Tips for Preparing and Practicing Thermal Ablation Therapy of Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Principles of Thermal Ablation and Treatment Devices
2.1. Radiofrequency Ablation (RFA)
2.2. Microwave Ablation (MWA)
2.3. Potential Immunomodulatory Effects
3. Clinical Indications and Proper Methodology
4. Tips for Beginning Tumor Ablation
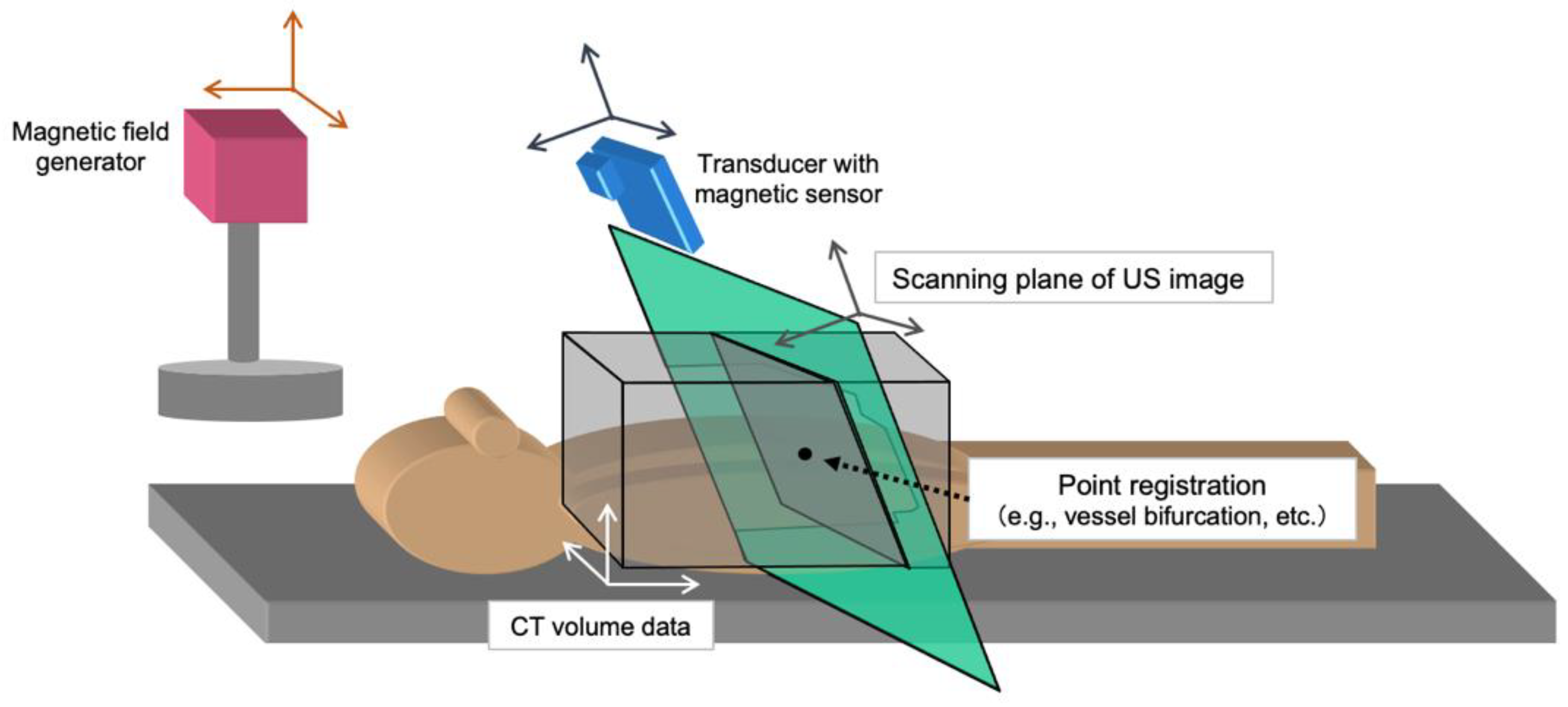
4.1. Planning US and Software-Based Planning
4.2. Grounding Pad on the Back
4.3. Body Position Change
4.4. Control of Needle Insertion on Clear US Images
4.5. Extreme Steam Popping
5. Risk Assessment for Tumor Ablation
5.1. Low Platelet Count (Thrombocytopenia)
5.2. Low Levels of Blood Clotting Factors (Coagulopathy)
5.3. Ascites, Esophagogastric Varices, and Hepatic Encephalopathy
5.4. History of Biliary Surgery/Interventions
5.5. Comorbidity of Renal Failure
6. Management of Factors Affecting the Visualization of Tumors in US
6.1. Artificial Ascites/Pleural Effusion Technique
6.2. Contrast-Enhanced US (CEUS) Guidance
6.3. Fusion Imaging Guidance
7. Treatment Response Assessment
8. Clinical Outcomes and Adverse Events
9. Clinical Implications of Thermal Ablation Therapies
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, M.; Brace, C.L.; Lee, F.T., Jr.; Goldberg, S.N. Principles of and Advances in Percutaneous Ablation. Radiology 2011, 258, 351–369. [Google Scholar] [CrossRef]
- Kuroda, H.; Nagasawa, T.; Fujiwara, Y.; Sato, H.; Abe, T.; Kooka, Y.; Endo, K.; Oikawa, T.; Sawara, K.; Takikawa, Y. Comparing the Safety and Efficacy of Microwave Ablation Using ThermosphereTM Technology versus Radiofrequency Ablation for Hepatocellular Carcinoma: A Propensity Score-Matched Analysis. Cancers 2021, 13, 1295. [Google Scholar] [CrossRef]
- Facciorusso, A.; Abd El Aziz, M.A.; Tartaglia, N.; Ramai, D.; Mohan, B.P.; Cotsoglou, C.; Pusceddu, S.; Giacomelli, L.; Ambrosi, A.; Sacco, R. Microwave Ablation Versus Radiofrequency Ablation for Treatment of Hepatocellular Carcinoma: A Meta-Analysis of Randomized Controlled Trials. Cancers 2020, 12, 3796. [Google Scholar] [CrossRef] [PubMed]
- Suwa, K.; Seki, T.; Aoi, K.; Yamashina, M.; Murata, M.; Yamashiki, N.; Nishio, A.; Shimatani, M.; Naganuma, M. Efficacy of microwave ablation versus radiofrequency ablation for hepatocellular carcinoma: A propensity score analysis. Abdom. Radiol. 2021, 46, 3790–3797. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Nishida, N.; Kudo, M. Radiofrequency ablation of liver metastasis: Potential impact on immune checkpoint inhibitor therapy. Eur. Radiol. 2019, 29, 5045–5051. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Takaki, H.; Yamakado, K.; Kudo, M. How Compatible Are Immune Checkpoint Inhibitors and Thermal Ablation for Liver Metastases? Cancers 2022, 14, 2206. [Google Scholar] [CrossRef]
- Shi, L.; Chen, L.; Wu, C.; Zhu, Y.; Xu, B.; Zheng, X.; Sun, M.; Wen, W.; Dai, X.; Yang, M.; et al. PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin. Cancer Res. 2016, 22, 1173–1184. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Kudo, M.; Kawamura, Y.; Hasegawa, K.; Tateishi, R.; Kariyama, K.; Shiina, S.; Toyoda, H.; Imai, Y.; Hiraoka, A.; Ikeda, M.; et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021, 10, 181–223. [Google Scholar] [CrossRef]
- Xie, D.; Shi, J.; Zhou, J.; Fan, J.; Gao, Q. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: A Chinese perspective. Clin. Mol. Hepatol. 2023, 29, 206–216. [Google Scholar] [CrossRef]
- Goh, M.J.; Sinn, D.H.; Kim, J.M.; Lee, M.W.; Hyun, D.H.; Yu, J.I.; Hong, J.Y.; Choi, M.S. Clinical practice guideline and real-life practice in hepatocellular carcinoma: A Korean perspective. Clin. Mol. Hepatol. 2023, 29, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Mirici-Cappa, F.; Gramenzi, A.; Santi, V.; Zambruni, A.; Micoli, A.D.; Frigerio, M.; Maraldi, F.; Di Nolfo, M.A.; Poggio, P.D.; Benvegnù, L.; et al. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: A 20-year multicentre experience. Gut 2010, 59, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Michitaka, K.; Horiike, N.; Hidaka, S.; Uehara, T.; Ichikawa, S.; Hasebe, A.; Miyamoto, Y.; Ninomiya, T.; Sogabe, I.; et al. Radiofrequency ablation therapy for hepatocellular carcinoma in elderly patients. J. Gastroenterol. Hepatol. 2010, 25, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.M.; Lin, C.J.; Lin, C.C.; Hsu, C.W.; Chen, Y.C. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 2005, 54, 1151–1156. [Google Scholar] [CrossRef]
- Rhim, H.; Lee, M.H.; Kim, Y.S.; Choi, D.; Lee, W.J.; Lim, H.K. Planning sonography to assess the feasibility of percutaneous radiofrequency ablation of hepatocellular carcinomas. AJR Am. J. Roentgenol. 2008, 190, 1324–1330. [Google Scholar] [CrossRef]
- Bae, J.W.; Lee, M.W.; Kang, T.W.; Song, K.D.; Cha, D.I.; Min, J.H.; Rhim, H. Percutaneous radiofrequency ablation for hepatic metastasis of colorectal cancer: Assessment of tumor visibility and the feasibility of the procedure with planning ultrasonography. Ultrasonography 2022, 41, 189–197. [Google Scholar] [CrossRef]
- Voglreiter, P.; Mariappan, P.; Pollari, M.; Flanagan, R.; Sequeiros, R.B.; Portugaller, R.H.; Fütterer, J.; Schmalstieg, D.; Kolesnik, M.; Moche, M. RFA Guardian: Comprehensive simulation of radiofrequency ablation treatment of liver tumors. Sci. Rep. 2018, 8, 787. [Google Scholar] [CrossRef]
- Ogawa, C.; Minami, Y.; Noda, T.; Arasawa, S.; Izuta, M.; Kubo, A.; Matsunaka, T.; Tamaki, H.; Shibatoge, M.; Kudo, M. Initial experience performing percutaneous ultrasound examination with real-time virtual sonography with color display. Oncology 2015, 89 (Suppl. S2), 11–18. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Solbiati, L.; Halpern, E.F.; Gazelle, G.S. Variables affecting proper system grounding for radiofrequency ablation in an animal model. J. Vasc. Interv. Radiol. 2000, 11, 1069–1075. [Google Scholar] [CrossRef]
- Nouso, K.; Oonishi, A.; Wakuta, A.; Kariyama, K. Modified radiofrequency ablation for the treatment of hepatocellular carcinoma. Hepatol. Res. 2016, 46, 1158–1161. [Google Scholar] [CrossRef]
- Ko, S.E.; Lee, M.W.; Lim, H.K.; Min, J.H.; Cha, D.I.; Kang, T.W.; Song, K.D.; Kim, M.J.; Rhim, H. The semi-erect position for better visualization of subphrenic hepatocellular carcinoma during ultrasonography examinations. Ultrasonography 2021, 40, 274–280. [Google Scholar] [CrossRef]
- Adams, R.B. Ultrasound scanning techniques. Surg. Open Sci. 2022, 10, 182–207. [Google Scholar] [CrossRef]
- Scholten, H.J.; Pourtaherian, A.; Mihajlovic, N.; Korsten, H.H.M.; Bouwman, R.A. Improving needle tip identification during ultrasound-guided procedures in anaesthetic practice. Anaesthesia 2017, 72, 889–904. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kudo, M.; Chung, H.; Minami, Y. Hepatocellular carcinoma that ruptured during radiofrequency ablation therapy. J. Gastroenterol. 2003, 39, 1015–1016. [Google Scholar] [CrossRef]
- Iida, H.; Aihara, T.; Ikuta, S.; Yamanaka, N. Effectiveness of impedance monitoring during radiofrequency ablation for predicting popping. World J. Gastroenterol. 2012, 18, 5870–5878. [Google Scholar] [CrossRef]
- Choe, J.; Kim, K.W.; Kim, Y.I.; Chung, J.W.; Huh, J.; Park, J.; Ham, S.J.; Jun, M.K.; Kim, P.N. Feasibility of a low-power radiofrequency ablation protocol to delay steam popping. J. Vasc. Interv. Radiol. 2016, 27, 268–274. [Google Scholar] [CrossRef]
- Goto, E.; Tateishi, R.; Shiina, S.; Masuzaki, R.; Enooku, K.; Sato, T.; Ohki, T.; Kondo, Y.; Goto, T.; Yoshida, H.; et al. Hemorrhagic complications of percutaneous radiofrequency ablation for liver tumors. J. Clin. Gastroenterol. 2010, 44, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, H.; Kurosaki, M.; Tanaka, H.; Kudo, M.; Abiru, S.; Igura, T.; Ishikawa, T.; Seike, M.; Katsube, T.; Ochiai, T.; et al. Lusutrombopag reduces need for platelet transfusion in patients with thrombocytopenia undergoing invasive procedures. Clin. Gastroenterol. Hepatol. 2019, 17, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Tateishi, R.; Hiroi, S.; Hongo, Y.; Fujiwara, M.; Kitanishi, Y.; Iwasaki, K.; Takeshima, T.; Igarashi, A. Effects of lusutrombopag on post-invasive procedural bleeding in thrombocytopenic patients with chronic liver disease. Adv. Ther. 2022, 39, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, D.; Caporaso, N.; Giannini, E.G.; Iacobellis, A.; Morelli, M.; Toniutto, P.; Violi, F.; PReBRiC (Procedure-Related Bleeding Risk in Cirrhosis) Group. Procedure-related bleeding risk in patients with cirrhosis and severe thrombocytopenia. Eur. J. Clin. Investig. 2021, 51, e13508. [Google Scholar] [CrossRef]
- Ronca, V.; Barabino, M.; Santambrogio, R.; Opocher, E.; Hodson, J.; Bertolini, E.; Birocchi, S.; Piccolo, G.; Battezzati, P.M.; Cattaneo, M.; et al. Impact of Platelet Count on Perioperative Bleeding in Patients With Cirrhosis Undergoing Surgical Treatments of Liver Cancer. Hepatol. Commun. 2022, 6, 423–434. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Greenberg, C.S.; Patton, H.M.; Caldwell, S.H. AGA Clinical Practice Update: Coagulation in Cirrhosis. Gastroenterology 2019, 157, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.O.; MacLaren, R. Comparison of Fresh-Frozen Plasma, Four-Factor Prothrombin Complex Concentrates, and Recombinant Factor VIIa to Facilitate Procedures in Critically Ill Patients with Coagulopathy from Liver Disease: A Retrospective Cohort Study. Pharmacotherapy 2016, 36, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J.; Patel, J.; Caldwell, H.; Davies, S.; Hebditch, V.; Hollywood, C.; Hubscher, S.; Karkhanis, S.; Lester, W.; Roslund, N.; et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020, 69, 1382–1403. [Google Scholar] [CrossRef]
- Little, A.F.; Ferris, J.V.; Dodd, G.D., 3rd; Baron, R.L. Image-guided percutaneous hepatic biopsy: Effect of ascites on the complication rate. Radiology 1996, 199, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.B.; Barefield, K.P.; Steinberg, H.V.; Bernardino, M.E. CT- or sonography-guided biopsy of the liver in the presence of ascites: Frequency of complications. AJR Am. J. Roentgenol. 1988, 151, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Kitai, S.; Kudo, M.; Nishida, N.; Izumi, N.; Sakamoto, M.; Matsuyama, Y.; Ichida, T.; Nakashima, O.; Matsui, O.; Ku, Y.; et al. Survival Benefit of Locoregional Treatment for Hepatocellular Carcinoma with Advanced Liver Cirrhosis. Liver Cancer 2016, 5, 175–189. [Google Scholar] [CrossRef]
- AChehab, M.A.; Thakor, A.S.; Tulin-Silver, S.; Connolly, B.L.; Cahill, A.M.; Ward, T.J.; Padia, S.A.; Kohi, M.P.; Midia, M.; Chaudry, G.; et al. Adult and Pediatric Antibiotic Prophylaxis during Vascular and IR Procedures: A Society of Interventional Radiology Practice Parameter Update Endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Association for Interventional Radiology. J. Vasc. Interv. Radiol. 2018, 29, 1483–1501. [Google Scholar]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am. J. Health Syst. Pharm. 2013, 70, 195–283. [Google Scholar] [CrossRef]
- Fang, C.; Cortis, K.; Yusuf, G.T.; Gregory, S.; Lewis, D.; Kane, P.; Peddu, P. Complications from percutaneous microwave ablation of liver tumours: A pictorial review. Br. J. Radiol. 2019, 92, 20180864. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Yamamoto, Y.; Yamamoto, N.; Maetani, Y.; Shibata, T.; Ikai, I.; Terajima, H.; Hatano, E.; Kubo, T.; Itoh, K.; et al. Cholangitis and liver abscess after percutaneous ablation therapy for liver tumors: Incidence and risk factors. J. Vasc. Interv. Radiol. 2003, 14, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Ocak, G.; Rookmaaker, M.B.; Algra, A.; de Borst, G.J.; Doevendans, P.A.; Kappelle, L.J.; Verhaar, M.C.; Visseren, F.L.; SMART Study Group. Chronic kidney disease and bleeding risk in patients at high cardiovascular risk: A cohort study. J. Thromb. Haemost. 2018, 16, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Hayaishi, S.; Kudo, M. Radiofrequency ablation for hepatic malignancies: Is needle tract cauterization necessary for preventing iatrogenic bleeding? Dig. Dis. 2013, 31, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Shimada, S.; Kamiyama, T.; Sugiyama, K.; Asahi, Y.; Nagatsu, A.; Orimo, T.; Kakisaka, T.; Kamachi, H.; Ito, Y.M.; et al. Impact of comorbid renal dysfunction in patients with hepatocellular carcinoma on long-term outcomes after curative resection. World J. Gastrointest. Surg. 2022, 14, 670–684. [Google Scholar] [CrossRef]
- Minami, Y.; Kudo, M.; Kawasaki, T.; Chung, H.; Ogawa, C.; Inoue, T.; Sakaguchi, Y.; Sakamoto, H.; Shiozaki, H. Percutaneous ultrasound-guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. J. Gastroenterol. 2003, 38, 1066–1070. [Google Scholar] [CrossRef]
- Uehara, T.; Hirooka, M.; Ishida, K.; Hiraoka, A.; Kumagi, T.; Kisaka, Y.; Hiasa, Y.; Onji, M. Percutaneous ultrasound-guided radiofrequency ablation of hepatocellular carcinoma with artificially induced pleural effusion and ascites. J. Gastroenterol. 2007, 42, 306–311. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Limquiaco, J.L.; Lin, C.C.; Chen, W.T.; Lin, S.M. Radiofrequency ablation following artificial ascites and pleural effusion creation may improve outcomes for hepatocellular carcinoma in high-risk locations. Abdom. Radiol. 2019, 44, 1141–1151. [Google Scholar] [CrossRef]
- Minami, Y.; Kudo, M.; Chung, H.; Kawasaki, T.; Yagyu, Y.; Shimono, T.; Shiozaki, H. Contrast harmonic sonography-guided radiofrequency ablation therapy versus B-mode sonography in hepatocellular carcinoma: Prospective randomized controlled trial. AJR Am. J. Roentgenol. 2007, 188, 489–494. [Google Scholar] [CrossRef]
- Minami, Y.; Kudo, M.; Hatanaka, K.; Kitai, S.; Inoue, T.; Hagiwara, S.; Chung, H.; Ueshima, K. Radiofrequency ablation guided by contrast harmonic sonography using perfluorocarbon microbubbles (Sonazoid) for hepatic malignancies: An initial experience. Liver Int. 2010, 30, 759–764. [Google Scholar] [CrossRef]
- Dohmen, T.; Kataoka, E.; Yamada, I.; Miura, K.; Ohshima, S.; Shibuya, T.; Segawa, D.; Sato, W.; Anezaki, Y.; Ishii, H.; et al. Efficacy of contrast-enhanced ultrasonography in radiofrequency ablation for hepatocellular carcinoma. Intern. Med. 2012, 51, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Minami, Y.; Chung, H.; Kudo, M.; Kitai, S.; Takahashi, S.; Inoue, T.; Ueshima, K.; Shiozaki, H. Radiofrequency ablation of hepatocellular carcinoma: Value of virtual CT sonography with magnetic navigation. AJR Am. J. Roentgenol. 2008, 190, W335–W341. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Rhim, H.; Cha, D.I.; Kim, Y.J.; Choi, D.; Kim, Y.; Lim, H.K. Percutaneous radiofrequency ablation of hepatocellular carcinoma: Fusion imaging guidance for management of lesions with poor conspicuity at conventional sonography. AJR Am. J. Roentgenol. 2012, 198, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Song, K.D.; Lee, M.W.; Rhim, H.; Cha, D.I.; Chong, Y.; Lim, H.K. Fusion imaging-guided radiofrequency ablation for hepatocellular carcinomas not visible on conventional ultrasound. AJR Am. J. Roentgenol. 2013, 201, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Minami, T.; Hagiwara, S.; Ida, H.; Ueshima, K.; Nishida, N.; Murakami, T.; Kudo, M. Ultrasound-ultrasound image overlay fusion improves real-time control of radiofrequency ablation margin in the treatment of hepatocellular carcinoma. Eur. Radiol. 2018, 28, 1986–1993. [Google Scholar] [CrossRef]
- Minami, Y.; Minami, T.; Takita, M.; Hagiwara, S.; Ida, H.; Ueshima, K.; Nishida, N.; Kudo, M. Radiofrequency ablation for hepatocellular carcinoma: Clinical value of ultrasound-ultrasound overlay fusion for optimal ablation and local controllability. Hepatol. Res. 2020, 50, 67–74. [Google Scholar] [CrossRef]
- Nouso, K.; Shiraga, K.; Uematsu, S.; Okamoto, R.; Harada, R.; Takayama, S.; Kawai, W.; Kimura, S.; Ueki, T.; Okano, N.; et al. Prediction of the ablated area by the spread of microbubbles during radiofrequency ablation of hepatocellular carcinoma. Liver Int. 2005, 25, 967–972. [Google Scholar] [CrossRef]
- Minami, Y.; Morita, M.; Chishina, H.; Aoki, T.; Takita, M.; Hagiwara, S.; Ida, H.; Ueshima, K.; Nishida, N.; Kudo, M. Can the entire ablative hyperechoic zone be regarded as a necrotic lesion after radiofrequency ablation of the liver? Ultrasound Med. Biol. 2021, 47, 2930–2935. [Google Scholar] [CrossRef]
- Limanond, P.; Zimmerman, P.; Raman, S.R.; Kadell, B.M.; Lu, D.S.K. Interpretation of CT and MRI after radiofrequency ablation of hepatic malignancies. AJR Am. J. Roentgenol. 2003, 181, 1635–1640. [Google Scholar] [CrossRef]
- Hasegawa, K.; Takemura, N.; Yamashita, T.; Watadani, T.; Kaibori, M.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Aikata, H.; et al. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2021 version (5th JSH-HCC Guidelines). Hepatol. Res. 2023, 53, 383–390. [Google Scholar] [CrossRef]
- Bouda, D.; Lagadec, M.; Alba, C.G.; Barrau, V.; Burgio, M.D.; Moussa, N.; Vilgrain, V.; Ronot, M. Imaging review of hepatocellular carcinoma after thermal ablation: The good, the bad, and the ugly. J. Magn. Reson. Imaging 2016, 44, 1070–1090. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Kudo, M. Imaging modalities for assessment of treatment response to nonsurgical hepatocellular carcinoma therapy: Contrast-Enhanced US, CT, and MRI. Liver Cancer 2015, 4, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Nishida, N.; Kudo, M. Imaging Diagnosis of Various Hepatocellular Carcinoma Subtypes and Its Hypervascular Mimics: Differential Diagnosis Based on Conventional Interpretation and Artificial Intelligence. Liver Cancer 2022, 12, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Kokubu, S.; Shibuya, A.; Ono, K.; Watanabe, M.; Hidaka, H.; Tsuchihashi, T.; Saigenji, K. Radiofrequency ablation of hepatocellular carcinoma: Correlation between local tumor progression after ablation and ablative margin. AJR Am. J. Roentgenol. 2007, 188, 480–488. [Google Scholar] [CrossRef]
- Shady, W.; Petre, E.N.; Do, K.G.; Gonen, M.; Yarmohammadi, H.; Brown, K.T.; Kemeny, N.E.; D’Angelica, M.; Kingham, P.T.; Solomon, S.B.; et al. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J. Vasc. Interv. Radiol. 2018, 29, 268–275. [Google Scholar] [CrossRef]
- Laimer, G.; Schullian, P.; Jaschke, N.; Putzer, D.; Eberle, G.; Alzaga, A.; Odisio, B.; Bale, R. Minimal ablative margin (MAM) assessment with image fusion: An independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur. Radiol. 2020, 30, 2463–2472. [Google Scholar] [CrossRef]
- Minami, Y.; Minami, T.; Ueshima, K.; Yagyu, Y.; Tsurusaki, M.; Okada, T.; Hori, M.; Kudo, M.; Murakami, T. Three-dimensional radiological assessment of ablative margins in hepatocellular carcinoma: Pilot study of overlay fused CT/MRI imaging with automatic registration. Cancers 2021, 13, 1460. [Google Scholar] [CrossRef]
- Bai, X.M.; Cui, M.; Yang, W.; Wang, H.; Wang, S.; Zhang, Z.Y.; Wu, W.; Chen, M.H.; Yan, K.; Goldberg, S.N. The 10-year Survival Analysis of Radiofrequency Ablation for Solitary Hepatocellular Carcinoma 5 cm or Smaller: Primary versus Recurrent HCC. Radiology 2021, 300, 458–469. [Google Scholar] [CrossRef]
- Ng, K.K.C.; Chok, K.S.H.; Chan, A.C.Y.; Cheung, T.T.; Wong, T.C.L.; Fung, J.Y.Y.; Yuen, J.; Poon, R.T.P.; Fan, S.T.; Lo, C.M. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br. J. Surg. 2017, 104, 1775–1784. [Google Scholar] [CrossRef]
- Kudo, M.; Izumi, N.; Kokudo, N.; Sakamoto, M.; Shiina, S.; Takayama, T.; Tateishi, R.; Nakashima, O.; Murakami, T.; Matsuyama, Y.; et al. Report of the 21st Nationwide Follow-up Survey of Primary Liver Cancer in Japan (2010–2011). Hepatol. Res. 2021, 51, 355–405. [Google Scholar] [CrossRef]
- Lin, S.M. Ultrasonography-guided radiofrequency ablation in hepatocellular carcinoma: Current status and future perspectives. J. Med. Ultrasound 2013, 21, 9–15. [Google Scholar] [CrossRef]
- Bertot, L.C.; Sato, M.; Tateishi, R.; Yoshida, H.; Koike, K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: A systematic review. Eur. Radiol. 2011, 21, 2584–2596. [Google Scholar] [CrossRef] [PubMed]
- Lahat, E.; Eshkenazy, R.; Zendel, A.; Zakai, B.B.; Maor, M.; Dreznik, Y.; Ariche, A. Complications after percutaneous ablation of liver tumors: A systematic review. Hepatobiliary Surg. Nutr. 2014, 3, 317–323. [Google Scholar] [PubMed]
- Maeda, M.; Saeki, I.; Sakaida, I.; Aikata, H.; Araki, Y.; Ogawa, C.; Kariyama, K.; Nouso, K.; Kitamoto, M.; Kobashi, H.; et al. Complications after radiofrequency ablation for hepatocellular carcinoma: A multicenter study involving 9,411 Japanese patients. Liver Cancer 2020, 9, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Shiratori, Y.; Kudo, M.; Shiina, S.; Mizuta, T.; Kojiro, M.; Yamamoto, K.; Koike, Y.; Saito, K.; Koyanagi, K.; et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology 2011, 54, 532–540. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Pinyol, R.; Montal, R.; Bassaganyas, L.; Sia, D.; Takayama, T.; Chau, G.Y.; Mazzaferro, V.; Roayaie, S.; Lee, H.C.; Kokudo, N.; et al. Molecular predictors of prevention of recurrence in HCC with sorafenib as adjuvant treatment and prognostic factors in the phase 3 STORM trial. Gut 2019, 68, 1065–1075. [Google Scholar] [CrossRef]
- Facciorusso, A.; Prete, V.D.; Crucinio, N.; Muscatiello, N.; Carr, B.I.; Leo, A.D.; Barone, M. Angiotensin receptor blockers improve survival outcomes after radiofrequency ablation in hepatocarcinoma patients. J. Gastroenterol. Hepatol. 2015, 30, 1643–1650. [Google Scholar] [CrossRef]
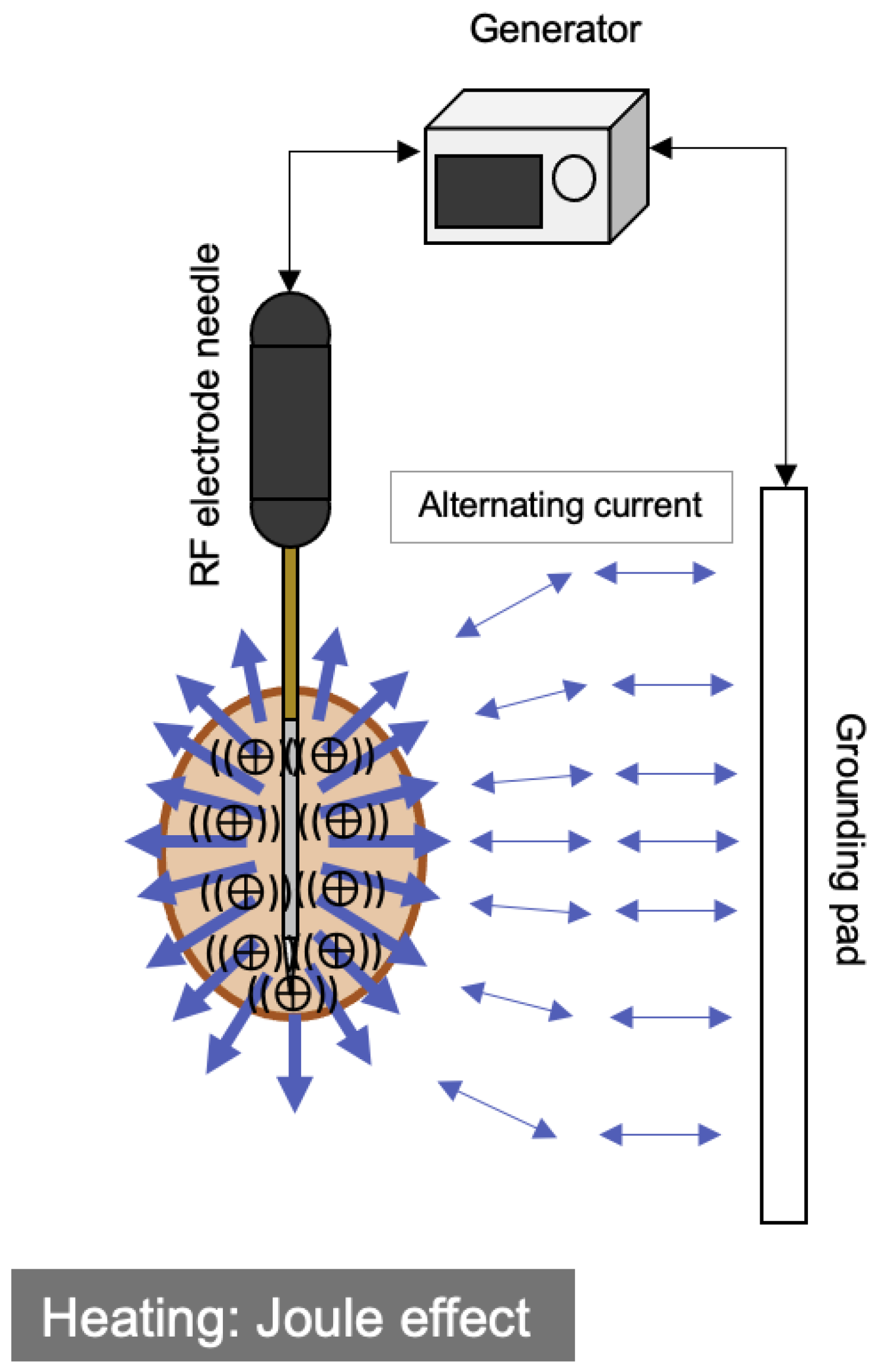
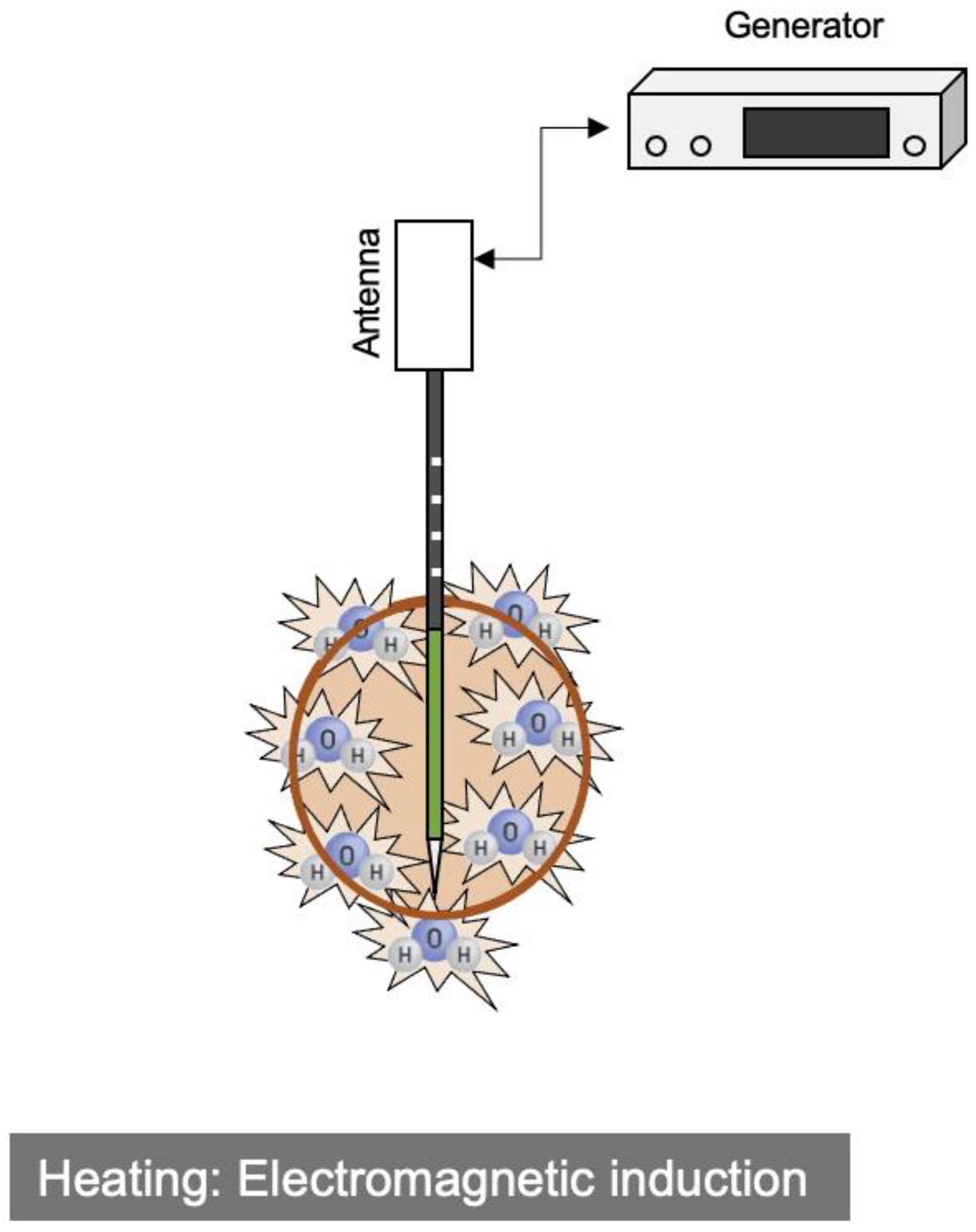

| RFA | MWA | |
|---|---|---|
| Heat generation | Joule effect | Induction heating |
| Energy | Alternating current (450 kHz) | Electromagnetic waves (2.45 GHz) |
| Needle gauge | 17 G | 13 G (Emprint®); 14 G, 17 G, 18 G (Mimapro®) |
| Output voltage, W | ~200 W | ~100 W |
| Temperature, °C | ~100 °C | ~150 °C |
| Ablation zone | Oval | (Oval~) Sphere |
| Heat-sink effect | Strong | Weak |
| Grounding pads | Necessary | Unnecessary |
| Parameters on ablation | Energization time, voltage, tissue impedance | Energization time, voltage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minami, Y.; Aoki, T.; Hagiwara, S.; Kudo, M. Tips for Preparing and Practicing Thermal Ablation Therapy of Hepatocellular Carcinoma. Cancers 2023, 15, 4763. https://doi.org/10.3390/cancers15194763
Minami Y, Aoki T, Hagiwara S, Kudo M. Tips for Preparing and Practicing Thermal Ablation Therapy of Hepatocellular Carcinoma. Cancers. 2023; 15(19):4763. https://doi.org/10.3390/cancers15194763
Chicago/Turabian StyleMinami, Yasunori, Tomoko Aoki, Satoru Hagiwara, and Masatoshi Kudo. 2023. "Tips for Preparing and Practicing Thermal Ablation Therapy of Hepatocellular Carcinoma" Cancers 15, no. 19: 4763. https://doi.org/10.3390/cancers15194763
APA StyleMinami, Y., Aoki, T., Hagiwara, S., & Kudo, M. (2023). Tips for Preparing and Practicing Thermal Ablation Therapy of Hepatocellular Carcinoma. Cancers, 15(19), 4763. https://doi.org/10.3390/cancers15194763









