macrometastases | 24 | 46.2 |
| Micrometastases | 14 | 26.9 |
| ITCs | 14 | 26.9 |
| Bilateral SLN involvement | ||
| Yes | 11 | 21.2 |
| No | 41 | 78.8 |
| Number of positive SLN | 1.36 ± 0.66 | [1–4] |
| Tumor size | ||
| Mean (mm) | 22.8 ± 13.4 | [4–70] |
| LVSI | ||
| Yes | 22 | 42.3 |
| No | 30 | 57.7 |
| Parametrial invasion | ||
| Yes | 6 | 11.5 |
| No | 46 | 88.5 |
| Vaginal invasion | ||
| Yes | 5 | 9.6 |
| No | 47 | 90.4 |
| Variable | Overall SLN | Overall Positive SLN | MAC | MIC | ITCs | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | [%] | n | [%] | n | [%] | n | [%] | n | [%] | ||
| Topography | |||||||||||
| Interiliac/External iliac area | 141 | 79.7 | 58 | 87.9 | 25 | 83.3 | 17 | 89.5 | 16 | 94.1 | 0.74 |
| Common iliac area | 18 | 10.2 | 3 | 4.6 | - | - | 2 | 10.5 | 1 | 5.9 | |
| Parametrial area | 8 | 4.5 | 3 | 4.6 | 3 | 10 | - | - | - | - | |
| Promontory area | 6 | 3.4 | 1 | 1.5 | 1 | 3.3 | - | - | - | - | |
| Paraaortic area | 4 | 2.3 | 1 | 1.5 | 1 | 3.3 | - | - | - | - | |
| Total | 177 | 100 | 66 | 100 | 30 | 100 | 19 | 100 | 17 | 100 | |
| Patient | Age | Histologic Type | Clinical 2018 FIGO Stage | Presence of LVSI | Parametrial Involvement | Number of Metastatic SLNs/Total SLNs | Location of Involved SLNs | Type of SLNs Involvement | Number of Metastatic Non-SLNs/Total Non-SLNs | Location of Involved Non-SLNs | Type of Non-SLNs Involvement |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77 | Squamous cell carcinoma | IB2 | Yes | Yes | 1/2 | Left interiliac | ITCs | 4/25 | Right interiliac (3) Right common iliac (1) | MIC and ITCs |
| 2 | 49 | Squamous cell carcinoma | IB2 | Yes | Yes | 3/5 | Right interiliac Left Parametrium Left interiliac | ITCs MAC MAC | 1/19 | Right interiliac | MAC |
| 3 | 45 | Squamous cell carcinoma | IB2 | Yes | Yes | 2/2 | Right interiliac Left interiliac | ITCs MAC | 1/7 | Left interiliac | MAC |
| 4 | 48 | Squamous cell carcinoma | IB1 | Yes | No | 2/9 | Right interiliac Left interiliac | MIC MIC | 1/18 | Right interiliac | MAC |
| 5 | 42 | Squamous cell carcinoma | IB1 | Yes | No | 1/2 | Right interiliac | MAC | 2/39 | - | - |
| 6 | 49 | Squamous cell carcinoma | IB1 | No | No | 1/2 | Left interiliac | MIC | 1/26 | Left paraaortic | MAC |
| 7 | 53 | Squamous cell carcinoma | IB2 | Yes | No | 1/3 | Right interiliac | MAC | 6/26 | Left interiliac (1) Right interiliac (5) | MAC MAC |
| Predictive Variable | Patient with Negative Non-SLN N = 45 | Patient with Positive Non-SLN N = 7 | p | ||
|---|---|---|---|---|---|
| n Mean ± SD | [%] [Range] | n Mean ± SD | [%] [Range] | ||
| Age [years] | |||||
| Mean | 40.8 ± 9.5 | [25–64] | 51.9 ± 11.6 | [42–77] | 0.01 |
| BMI [kg/m2] | |||||
| Mean | 22.6 ± 4.2 | [17.0–33.7] | 26.5 ± 7.0 | [19.3–38.8] | 0.11 |
| Parity status | |||||
| 0 | 13 | 28.9 | 1 | 14.3 | 0.66 |
| ≥1 | 32 | 71.1 | 6 | 85.7 | |
| Histology | |||||
| Squamous cell carcinoma | 35 | 77.8 | 7 | 100.0 | 0.32 |
| Adenocarcinoma | 10 | 22.2 | 0 | 0.0 | |
| Preoperative brachytherapy | |||||
| Yes | 8 | 21.1 | 2 | 40.0 | 0.57 |
| No | 30 | 78.9 | 3 | 60.0 | |
| Not specified | 7 | 2 | |||
| Preoperative conization | |||||
| Yes | 20 | 44.4 | 2 | 28.6 | 0.68 |
| No | 25 | 55.6 | 5 | 9.6 | |
| Clinical 2018 FIGO stage | |||||
| IA | 3 | 6.7 | 0 | 0.0 | 0.23 |
| IB1 | 32 | 71.1 | 3 | 42.9 | |
| IB2 | 9 | 20.0 | 4 | 57.1 | |
| IIA | 1 | 2.2 | 0 | 0.0 | |
| SLN status | |||||
| Macrometastases | 20 | 44.4 | 4 | 57.1 | 0.87 |
| Micrometastases | 12 | 26.7 | 2 | 28.6 | |
| ITCs | 13 | 28.9 | 1 | 14.3 | |
| Bilateral SLN involvement | |||||
| Yes | 8 | 17.8 | 3 | 42.9 | 0.15 |
| No | 37 | 82.2 | 4 | 57.1 | |
| Number of positive SLN | 1.29 ± 0.55 | [1–3] | 1.86 ± 1.07 | [1–4] | 0.07 |
| Tumor size | |||||
| Mean (mm) | 21.9 ± 13.0 | [4–70] | 28.1 ± 15.4 | [7–51] | 0.20 |
| LVSI | |||||
| Yes | 16 | 35.6 | 6 | 85.7 | 0.03 |
| No | 29 | 64.4 | 1 | 14.3 | |
| Parametrial invasion | |||||
| Yes | 3 | 6.7 | 3 | 42.9 | 0.03 |
| No | 42 | 93.3 | 4 | 57.1 | |
| Vaginal invasion | |||||
| Yes | 3 | 6.7 | 2 | 28.6 | 0.13 |
| No | 42 | 93.3 | 5 | 9.6 | |
| Variable | ORa | IC 95% | p |
|---|---|---|---|
| Age [years] | |||
| 1.16 | 1.01–1.32 | 0.03 | |
| BMI (kg/m2) | |||
| 1.25 | 0.94–1.66 | 0.12 | |
| Bilateral SLN involvement | |||
| No | 1 | ||
| Yes | 2.65 | 0.01–523.29 | 0.72 |
| Number of positive SLN | |||
| 0.60 | 0.01–38.01 | 0.81 | |
| LVSI | |||
| No | 1 | ||
| Yes | 25.97 | 1.16–582.1 | 0.04 |
| Parametrial invasion | |||
| No | 1 | ||
| Yes | 1.20 | 0.01–121.64 | 0.94 |
| Vaginal invasion | |||
| No | 1 | ||
| Yes | 0.65 | 0.01–117.25 | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pache, B.; Tantari, M.; Guani, B.; Mathevet, P.; Magaud, L.; Lecuru, F.; Balaya, V., on behalf of the SENTICOL Group. Predictors of Non-Sentinel Lymph Node Metastasis in Patients with Positive Sentinel Lymph Node in Early-Stage Cervical Cancer: A SENTICOL GROUP Study. Cancers 2023, 15, 4737. https://doi.org/10.3390/cancers15194737
Pache B, Tantari M, Guani B, Mathevet P, Magaud L, Lecuru F, Balaya V on behalf of the SENTICOL Group. Predictors of Non-Sentinel Lymph Node Metastasis in Patients with Positive Sentinel Lymph Node in Early-Stage Cervical Cancer: A SENTICOL GROUP Study. Cancers. 2023; 15(19):4737. https://doi.org/10.3390/cancers15194737
Chicago/Turabian StylePache, Basile, Matteo Tantari, Benedetta Guani, Patrice Mathevet, Laurent Magaud, Fabrice Lecuru, and Vincent Balaya on behalf of the SENTICOL Group. 2023. "Predictors of Non-Sentinel Lymph Node Metastasis in Patients with Positive Sentinel Lymph Node in Early-Stage Cervical Cancer: A SENTICOL GROUP Study" Cancers 15, no. 19: 4737. https://doi.org/10.3390/cancers15194737
APA StylePache, B., Tantari, M., Guani, B., Mathevet, P., Magaud, L., Lecuru, F., & Balaya, V., on behalf of the SENTICOL Group. (2023). Predictors of Non-Sentinel Lymph Node Metastasis in Patients with Positive Sentinel Lymph Node in Early-Stage Cervical Cancer: A SENTICOL GROUP Study. Cancers, 15(19), 4737. https://doi.org/10.3390/cancers15194737








