Blockade of IL-10 Signaling Ensures Mifamurtide Efficacy in Metastatic Osteosarcoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Cell Culture
2.3. Co-Culture and Treatment
2.4. Measurement of Intracellular and Mitochondrial ROS
2.5. Flow Cytometric Analysis
2.6. Real-Time PCR
2.7. ELISA and AlphaLISA Assay
2.8. Luminex Cytokines Quantification
2.9. In Vivo Lung Retention Assay
2.10. Statistical Analysis
3. Results
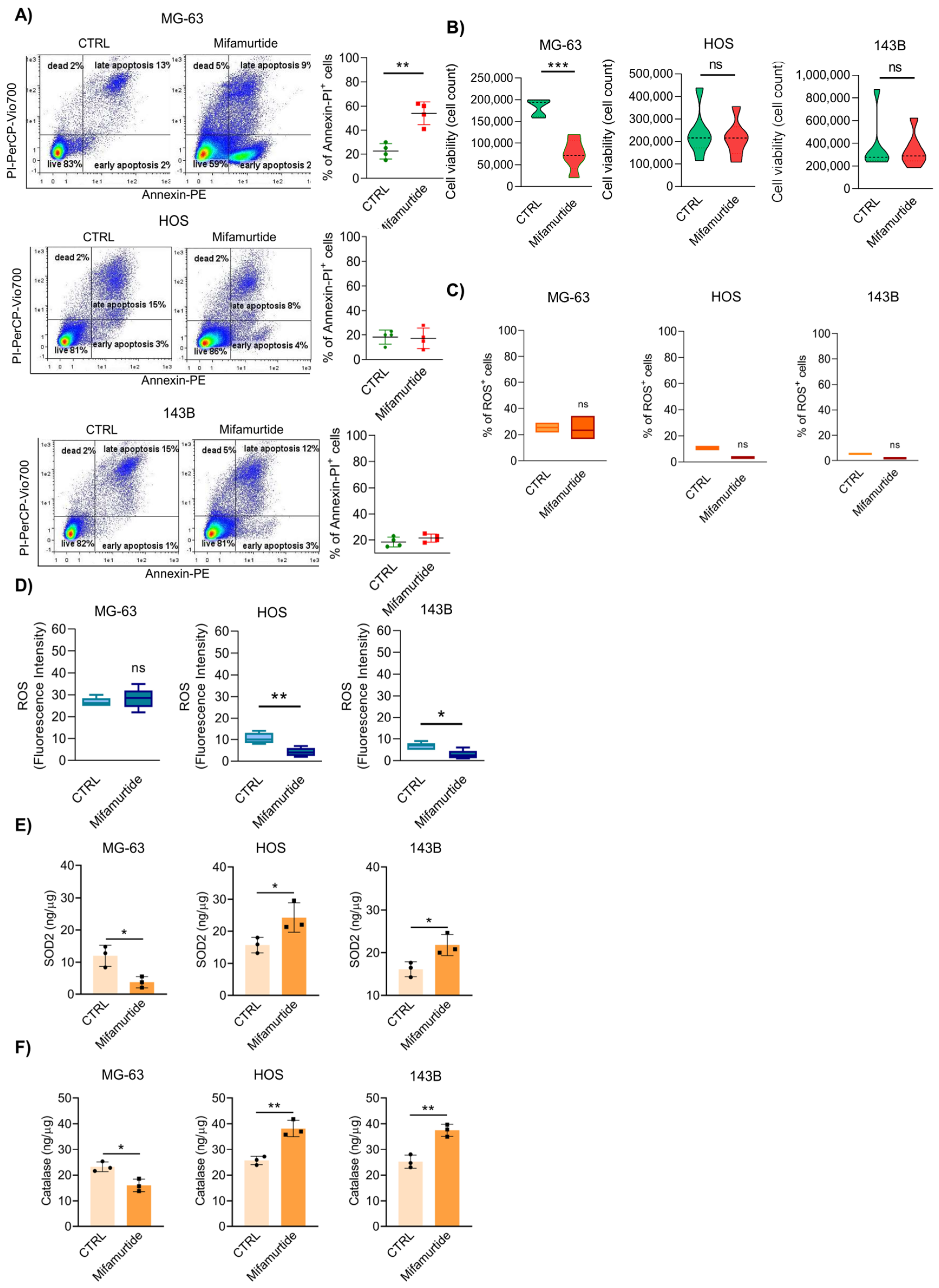
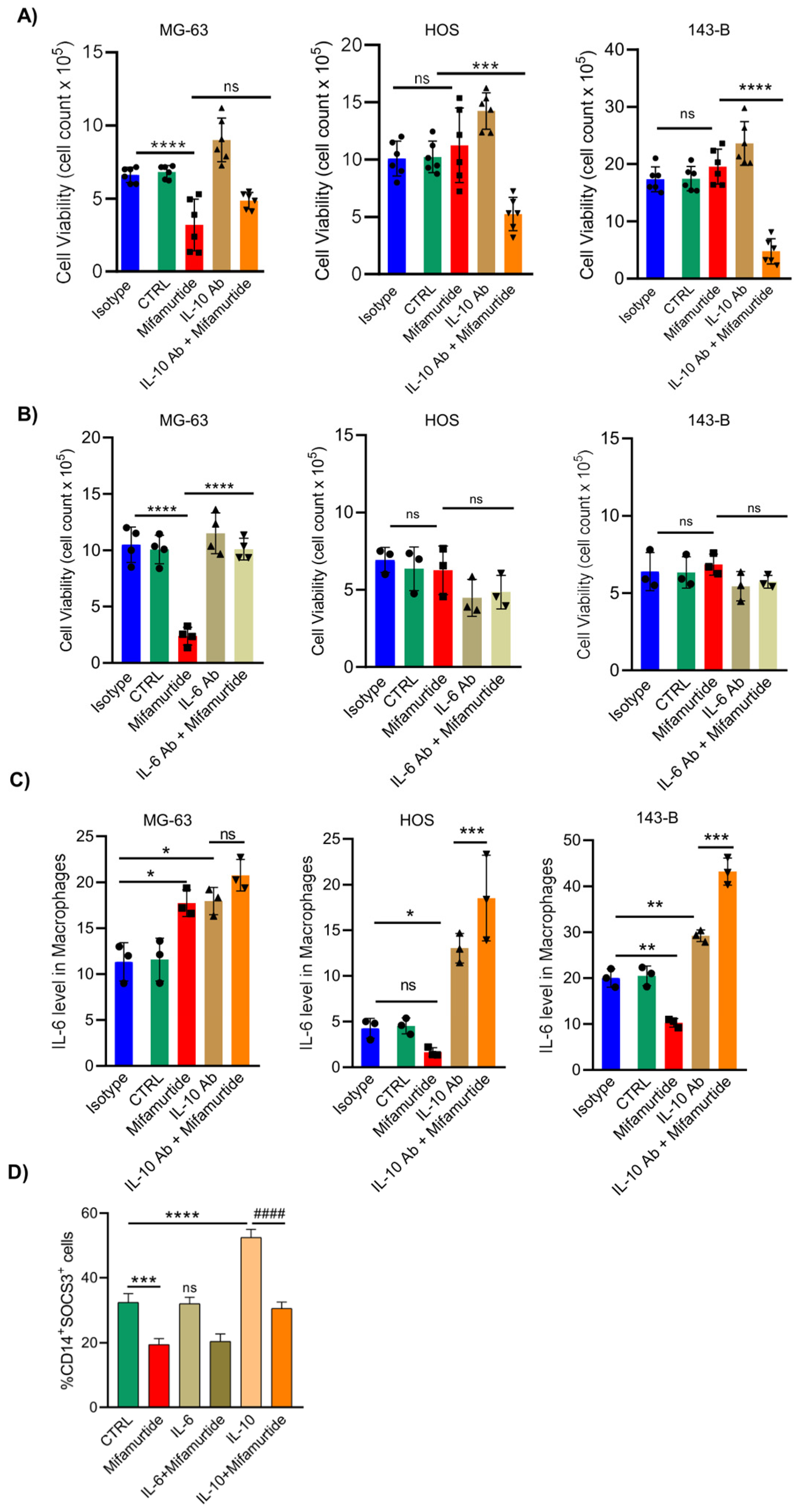
3.1. Mifamurtide Is Unable to Exert Its Antitumoral Action in the Most Aggressive Osteosarcoma Cell Lines
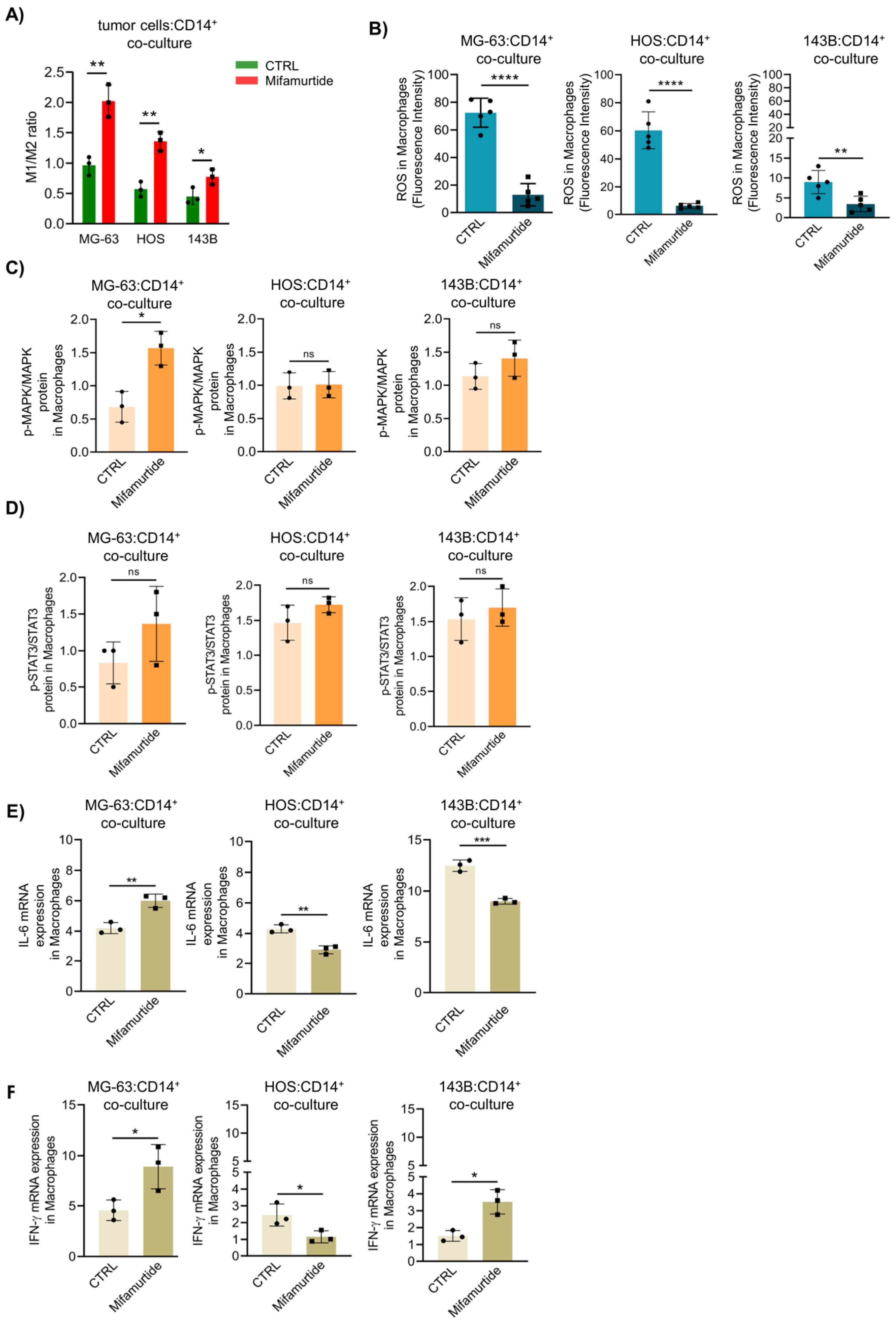
3.2. Mifamurtide Triggers MAPK and STAT3 Signaling Pathways Only in Macrophages Co-cultured with the Less Aggressive MG-63 Cells
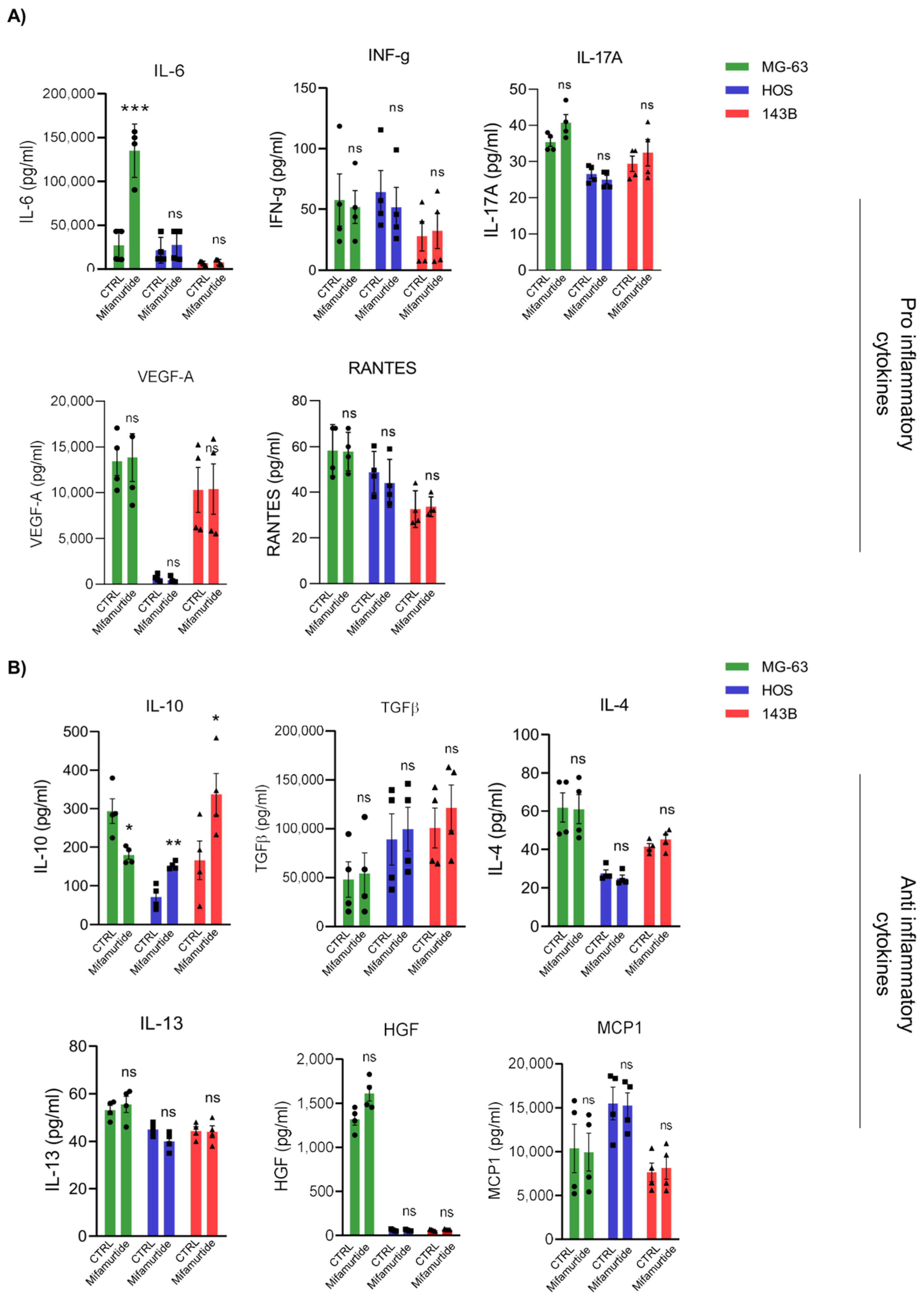
3.3. IL-6 and IL-10 Are Differentially Secreted in Macrophages-Tumor Co-Culture Medium Depending on OS Cell Aggressiveness
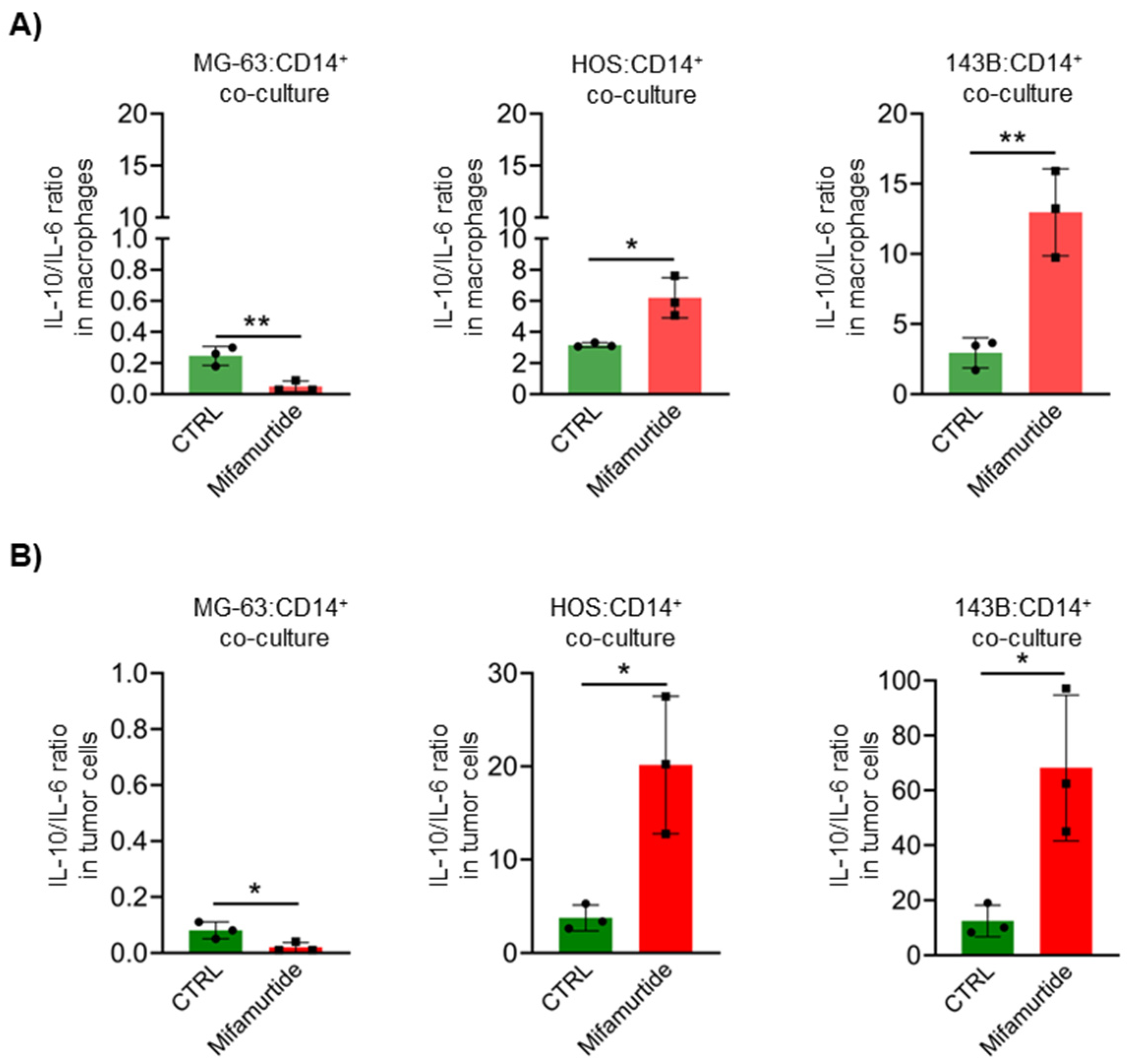
3.4. Mifamurtide Treatment Causes a Different Trend of IL-10/IL-6 Ratio on OS Cell Lines Depending on the Different Stage of Malignancy
3.5. An Anti-IL-10 Blocking Antibody Triggers the Anti-Tumoral Activity of Mifamurtide on Aggressive OS Cells
3.6. IL-10 Blocking Antibody Converts the Pro-Tumoral IL-10 Cellular Response into a Tumoricidal Action That Synergize with Mifamurtide
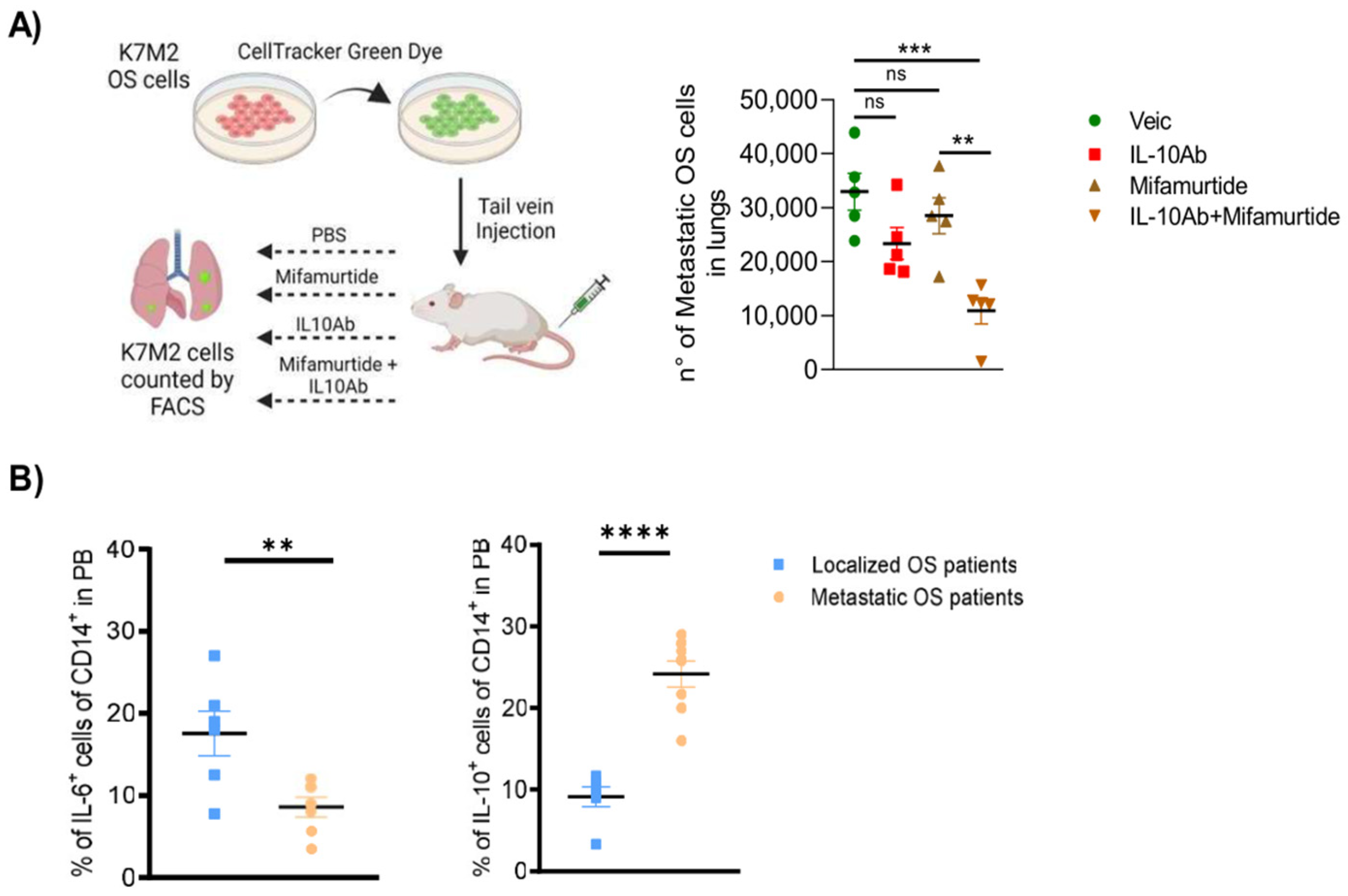
3.7. Administration of Anti-IL-10 Blocking Antibody Synergizes with Mifamurtide in Decreasing Lung Metastasis Dissemination in a Syngeneic OS Model In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Durfee, R.A.; Mohammed, M.; Luu, H.H. Review of Osteosarcoma and Current Management. Rheumatol. Ther. 2016, 3, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Damron, T.; Ward, W.G.; Stewart, A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin. Orthop. Relat. Res. 2007, 459, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Valery, P.C.; Laversanne, M.; Bray, F. Bone cancer incidence by morphological subtype: A global assessment. Cancer Causes Control 2015, 26, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.J.; Frezza, A.M.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; Bonvalot, S.; et al. Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. 7), 1520–1536. [Google Scholar]
- Hansen, M.F.; Seton, M.; Merchant, A. Osteosarcoma in Paget’s disease of bone. J. Bone Miner. Res. 2006, 21 (Suppl. 2), P58–P63. [Google Scholar] [CrossRef] [PubMed]
- Luetke, A.; Meyer, P.A.; Lewis, I.; Juergens, H. Osteosarcoma treatment—Where do we stand? A state of the art review. Cancer Treat. Rev. 2014, 40, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A.; Gorlick, R. Osteosarcoma. Pediatr. Clin. N. Am. 1997, 44, 973–989. [Google Scholar] [CrossRef]
- Neyssa, M.; Gebhardt, M.; Teot, L.; Gorlick, R. Biology and Therapeutic Advances for Pediatric Osteosarcoma. Oncologist 2004, 4, 422–441. [Google Scholar]
- Wunder, J.S.; Gokgoz, N.; Parkes, R.; Bull, S.B.; Eskandarian, S.; Davis, A.M.; Beauchamp, C.P.; Conrad, E.U.; Grimer, R.J.; Healey, J.H.; et al. TP53 Mutations and Outcome in Osteosarcoma: A Prospective, Multicenter Study. J. Clin. Oncol. 2007, 23, 15735124. [Google Scholar] [CrossRef]
- Chen, X.; Bahrami, A.; Pappo, A.; Easton, J.; Dalton, J.; Hedlund, E.; Ellison, D.; Shurtleff, S.; Wu, G.; Wei, L.; et al. Recurrent Somatic Structural Variations Contribute to Tumorigenesis in Pediatric Osteosarcoma. Cell Rep. 2014, 7, 104–112. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, J.; Zhang, K.; Guo, Y. TP53 Mutations and Survival in Osteosarcoma Patients: A Meta-Analysis of Published Data. Dis. Markers 2016, 2016, 4639575. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Song, W.X.; Luo, J.; Haydon, R.C.; He, T.C. Osteosarcoma Development and Stem Cell Differentiation. Clin. Orthop. Relat. Res. 2008, 466, 2114–2130. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A. Muramyl tripeptide (mifamurtide) for the treatment of osteosarcoma. Expert Rev. Anticancer Ther. 2009, 9, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Mifamurtide: A review of its use in the treatment of osteosarcoma. Paediatr. Drugs 2010, 12, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Kleinerman, E.S.; Jia, S.F.; Griffin, J.; Seibel, L.N.; Benjamin, R.S.; Jaffe, N. Phase-II Study of Liposomal Muramyl Tripeptide in Osteosarcoma—The Cytokine Cascade and Monocyte Activation Following Administration. J. Clin. Oncol. 1992, 10, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Balakrishnan, L.; Sharma, K.; Khan, A.A.; Advani, J.; Gowda, H.; Triphathy, S.P.; Suar, M.; Pandey, A.; Gandotra, S.; et al. A network map of Interleukin-10 signaling pathway. J. Cell Commun. Signal. 2016, 10, 61–67. [Google Scholar] [CrossRef]
- Braun, D.A.; Fribourg, M.; Sealfon, S.C. Cytokine response is determined by duration of receptor and signal transducers and activators of transcription 3 (STAT3) activation. J. Biol. Chem. 2013, 288, 2986–2993. [Google Scholar] [CrossRef]
- Tacyildiz, N.; Ozdemir, S.I.; Una, E.; Berber, M.; Dinclasan, H.; Yavuz, G. The Efficiency and Toxicity of Mifamurtide in Childhood Osteosarcoma. J. Pediatr. Hemat. Oncol. 2018, 40, e373–e376. [Google Scholar] [CrossRef]
- Meyers, P.A.; Schwartz, C.L.; Krailo, M.D.; Healey, J.H.; Bernstein, M.L.; Betcher, D.; Ferguson, W.S.; Gebhardt, M.C.; Goorin, A.M.; Harris, M.; et al. Children’s Oncology Group. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J. Clin. Oncol. 2008, 26, 633–638. [Google Scholar] [CrossRef]
- Jimmy, R.; Stern, C.; Lisy, K.; White, S. Effectiveness of mifamurtide in addition to standard chemotherapy for high-grade osteosarcoma: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2017, 15, 2113–2152. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A.; Schwartz, C.L.; Krailo, M.D.; Kleinerman, E.S.; Betcher, D.; Bernstein, M.L.; Conrad, E.; Ferguson, W.; Gebhardt, M.; Goorin, A.M.; et al. Osteosarcoma: A randomized, prospective trial of the addition of mifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J. Clin. Oncol. 2005, 23, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, K.; Vegna, S.; Ramirez, C.; Akkari, L. Understanding the Origin and Diversity of Macrophages to Tailor Their Targeting in Solid Cancers. Front. Immunol. 2019, 10, 2215. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.W.; Liu, P.P.; Wang, Z.X.; Chen, C.Y.; Xie, H. Macrophages in Osteosarcoma Immune Microenvironment: Implications for Immunotherapy. Front. Oncol. 2020, 10, 586580. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Dennen, K.; Gordon, N.; Kleinerman, E.S. Exosomal communication by metastatic osteosarcoma cells modulates alveolar macrophages to an M2 tumor-promoting phenotype and inhibits tumoricidal functions. Oncoimmunology 2020, 9, 1747677. [Google Scholar] [CrossRef] [PubMed]
- Venetsanakos, E.; Beckman, I.; Bradley, J.; Skinner, J.M. High incidence of interleukin 10 mRNA but not interleukin 2 mRNA detected in human breast tumours. Br. J. Cancer 1997, 75, 1826–1830. [Google Scholar] [CrossRef][Green Version]
- Knoefel, B.; Nuske, K.; Steiner, T.; Junker, K.; Kosmehl, H.; Rebstock, K.; Reihnold, D.; Junker, U. Renal cell carcinomas produce IL-6, IL-10, IL-11, and TGF-beta 1 in primary cultures and modulate T lymphocyte blast transformation. J. Interferon Cytokine Res. 1997, 17, 95–102. [Google Scholar] [CrossRef]
- Huang, M.; Wang, J.; Lee, P.; Sharma, S.; Mao, J.T.; Meissner, H.; Uyemura, K.; Modlin, R.; Wollman, J.; Dubinett, S.M. Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res. 1995, 55, 3847–3853. [Google Scholar]
- Kim, J.; Modlin, R.L.; Moy, R.L.; Dubinett, S.M.; McHugh, T.; Nickoloff, B.J.; Uyemura, K. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J. Immunol. 1995, 155, 2240–2247. [Google Scholar] [CrossRef]
- Sato, T.; McCue, P.; Masuoka, K.; Salen, S.; Lattime, E.C.; Berd, D. Interleukin 10 production by human melanoma. Clin. Cancer Res. 1996, 2, 1383–1390. [Google Scholar]
- Mahipal, A.; Terai, M.; Berd, D.; Chervoneva, I.; Patel, K.; Mastrangelo, M.J.; Sato, T. Tumor-derived interleukin-10 as a prognostic factor in stage III patients undergoing adjuvant treatment with an autologous melanoma cell vaccine. Cancer Immunol. Immunother. 2011, 60, 1039–1045. [Google Scholar] [CrossRef]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef]
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.T.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 2014, 26, 623–637. [Google Scholar] [CrossRef]
- Ruscica, M.; Ricci, C.; Macchi, C.; Magni, P.; Cristofani, C.; Liu, J.; Corsini, A.; Ferri, N. Suppressor of Cytokine Signaling-3 (SOCS-3) Induces Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Expression in Hepatic HepG2 Cell Line. J. Biol. Chem. 2016, 291, 3508–3519. [Google Scholar] [CrossRef]
| Cytokine | ALIVE | DEAD |
|---|---|---|
| Low IL-6 | n = 12 | n = 0 |
| High IL-6 | n = 4 | n = 8 |
| Low IL-10 | n = 12 | n = 0 |
| High IL-10 | n = 4 | n = 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastasi, N.; Pasha, A.; Bruno, G.; Subbiani, A.; Pietrovito, L.; Leo, A.; Scala, L.; de Simone, L.; Casazza, G.; Lunardi, F.; et al. Blockade of IL-10 Signaling Ensures Mifamurtide Efficacy in Metastatic Osteosarcoma. Cancers 2023, 15, 4744. https://doi.org/10.3390/cancers15194744
Nastasi N, Pasha A, Bruno G, Subbiani A, Pietrovito L, Leo A, Scala L, de Simone L, Casazza G, Lunardi F, et al. Blockade of IL-10 Signaling Ensures Mifamurtide Efficacy in Metastatic Osteosarcoma. Cancers. 2023; 15(19):4744. https://doi.org/10.3390/cancers15194744
Chicago/Turabian StyleNastasi, Nicoletta, Amada Pasha, Gennaro Bruno, Angela Subbiani, Laura Pietrovito, Angela Leo, Lucia Scala, Lorena de Simone, Gabriella Casazza, Federica Lunardi, and et al. 2023. "Blockade of IL-10 Signaling Ensures Mifamurtide Efficacy in Metastatic Osteosarcoma" Cancers 15, no. 19: 4744. https://doi.org/10.3390/cancers15194744
APA StyleNastasi, N., Pasha, A., Bruno, G., Subbiani, A., Pietrovito, L., Leo, A., Scala, L., de Simone, L., Casazza, G., Lunardi, F., Taddei, M. L., Tamburini, A., Tondo, A., Favre, C., & Calvani, M. (2023). Blockade of IL-10 Signaling Ensures Mifamurtide Efficacy in Metastatic Osteosarcoma. Cancers, 15(19), 4744. https://doi.org/10.3390/cancers15194744






