Mistletoe Extracts from Different Host Trees Disparately Inhibit Bladder Cancer Cell Growth and Proliferation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Mistletoe Extracts
2.3. Tumor Cell Growth
2.4. Tumor Cell Proliferation
2.5. Integrin and CD44 Surface Expression
2.6. Apoptosis
2.7. Cell Cycling
2.8. Western Blot Analysis
2.9. Blocking Studies
2.10. Knockdown Studies
2.11. Statistics
3. Results
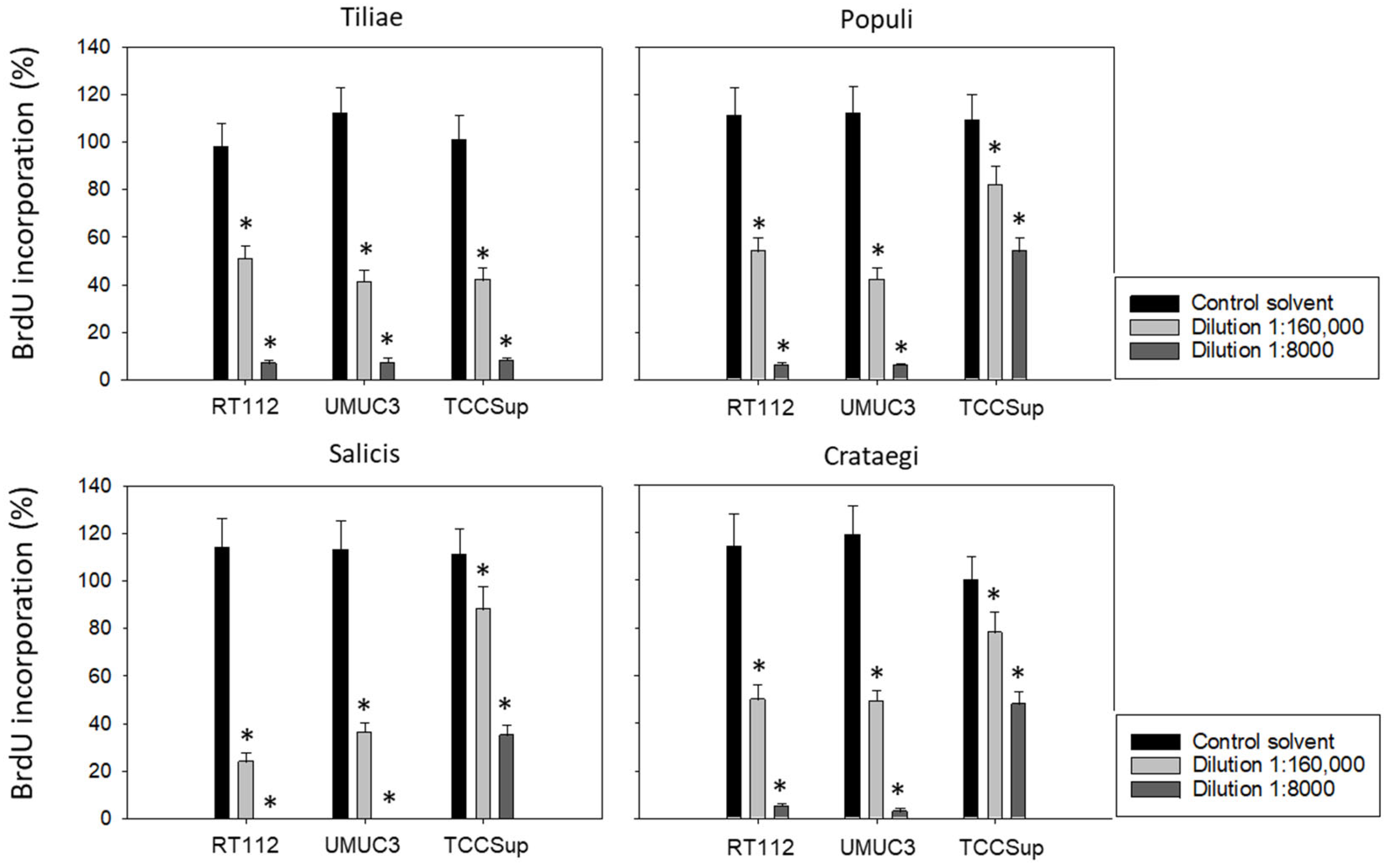
3.1. Influence of Mistletoe Extracts on Tumor Growth and Proliferation
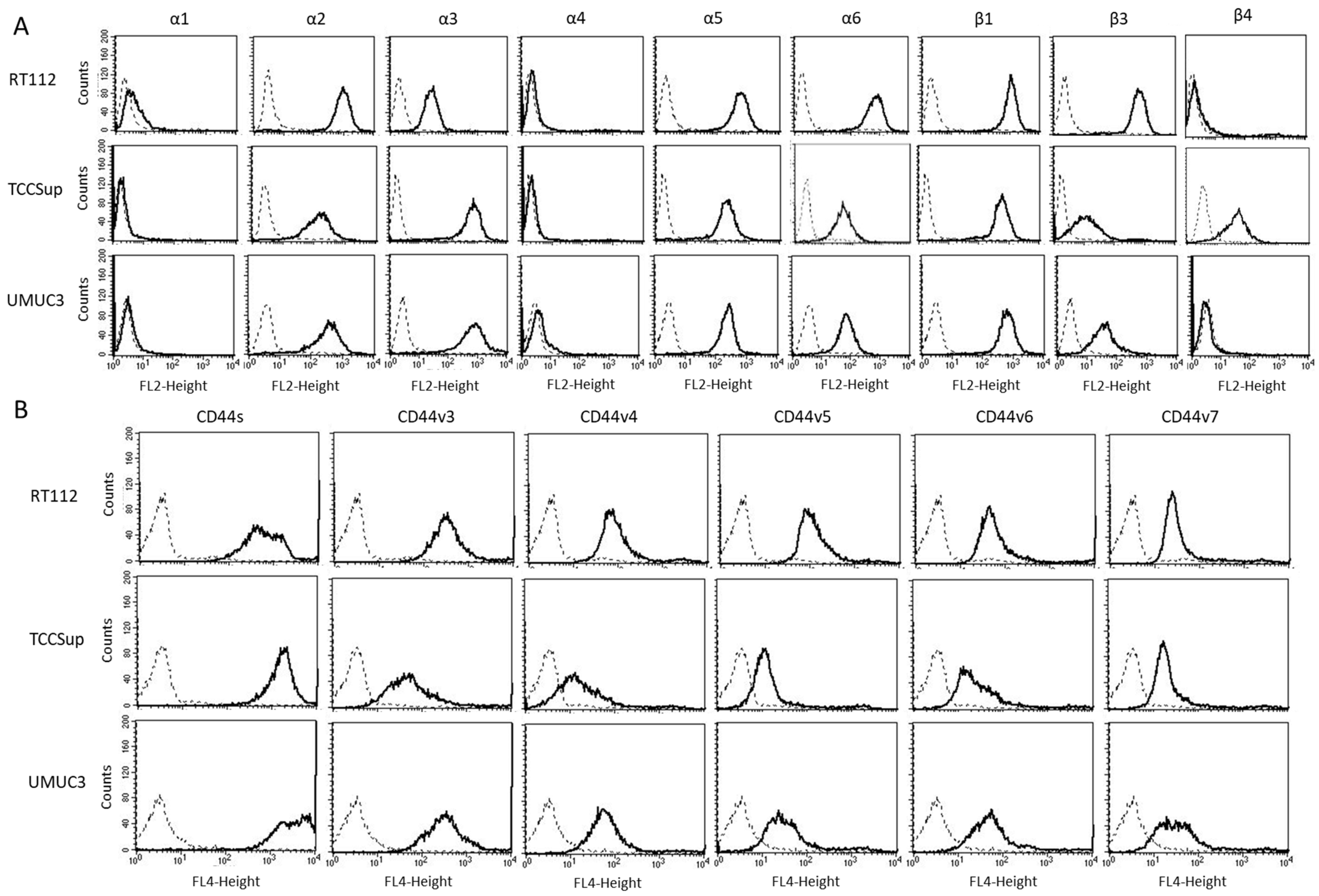
3.2. Mistletoe Extracts Alter Integrin α and β and CD44s and CD44v Expression
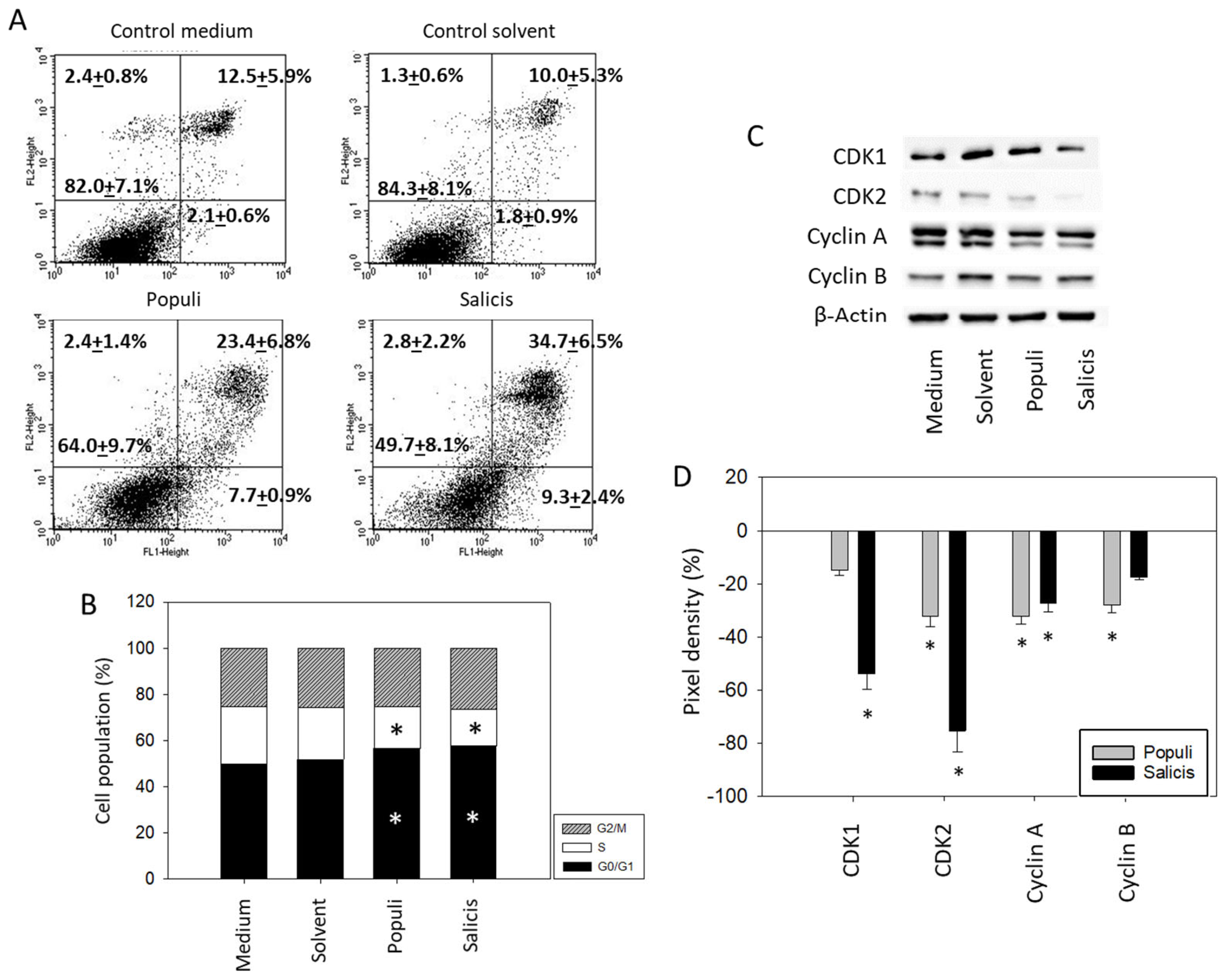
3.3. Influence of Mistletoe Extracts on Apoptosis and Cell Cycling
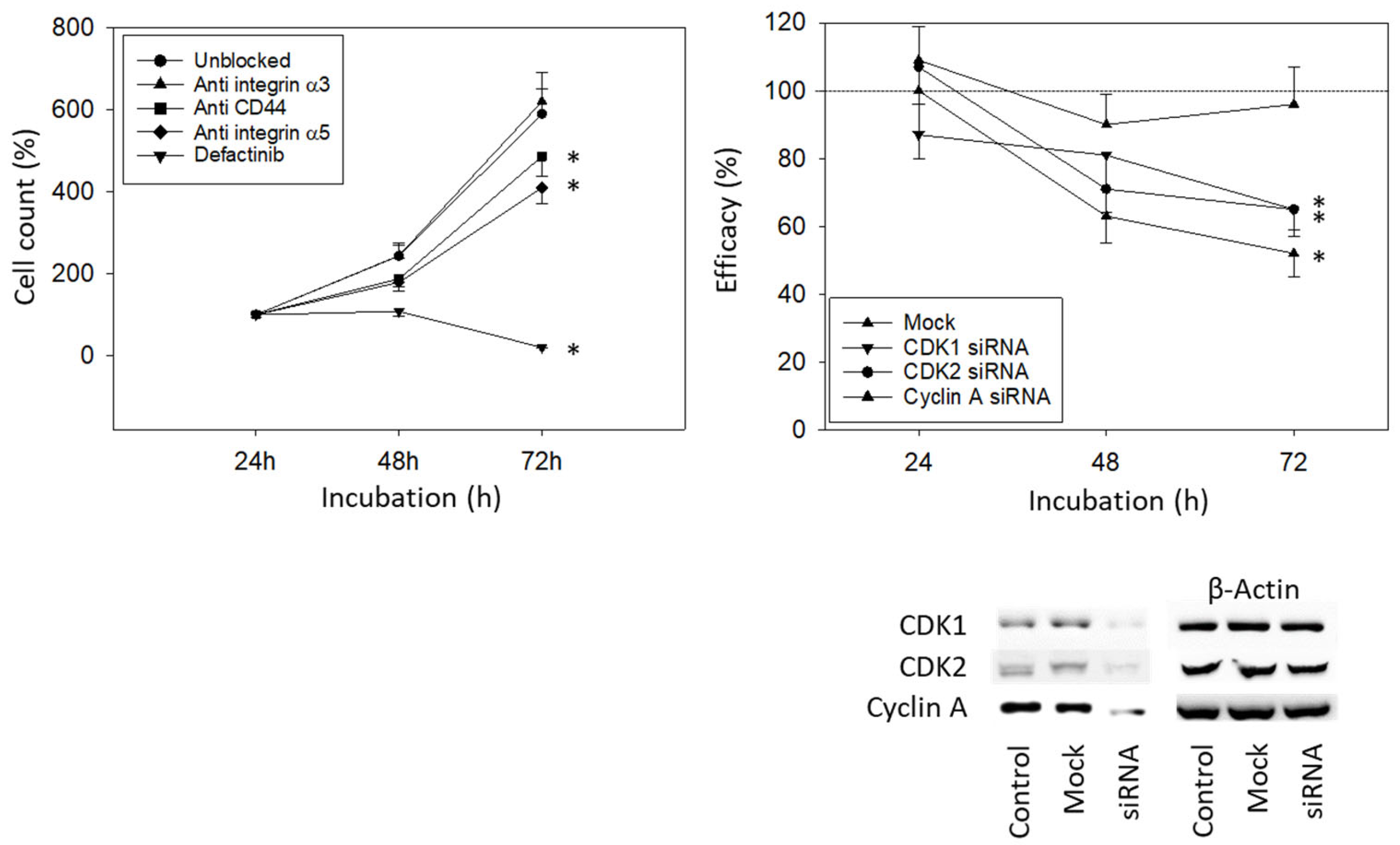
3.4. Blocking Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, V.G.; Oh, W.K.; Galsky, M.D. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA A Cancer J. Clin. 2020, 70, 404–423. [Google Scholar] [CrossRef] [PubMed]
- Bilim, V.; Kuroki, H.; Shirono, Y.; Murata, M.; Hiruma, K.; Tomita, Y. Advanced Bladder Cancer: Changing the Treatment Landscape. J. Pers. Med. 2022, 12, 1745. [Google Scholar] [CrossRef] [PubMed]
- Cockrell, D.C.; Rose, T.L. Current Status of Perioperative Therapy in Muscle-Invasive Bladder Cancer and Future Directions. Curr. Oncol. Rep. 2023, 25, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Beltran, A.; Cimadamore, A.; Blanca, A.; Massari, F.; Vau, N.; Scarpelli, M.; Cheng, L.; Montironi, R. Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer. Cancers 2021, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Cohen, A.J.; Lui, H.; Mmonu, N.A.; Brody, H.; Patino, G.; Liaw, A.; Butler, C.; Fergus, K.B.; Mena, J.; et al. Use of GoFundMe® to crowdfund complementary and alternative medicine treatments for cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann-Klemd, A.M.; Reinhardt, J.K.; Winker, M.; Gründemann, C. Phytotherapy in Integrative Oncology—An Update of Promising Treatment Options. Molecules 2022, 27, 3209. [Google Scholar] [CrossRef] [PubMed]
- Oei, S.L.; Thronicke, A.; Schad, F. Mistletoe and Immunomodulation: Insights and Implications for Anticancer Therapies. Evid.-Based Complement. Altern. Med. 2019, 2019, 5893017. [Google Scholar] [CrossRef]
- PDQ Integrative, Alternative, and Complementary Therapies Editorial Board. Mistletoe Extracts (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries [Internet]; National Cancer Institute: Bethesda, MD, USA, 2002. [Google Scholar] [PubMed]
- Weissenstein, U.; Kunz, M.; Urech, K.; Baumgartner, S. Interaction of standardized mistletoe (Viscum album) extracts with chemotherapeutic drugs regarding cytostatic and cytotoxic effects in vitro. BMC Complement. Altern. Med. 2014, 14, 6. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.-C.; Lee, C. Mistletoe (Viscum album) extract targets Axl to suppress cell proliferation and overcome cisplatin- and erlotinib-resistance in non-small cell lung cancer cells. Phytomedicine 2017, 36, 183–193. [Google Scholar] [CrossRef]
- Urech, K.; Büssing, A.; Thalmann, G.; Schaefermeyer, H.; Heusser, P. Antiproliferative effects of mistletoe (Viscum album L.) extract in urinary bladder carcinoma cell lines. Anticancer Res. 2006, 26, 3049–3055. [Google Scholar]
- Mengs, U.; Schwarz, T.; Bulitta, M.; Weber, K. Antitumoral effects of an intravesically applied aqueous mistletoe extract on urinary bladder carcinoma MB49 in mice. Anticancer Res. 2000, 20, 3565–3568. [Google Scholar] [PubMed]
- Kunze, E.; Schulz, H.; Adamek, M.; Gabius, H.-J. Long-term administration of galactoside-specific mistletoe lectin in an animal model: No protection against N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder carcinogenesis in rats and no induction of a relevant local cellular immune response. J. Cancer Res. Clin. Oncol. 2000, 126, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.; El-Leithy, T.; Dorp, F.V.; Zakaria, A.; Eisenhardt, A.; Tschirdewahn, S.; Rübben, H. Mistletoe Plant Extract in Patients with Nonmuscle Invasive Bladder Cancer: Results of a Phase Ib/IIa Single Group Dose Escalation Study. J. Urol. 2015, 194, 939–943. [Google Scholar] [CrossRef] [PubMed]
- von Schoen-Angerer, T.; Wilkens, J.; Kienle, G.S.; Kiene, H.; Vagedes, J. High-Dose Viscum album Extract Treatment in the Prevention of Recurrent Bladder Cancer: A Retrospective Case Series. Perm. J. 2015, 19, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Elsässer-Beile, U.; Leiber, C.; Wolf, P.; Lucht, M.; Mengs, U.; Wetterauer, U. Adjuvant intravesical treatment of superficial bladder cancer with a standardized mistletoe extract. J. Urol. 2005, 174, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Goebell, P.J.; Otto, T.; Suhr, J.; Rübben, H. Evaluation of an Unconventional Treatment Modality with Mistletoe Lectin to Prevent Recurrence of Superficial Bladder Cancer: A Randomized Phase ii Trial. J. Urol. 2002, 168, 72–75. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Yang, C.; Kuang, R.; Kazobinka, G.; Pang, Z.; Hou, T. Prognostic Implication of Urothelial Stem Cell Markers Differs According to Primary Tumour Location in Non-Muscle-Invasive Bladder Cancer. Cell. Physiol. Biochem. 2018, 48, 2364–2373. [Google Scholar] [CrossRef]
- Felenda, J.E.; Turek, C.; Stintzing, F.C. Antiproliferative potential from aqueous Viscum album L. preparations and their main constituents in comparison with ricin and purothionin on human cancer cells. J. Ethnopharmacol. 2019, 236, 100–107. [Google Scholar] [CrossRef]
- Zuzak, T.; Rist, L.; Viviani, A.; Eggenschwiler, J.; Mol, C.; Riegert, U.; Meyer, U. Das Mistelpräparat Iscucin®–Herstellung, Analytik, Wirkung in vitro. J. Anthr. Med. 2004, 57, 467–473. [Google Scholar]
- Eggenschwiler, J.; von Balthazar, L.; Stritt, B.; Pruntsch, D.; Ramos, M.; Urech, K.; Rist, L.; Simões-Wüst, A.P.; Viviani, A. Mistletoe lectin is not the only cytotoxic component in fermented preparations of Viscum album from white fir (Abies pectinata). BMC Complement. Altern. Med. 2007, 7, 14. [Google Scholar] [CrossRef]
- Hunziker-Basler, N.; Zuzak, T.J.; Eggenschwiler, J.; Rist, L.; Simões-Wüst, A.P.; Viviani, A. Prolonged cytotoxic effect of aqueous extracts from dried Viscum album on bladder cancer cells. Pharmazie 2007, 62, 237–238. [Google Scholar] [PubMed]
- Holandino, C.; de Oliveira Melo, M.N.; Oliveira, A.P.; da Costa Batista, J.V.; Capella, M.A.M.; Garrett, R.; Grazi, M.; Ramm, H.; Torre, C.D.; Schaller, G.; et al. Phytochemical analysis and in vitro anti-proliferative activity of Viscum album ethanolic extracts. BMC Complement. Med. Ther. 2020, 20, 215. [Google Scholar] [CrossRef] [PubMed]
- Juengel, E.; Dos Santos, S.M.; Schneider, T.; Makarević, J.; Hudak, L.; Bartsch, G.; Haferkamp, A.; Wiesner, C.; Blaheta, R.A. HDAC inhibition suppresses bladder cancer cell adhesion to collagen under flow conditions. Exp. Biol. Med. 2013, 238, 1297–1304. [Google Scholar] [CrossRef]
- Jiang, P.; Zhu, Y.; Xu, Z.; Chen, X.; Xie, L. Interference with circBC048201 inhibits the proliferation, migration, and invasion of bladder cancer cells through the miR-1184/ITGA3 axis. Mol. Cell. Biochem. 2020, 474, 83–94. [Google Scholar] [CrossRef]
- Juengel, E.; Natsheh, I.; Najafi, R.; Rutz, J.; Tsaur, I.; Haferkamp, A.; Chun, F.K.; Blaheta, R.A. Mechanisms behind Temsirolimus Resistance Causing Reactivated Growth and Invasive Behavior of Bladder Cancer Cells In Vitro. Cancers 2019, 11, 777. [Google Scholar] [CrossRef]
- Justin, S.; Rutz, J.; Maxeiner, S.; Chun, F.K.; Juengel, E.; Blaheta, R.A. Bladder Cancer Metastasis Induced by Chronic Everolimus Application Can Be Counteracted by Sulforaphane In Vitro. Int. J. Mol. Sci. 2020, 21, 5582. [Google Scholar] [CrossRef] [PubMed]
- Lityńska, A.; Przybyło, M.; Pocheć, E.; Laidler, P. Adhesion properties of human bladder cell lines with extracellular matrix components: The role of integrins and glycosylation. Acta Biochim. Pol. 2002, 49, 643–650. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Yoshino, H.; Yonemori, M.; Miyamoto, K.; Sugita, S.; Matsushita, R.; Itesako, T.; Tatarano, S.; Nakagawa, M.; Enokida, H. Regulation of ITGA3 by the dual-stranded microRNA-199 family as a potential prognostic marker in bladder cancer. Br. J. Cancer 2017, 116, 1077–1087. [Google Scholar] [CrossRef]
- Xiong, Y.; Song, X.; Kudusi; Zu, X.; Chen, M.; He, W.; Qi, L. Oncogenic GBX2 promotes the malignant behaviors of bladder cancer cells by binding to the ITGA5 promoter and activating its transcription. Funct. Integr. Genom. 2022, 22, 937–950. [Google Scholar] [CrossRef]
- Yan, T.; Ye, X.-X. MicroRNA-328-3p inhibits the tumorigenesis of bladder cancer through targeting ITGA5 and inactivating PI3K/AKT pathway. Eur. Rev. Med. Pharm. Sci. 2019, 23, 5139–5148. [Google Scholar]
- Wang, J.-F.; Chen, Y.-Y.; Zhang, S.-W.; Zhao, K.; Qiu, Y.; Wang, Y.; Wang, J.-C.; Yu, Z.; Li, B.-P.; Wang, Z.; et al. ITGA5 Promotes Tumor Progression through the Activation of the FAK/AKT Signaling Pathway in Human Gastric Cancer. Oxidative Med. Cell. Longev. 2022, 2022, 8611306. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-K.; Liao, S.-K. Differential expression of CD44 variant isoforms by cell lines and tissue specimens of transitional cell carcinomas. Anticancer Res. 2003, 23, 4635–4639. [Google Scholar] [PubMed]
- Anand, V.; Khandelwal, M.; Appunni, S.; Gupta, N.; Seth, A.; Singh, P.; Mathur, S.; Sharma, A. CD44 splice variant (CD44v3) promotes progression of urothelial carcinoma of bladder through Akt/ERK/STAT3 pathways: Novel therapeutic approach. J. Cancer Res. Clin. Oncol. 2019, 145, 2649–2661. [Google Scholar] [CrossRef] [PubMed]
- Toma, V.; Hauri, D.; Schmid, U.; Ackermann, D.; Maurer, R.; Alund, G.; Knönagel, H.; Rist, M.; Gasser, T.C.; Sauter, G.; et al. Focal Loss of CD44 Variant Protein Expression is Related to Recurrence in Superficial Bladder Carcinoma. Am. J. Pathol. 1999, 155, 1427–1432. [Google Scholar] [CrossRef]
- Gaiteiro, C.; Soares, J.; Relvas-Santos, M.; Peixoto, A.; Ferreira, D.; Paulo, P.; Brandão, A.; Fernandes, E.; Azevedo, R.; Palmeira, C.; et al. Glycoproteogenomics characterizes the CD44 splicing code associated with bladder cancer invasion. Theranostics 2022, 12, 3150–3177. [Google Scholar] [CrossRef]
- Zhang, H.; Brown, R.L.; Wei, Y.; Zhao, P.; Liu, S.; Liu, X.; Deng, Y.; Hu, X.; Zhang, J.; Gao, X.D.; et al. CD44 splice isoform switching determines breast cancer stem cell state. Genes Dev. 2019, 33, 166–179. [Google Scholar] [CrossRef]
- Rutz, J.; Thaler, S.; Maxeiner, S.; Chun, F.K.; Blaheta, R.A. Sulforaphane Reduces Prostate Cancer Cell Growth and Proliferation In Vitro by Modulating the Cdk-Cyclin Axis and Expression of the CD44 Variants 4, 5, and 7. Int. J. Mol. Sci. 2020, 21, 8724. [Google Scholar] [CrossRef]
- Shatat, M.A.; Gauthier, B.; Yoon, S.; Yuan, E.; Yang, P.; Narla, G.; Dowlati, A.; Lee, R.T. Mistletoe lectin inhibits growth of Myc-amplified small-cell lung cancer. Cancer Med. 2022, 12, 8378–8387. [Google Scholar] [CrossRef]
- Kwon, Y.-S.; Chun, S.-Y.; Kim, M.-K.; Nan, H.-Y.; Lee, C.; Kim, S. Mistletoe Extract Targets the STAT3-FOXM1 Pathway to Induce Apoptosis and Inhibits Metastasis in Breast Cancer Cells. Am. J. Chin. Med. 2021, 49, 487–504. [Google Scholar] [CrossRef]
- Beztsinna, N.; de Matos, M.B.C.; Walther, J.; Heyder, C.; Hildebrandt, E.; Leneweit, G.; Mastrobattista, E.; Kok, R.J. Quantitative analysis of receptor-mediated uptake and pro-apoptotic activity of mistletoe lectin-1 by high content imaging. Sci. Rep. 2018, 8, 2768. [Google Scholar] [CrossRef]
- Harmsma, M.; Ummelen, M.; Dignef, W.; Tusenius, K.J.; Ramaekers, F.C.S. Effects of Mistletoe (Viscum album L.) Extracts Iscador on Cell Cycle and Survival of Tumor Cells. Arzneimittelforschung 2006, 56, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Justin, S.; Rutz, J.; Maxeiner, S.; Chun, F.K.; Juengel, E.; Blaheta, R.A. Chronic Sulforaphane Administration Inhibits Resistance to the mTOR-Inhibitor Everolimus in Bladder Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4026. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Yuan, H.; Wang, W.; Zhu, Z.; Lu, Y.; Wang, J.; Feng, F.; Wu, J. Importance of PNO1 for growth and survival of urinary bladder carcinoma: Role in core-regulatory circuitry. J. Cell. Mol. Med. 2020, 24, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, J.F.; Kim, Y.S.; Lumbera, W.M.L.; Hwang, S.G. Viscum album var Hot Water Extract Mediates Anti-cancer Effects through G1 Phase Cell Cycle Arrest in SK-Hep1 Human Hepatocarcinoma cells. Asian Pac. J. Cancer Prev. 2015, 16, 6417–6421. [Google Scholar] [CrossRef] [PubMed]
- Weissenstein, U.; Kunz, M.; Urech, K.; Regueiro, U.; Baumgartner, S. Interaction of a standardized mistletoe (Viscum album) preparation with antitumor effects of Trastuzumab in vitro. BMC Complement. Altern. Med. 2016, 16, 271. [Google Scholar] [CrossRef]
- Kleinsimon, S.; Longmuss, E.; Rolff, J.; Jäger, S.; Eggert, A.; Delebinski, C.; Seifert, G. GADD45A and CDKN1A are involved in apoptosis and cell cycle modulatory effects of viscumTT with further inactivation of the STAT3 pathway. Sci. Rep. 2018, 8, 5750. [Google Scholar] [CrossRef]
- Schad, F.; Thronicke, A. Safety of Combined Targeted and Helixor® Viscum album L. Therapy in Breast and Gynecological Cancer Patients, a Real-World Data Study. Int. J. Environ. Res. Public Health 2023, 20, 2565. [Google Scholar] [CrossRef]
- Bryant, S.; Duncan, L.; Feder, G.; Huntley, A.L. A pilot study of the mistletoe and breast cancer (MAB) trial: A protocol for a randomised double-blind controlled trial. Pilot Feasibility Stud. 2022, 8, 78. [Google Scholar] [CrossRef]
- Paller, C.J.; Wang, L.; Fu, W.; Kumar, R.; Durham, J.N.; Azad, N.S.; Laheru, D.A.; Browner, I.; Kachhap, S.K.; Boyapati, K.; et al. Phase I Trial of Intravenous Mistletoe Extract in Advanced Cancer. Cancer Res. Commun. 2023, 3, 338–346. [Google Scholar] [CrossRef]
- Schnell-Inderst, P.; Steigenberger, C.; Mertz, M.; Otto, I.; Flatscher-Thöni, M.; Siebert, U. Additional treatment with mistletoe extracts for patients with breast cancer compared to conventional cancer therapy alone–efficacy and safety, costs and cost-effectiveness, patients and social aspects, and ethical assessment. Ger. Med. Sci. 2022, 20, Doc10. [Google Scholar] [CrossRef]
- Lee, R.T.; Yang, P.; Alahmadi, A.; McQuade, J.; Yuan, E.; Difeo, A.; Narla, G.; Kaseb, A. Mistletoe Extract Viscum Fraxini-2 for Treatment of Advanced Hepatocellular Carcinoma: A Case Series. Case Rep. Oncol. 2021, 14, 224–231. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juengel, E.; Rutz, J.; Meiborg, M.; Markowitsch, S.D.; Maxeiner, S.; Grein, T.; Thomas, A.; Chun, F.K.-H.; Haferkamp, A.; Tsaur, I.; et al. Mistletoe Extracts from Different Host Trees Disparately Inhibit Bladder Cancer Cell Growth and Proliferation. Cancers 2023, 15, 4849. https://doi.org/10.3390/cancers15194849
Juengel E, Rutz J, Meiborg M, Markowitsch SD, Maxeiner S, Grein T, Thomas A, Chun FK-H, Haferkamp A, Tsaur I, et al. Mistletoe Extracts from Different Host Trees Disparately Inhibit Bladder Cancer Cell Growth and Proliferation. Cancers. 2023; 15(19):4849. https://doi.org/10.3390/cancers15194849
Chicago/Turabian StyleJuengel, Eva, Jochen Rutz, Moritz Meiborg, Sascha D. Markowitsch, Sebastian Maxeiner, Timothy Grein, Anita Thomas, Felix K.-H. Chun, Axel Haferkamp, Igor Tsaur, and et al. 2023. "Mistletoe Extracts from Different Host Trees Disparately Inhibit Bladder Cancer Cell Growth and Proliferation" Cancers 15, no. 19: 4849. https://doi.org/10.3390/cancers15194849
APA StyleJuengel, E., Rutz, J., Meiborg, M., Markowitsch, S. D., Maxeiner, S., Grein, T., Thomas, A., Chun, F. K.-H., Haferkamp, A., Tsaur, I., Vakhrusheva, O., & Blaheta, R. A. (2023). Mistletoe Extracts from Different Host Trees Disparately Inhibit Bladder Cancer Cell Growth and Proliferation. Cancers, 15(19), 4849. https://doi.org/10.3390/cancers15194849






