Activity in Group-Housed Home Cages of Mice as a Novel Preclinical Biomarker in Oncology Studies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and DVC-Based Activity Monitoring
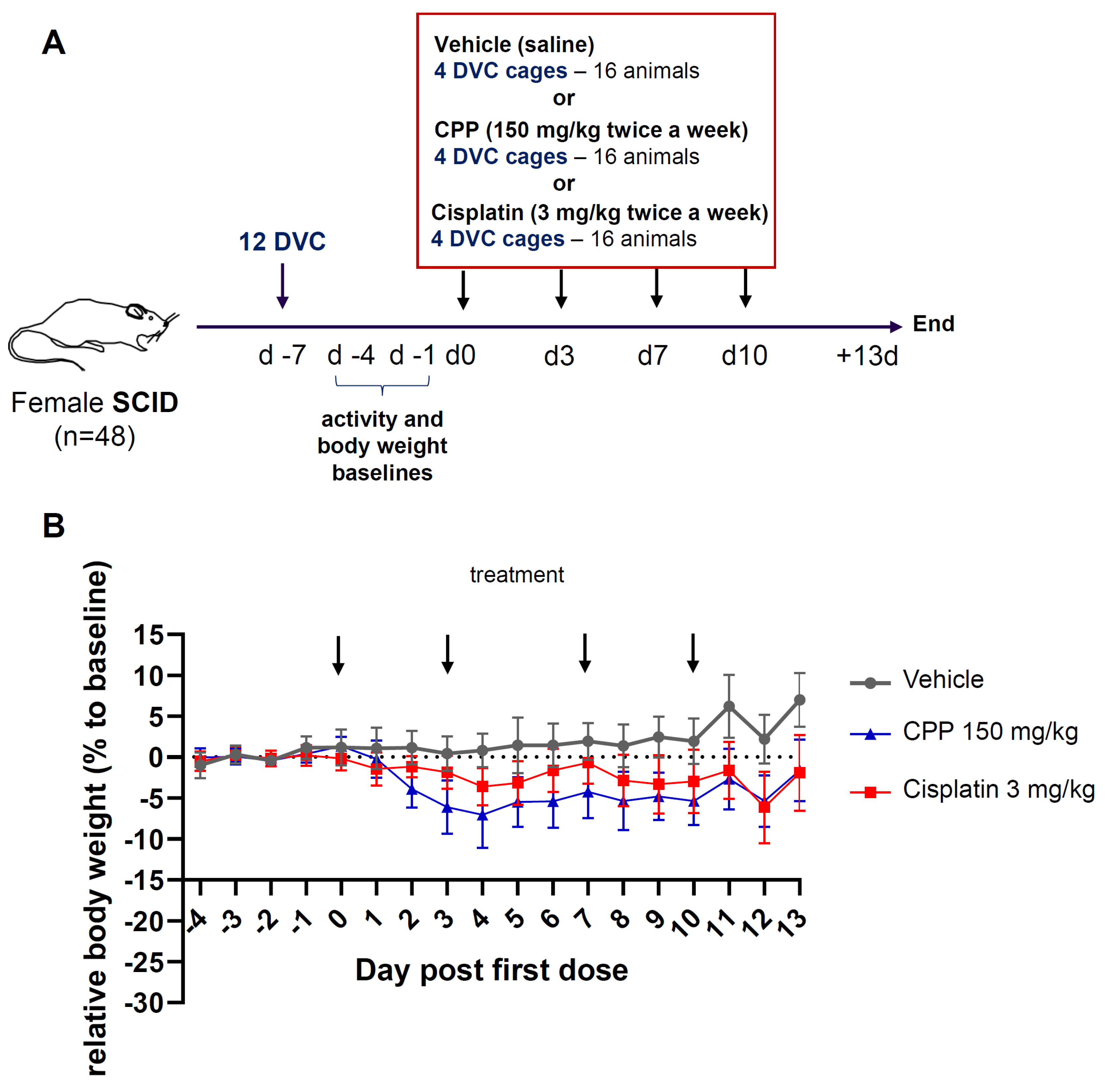
2.2. Study Design
2.3. Clinical Examination
2.4. Statistical Analysis
3. Results
3.1. Clinical Evaluation of Body Weight Change Revealed Mild Alterations after Chemotherapy Treatment
3.2. Clinical Assessment by Staff Revealed Signs of Discomfort Following Chemotherapy Treatment, but Little Evidence of Altered Activity
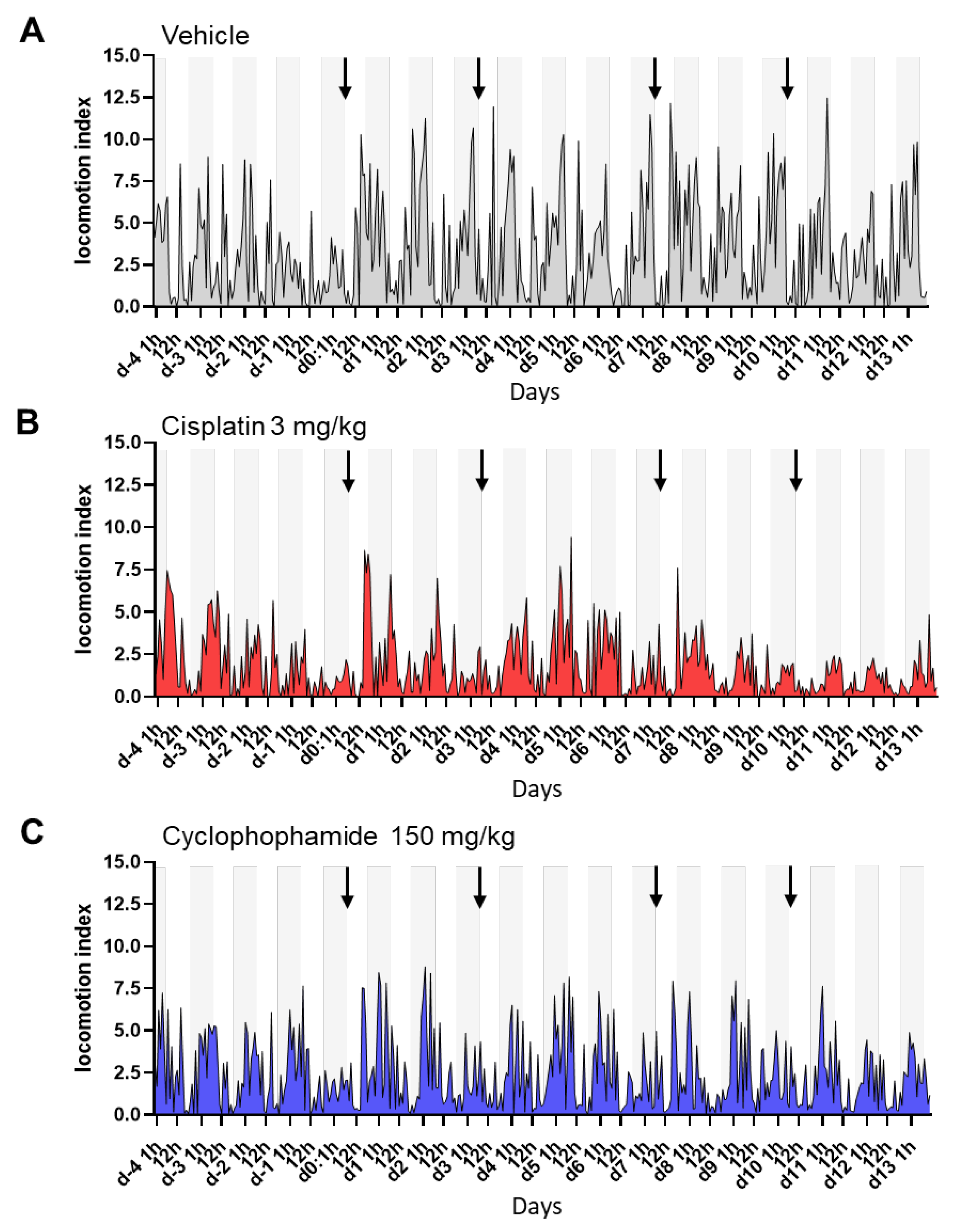
3.3. Digital Biomarker Detected Alterations of Animal Activity during Nighttime in Animals Treated with Chemotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Kalbasi, A.; Ribas, A. Tumour-Intrinsic Resistance to Immune Checkpoint Blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef]
- Pérez-Ruiz, E.; Melero, I.; Kopecka, J.; Sarmento-Ribeiro, A.B.; García-Aranda, M.; De Las Rivas, J. Cancer Immunotherapy Resistance Based on Immune Checkpoints Inhibitors: Targets, Biomarkers, and Remedies. Drug Resist. Updates 2020, 53, 100718. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity 2020, 52, 55–81. [Google Scholar] [CrossRef]
- Denayer, T.; Stöhr, T.; Roy, M.V. Animal Models in Translational Medicine: Validation and Prediction. Eur. J. Mol. Clin. Med. 2014, 2, 5. [Google Scholar] [CrossRef]
- Zuberi, A.; Lutz, C. Mouse Models for Drug Discovery. Can New Tools and Technology Improve Translational Power? ILAR J. 2016, 57, 178–185. [Google Scholar] [CrossRef]
- Cook, D.; Brown, D.; Alexander, R.; March, R.; Morgan, P.; Satterthwaite, G.; Pangalos, M.N. Lessons Learned from the Fate of AstraZeneca’s Drug Pipeline: A Five-Dimensional Framework. Nat. Rev. Drug Discov. 2014, 13, 419–431. [Google Scholar] [CrossRef]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to Improve R&D Productivity: The Pharmaceutical Industry’s Grand Challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [CrossRef]
- Seyhan, A.A. Lost in Translation: The Valley of Death across Preclinical and Clinical Divide—Identification of Problems and Overcoming Obstacles. Transl. Med. Commun. 2019, 4, 18. [Google Scholar] [CrossRef]
- Atkins, J.T.; George, G.C.; Hess, K.; Marcelo-Lewis, K.L.; Yuan, Y.; Borthakur, G.; Khozin, S.; LoRusso, P.; Hong, D.S. Pre-Clinical Animal Models Are Poor Predictors of Human Toxicities in Phase 1 Oncology Clinical Trials. Br. J. Cancer 2020, 123, 1496–1501. [Google Scholar] [CrossRef]
- Wells, D.J. Animal Welfare and the 3Rs in European Biomedical Research. Ann. N. Y. Acad. Sci. 2011, 1245, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Canadian Council on Animal Care. CCAC GUIDELINES. Can. Vet. J. 2003, 44, 631. [Google Scholar]
- Stokes, W.S. Humane Endpoints for Laboratory Animals Used in Regulatory Testing. ILAR J. 2002, 43, S31–S38. [Google Scholar] [PubMed]
- Hamm, T.E.; Dell, R.B.; Van Sluyters, R.C. Laboratory Animal Care Policies and Regulations: United States. ILAR J. 1995, 37, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Zentrich, E.; Talbot, S.R.; Bleich, A.; Häger, C. Automated Home-Cage Monitoring During Acute Experimental Colitis in Mice. Front. Neurosci. 2021, 15, 760606. [Google Scholar] [CrossRef]
- Baran, S.W.; Gupta, A.D.; Lim, M.A.; Mathur, A.; Rowlands, D.J.; Schaevitz, L.R.; Shanmukhappa, S.K.; Walker, D.B. Continuous, Automated Breathing Rate and Body Motion Monitoring of Rats with Paraquat-Induced Progressive Lung Injury. Front. Physiol. 2020, 11, 569001. [Google Scholar] [CrossRef]
- Lim, M.A.; Louie, B.; Ford, D.; Heath, K.; Cha, P.; Betts-Lacroix, J.; Lum, P.Y.; Robertson, T.L.; Schaevitz, L. Development of the Digital Arthritis Index, a Novel Metric to Measure Disease Parameters in a Rat Model of Rheumatoid Arthritis. Front. Pharmacol. 2017, 8, 818. [Google Scholar] [CrossRef]
- Golini, E.; Rigamonti, M.; Iannello, F.; De Rosa, C.; Scavizzi, F.; Raspa, M.; Mandillo, S. A Non-Invasive Digital Biomarker for the Detection of Rest Disturbances in the SOD1G93A Mouse Model of ALS. Front. Neurosci. 2020, 14, 896. [Google Scholar] [CrossRef]
- Baran, S.W.; Lim, M.A.; Do, J.P.; Stolyar, P.; Rabe, M.D.; Schaevitz, L.R.; Cadena, S.M. Digital Biomarkers Enable Automated, Longitudinal Monitoring in a Mouse Model of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1206–1213. [Google Scholar] [CrossRef]
- Winn, C.B.; Hwang, S.-K.; Morin, J.; Bluette, C.T.; Manickam, B.; Jiang, Z.K.; Giddabasappa, A.; Liu, C.-N.; Matthews, K. Automated Monitoring of Respiratory Rate as a Novel Humane Endpoint: A Refinement in Mouse Metastatic Lung Cancer Models. PLoS ONE 2021, 16, e0257694. [Google Scholar] [CrossRef]
- Jones, J.M.; Foster, W.; Twomey, C.R.; Burdge, J.; Ahmed, O.M.; Pereira, T.D.; Wojick, J.A.; Corder, G.; Plotkin, J.B.; Abdus-Saboor, I. A Machine-Vision Approach for Automated Pain Measurement at Millisecond Timescales. eLife 2020, 9, e57258. [Google Scholar] [CrossRef]
- Iannello, F. Non-Intrusive High Throughput Automated Data Collection from the Home Cage. Heliyon 2019, 5, e01454. [Google Scholar] [CrossRef] [PubMed]
- Pernold, K.; Iannello, F.; Low, B.E.; Rigamonti, M.; Rosati, G.; Scavizzi, F.; Wang, J.; Raspa, M.; Wiles, M.V.; Ulfhake, B. Towards Large Scale Automated Cage Monitoring—Diurnal Rhythm and Impact of Interventions on in-Cage Activity of C57BL/6J Mice Recorded 24/7 with a Non-Disrupting Capacitive-Based Technique. PLoS ONE 2019, 14, e0211063. [Google Scholar] [CrossRef] [PubMed]
- Voikar, V.; Gaburro, S. Three Pillars of Automated Home-Cage Phenotyping of Mice: Novel Findings, Refinement, and Reproducibility Based on Literature and Experience. Front. Behav. Neurosci. 2020, 14, 575434. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.H. Automated Home-Cage Testing as a Tool to Improve Reproducibility of Behavioral Research? Front. Neurosci. 2020, 14, 383. [Google Scholar] [CrossRef]
- Baran, S.W.; Bratcher, N.; Dennis, J.; Gaburro, S.; Karlsson, E.M.; Maguire, S.; Makidon, P.; Noldus, L.P.J.J.; Potier, Y.; Rosati, G.; et al. Emerging Role of Translational Digital Biomarkers Within Home Cage Monitoring Technologies in Preclinical Drug Discovery and Development. Front. Behav. Neurosci. 2022, 15, 758274. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Bissery, M.C.; Guénard, D.; Guéritte-Voegelein, F.; Lavelle, F. Experimental Antitumor Activity of Taxotere (RP 56976, NSC 628503), a Taxol Analogue. Cancer Res. 1991, 51, 4845–4852. [Google Scholar]
- Athira, V.R.; Shivanandappa, T.; Yajurvedi, H.N. Cyclophosphamide, a Cancer Chemotherapy Drug-Induced Early Onset of Reproductive Senescence and Alterations in Reproductive Performance and Their Prevention in Mice. Drug Chem. Toxicol. 2022, 45, 760–766. [Google Scholar] [CrossRef]
- Florea, A.-M.; Büsselberg, D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef] [PubMed]
- Roque-Diaz, Y.; Sanadar, M.; Han, D.; López-Mesas, M.; Valiente, M.; Tolazzi, M.; Melchior, A.; Veclani, D. The Dark Side of Platinum Based Cytostatic Drugs: From Detection to Removal. Processes 2021, 9, 1873. [Google Scholar] [CrossRef]
- Matsos, A.; Johnston, I.N. Chemotherapy-Induced Cognitive Impairments: A Systematic Review of the Animal Literature. Neurosci. Biobehav. Rev. 2019, 102, 382–399. [Google Scholar] [CrossRef]
- John, J.; Kinra, M.; Mudgal, J.; Viswanatha, G.L.; Nandakumar, K. Animal Models of Chemotherapy-Induced Cognitive Decline in Preclinical Drug Development. Psychopharmacology 2021, 238, 3025–3053. [Google Scholar] [CrossRef] [PubMed]
- Was, H.; Borkowska, A.; Bagues, A.; Tu, L.; Liu, J.Y.H.; Lu, Z.; Rudd, J.A.; Nurgali, K.; Abalo, R. Mechanisms of Chemotherapy-Induced Neurotoxicity. Front. Pharmacol. 2022, 13, 750507. [Google Scholar] [CrossRef]
- Bains, R.S.; Wells, S.; Sillito, R.R.; Armstrong, J.D.; Cater, H.L.; Banks, G.; Nolan, P.M. Assessing Mouse Behaviour throughout the Light/Dark Cycle Using Automated in-Cage Analysis Tools. J. Neurosci. Methods 2018, 300, 37–47. [Google Scholar] [CrossRef]
- Krarup-Hansen, A.; Helweg-Larsen, S.; Schmalbruch, H.; Rørth, M.; Krarup, C. Neuronal Involvement in Cisplatin Neuropathy: Prospective Clinical and Neurophysiological Studies. Brain 2007, 130, 1076–1088. [Google Scholar] [CrossRef]
- Tay, N.; Laakso, E.-L.; Schweitzer, D.; Endersby, R.; Vetter, I.; Starobova, H. Chemotherapy-Induced Peripheral Neuropathy in Children and Adolescent Cancer Patients. Front. Mol. Biosci. 2022, 9, 1015746. [Google Scholar] [CrossRef]
- Gan, H.K.; Bernstein, L.J.; Brown, J.; Ringash, J.; Vakilha, M.; Wang, L.; Goldstein, D.; Kim, J.; Hope, A.; O’Sullivan, B.; et al. Cognitive Functioning after Radiotherapy or Chemoradiotherapy for Head-and-Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 126–134. [Google Scholar] [CrossRef]
- Seigers, R.; Schagen, S.B.; Van Tellingen, O.; Dietrich, J. Chemotherapy-Related Cognitive Dysfunction: Current Animal Studies and Future Directions. Brain Imaging Behav. 2013, 7, 453–459. [Google Scholar] [CrossRef]
- Jangra, A.; Kwatra, M.; Singh, T.; Pant, R.; Kushwah, P.; Ahmed, S.; Dwivedi, D.; Saroha, B.; Lahkar, M. Edaravone Alleviates Cisplatin-Induced Neurobehavioral Deficits via Modulation of Oxidative Stress and Inflammatory Mediators in the Rat Hippocampus. Eur. J. Pharmacol. 2016, 791, 51–61. [Google Scholar] [CrossRef]
- Chiu, G.S.; Maj, M.A.; Rizvi, S.; Dantzer, R.; Vichaya, E.G.; Laumet, G.; Kavelaars, A.; Heijnen, C.J. Pifithrin-μ Prevents Cisplatin-Induced Chemobrain by Preserving Neuronal Mitochondrial Function. Cancer Res. 2017, 77, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Kavelaars, A.; Heijnen, C.J. Metformin Prevents Cisplatin-Induced Cognitive Impairment and Brain Damage in Mice. PLoS ONE 2016, 11, e0151890. [Google Scholar] [CrossRef] [PubMed]
- Lomeli, N.; Di, K.; Czerniawski, J.; Guzowski, J.F.; Bota, D.A. Cisplatin-Induced Mitochondrial Dysfunction Is Associated with Impaired Cognitive Function in Rats. Free. Radic. Biol. Med. 2017, 102, 274–286. [Google Scholar] [CrossRef]
- Huo, X.; Reyes, T.M.; Heijnen, C.J.; Kavelaars, A. Cisplatin Treatment Induces Attention Deficits and Impairs Synaptic Integrity in the Prefrontal Cortex in Mice. Sci. Rep. 2018, 8, 17400. [Google Scholar] [CrossRef] [PubMed]
- Boehmerle, W.; Huehnchen, P.; Peruzzaro, S.; Balkaya, M.; Endres, M. Electrophysiological, Behavioral and Histological Characterization of Paclitaxel, Cisplatin, Vincristine and Bortezomib-Induced Neuropathy in C57Bl/6 Mice. Sci. Rep. 2014, 4, 6370. [Google Scholar] [CrossRef]
- Iqubal, A.; Sharma, S.; Najmi, A.K.; Syed, M.A.; Ali, J.; Alam, M.M.; Haque, S.E. Nerolidol Ameliorates Cyclophosphamide-Induced Oxidative Stress, Neuroinflammation and Cognitive Dysfunction: Plausible Role of Nrf2 and NF- ΚB. Life Sci. 2019, 236, 116867. [Google Scholar] [CrossRef]
- Oyagbemi, A.A.; Omobowale, T.O.; Saba, A.B.; Olowu, E.R.; Dada, R.O.; Akinrinde, A.S. Gallic Acid Ameliorates Cyclophosphamide-Induced Neurotoxicity in Wistar Rats Through Free Radical Scavenging Activity and Improvement in Antioxidant Defense System. J. Diet Suppl. 2016, 13, 402–419. [Google Scholar] [CrossRef]
- Yang, M.; Kim, J.-S.; Song, M.-S.; Kim, S.-H.; Kang, S.S.; Bae, C.-S.; Kim, J.-C.; Wang, H.; Shin, T.; Moon, C. Cyclophosphamide Impairs Hippocampus-Dependent Learning and Memory in Adult Mice: Possible Involvement of Hippocampal Neurogenesis in Chemotherapy-Induced Memory Deficits. Neurobiol. Learn. Mem. 2010, 93, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Heckler, C.E.; Thompson, B.D.; Gross, R.A.; Opanashuk, L.A.; Cory-Slechta, D.A. A Clinically Relevant Dose of Cyclophosphamide Chemotherapy Impairs Memory Performance on the Delayed Spatial Alternation Task That Is Sustained over Time as Mice Age. Neurotoxicology 2016, 56, 287–293. [Google Scholar] [CrossRef]
- Wu, L.; Guo, D.; Liu, Q.; Gao, F.; Wang, X.; Song, X.; Wang, F.; Zhan, R.-Z. Abnormal Development of Dendrites in Adult-Born Rat Hippocampal Granule Cells Induced by Cyclophosphamide. Front. Cell. Neurosci. 2017, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Jhuang, H.; Garrote, E.; Yu, X.; Khilnani, V.; Poggio, T.; Steele, A.D.; Serre, T. Automated Home-Cage Behavioural Phenotyping of Mice. Nat. Commun. 2010, 1, 68. [Google Scholar] [CrossRef] [PubMed]
- Gettayacamin, M.; Retnam, L. AAALAC International Standards and Accreditation Process. Toxicol. Res. 2017, 33, 183–189. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terry, S.; Gommet, C.; Kerangueven, A.-C.; Leguet, M.; Thévenin, V.; Berthelot, M.; Begoud, L.; Windenberger, F.; Lainee, P. Activity in Group-Housed Home Cages of Mice as a Novel Preclinical Biomarker in Oncology Studies. Cancers 2023, 15, 4798. https://doi.org/10.3390/cancers15194798
Terry S, Gommet C, Kerangueven A-C, Leguet M, Thévenin V, Berthelot M, Begoud L, Windenberger F, Lainee P. Activity in Group-Housed Home Cages of Mice as a Novel Preclinical Biomarker in Oncology Studies. Cancers. 2023; 15(19):4798. https://doi.org/10.3390/cancers15194798
Chicago/Turabian StyleTerry, Stéphane, Céline Gommet, Anne-Cécile Kerangueven, Mickaël Leguet, Vincent Thévenin, Mickaël Berthelot, Laurent Begoud, Fanny Windenberger, and Pierre Lainee. 2023. "Activity in Group-Housed Home Cages of Mice as a Novel Preclinical Biomarker in Oncology Studies" Cancers 15, no. 19: 4798. https://doi.org/10.3390/cancers15194798
APA StyleTerry, S., Gommet, C., Kerangueven, A.-C., Leguet, M., Thévenin, V., Berthelot, M., Begoud, L., Windenberger, F., & Lainee, P. (2023). Activity in Group-Housed Home Cages of Mice as a Novel Preclinical Biomarker in Oncology Studies. Cancers, 15(19), 4798. https://doi.org/10.3390/cancers15194798





