Focal Boost in Prostate Cancer Radiotherapy: A Review of Planning Studies and Clinical Trials
Abstract
:Simple Summary
Abstract
1. Introduction
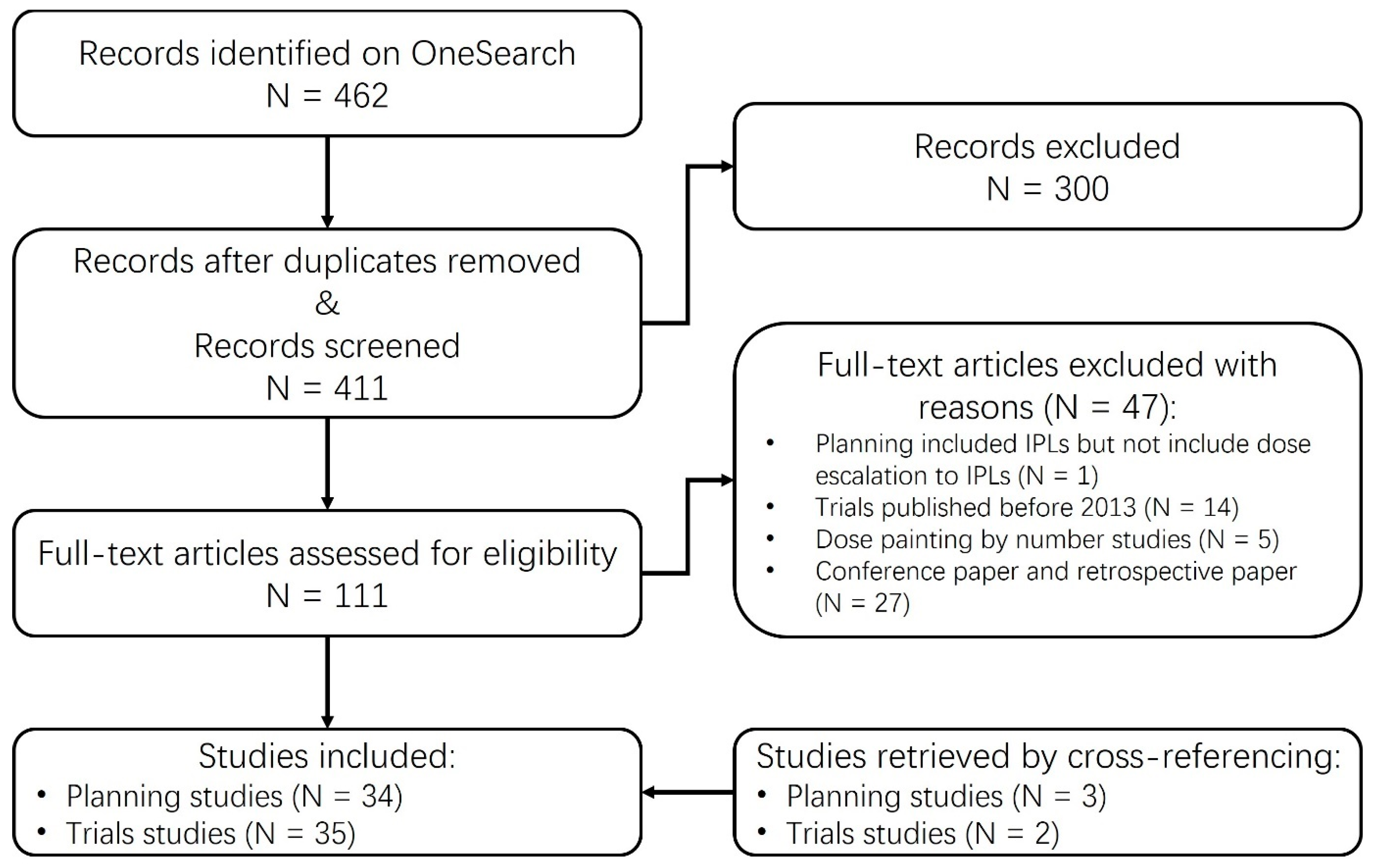
2. Methods
Study Inclusion Criteria
- The studies had to include the defined GTV within the prostate gland.
- The studies had to incorporate dose escalation specifically to the GTV, in comparison to the prescribed dose administered to the remaining prostate.
3. Topical Review
Statistics of Reviewed Studies
4. Overview of Planning Methodology
4.1. GTV Identification
4.1.1. GTV Identification by MRI
4.1.2. GTV Identification by PET-CT
4.2. Comparison and Utilization of MRI and PET-CT in GTV Identification
4.3. GTV Margin
4.4. Dose Prescription
4.5. Derivation of Dose Prescription from Conventional Radiotherapy
4.6. Derivation of Dose Prescription by Radio-Biological Features
5. Overview of Results
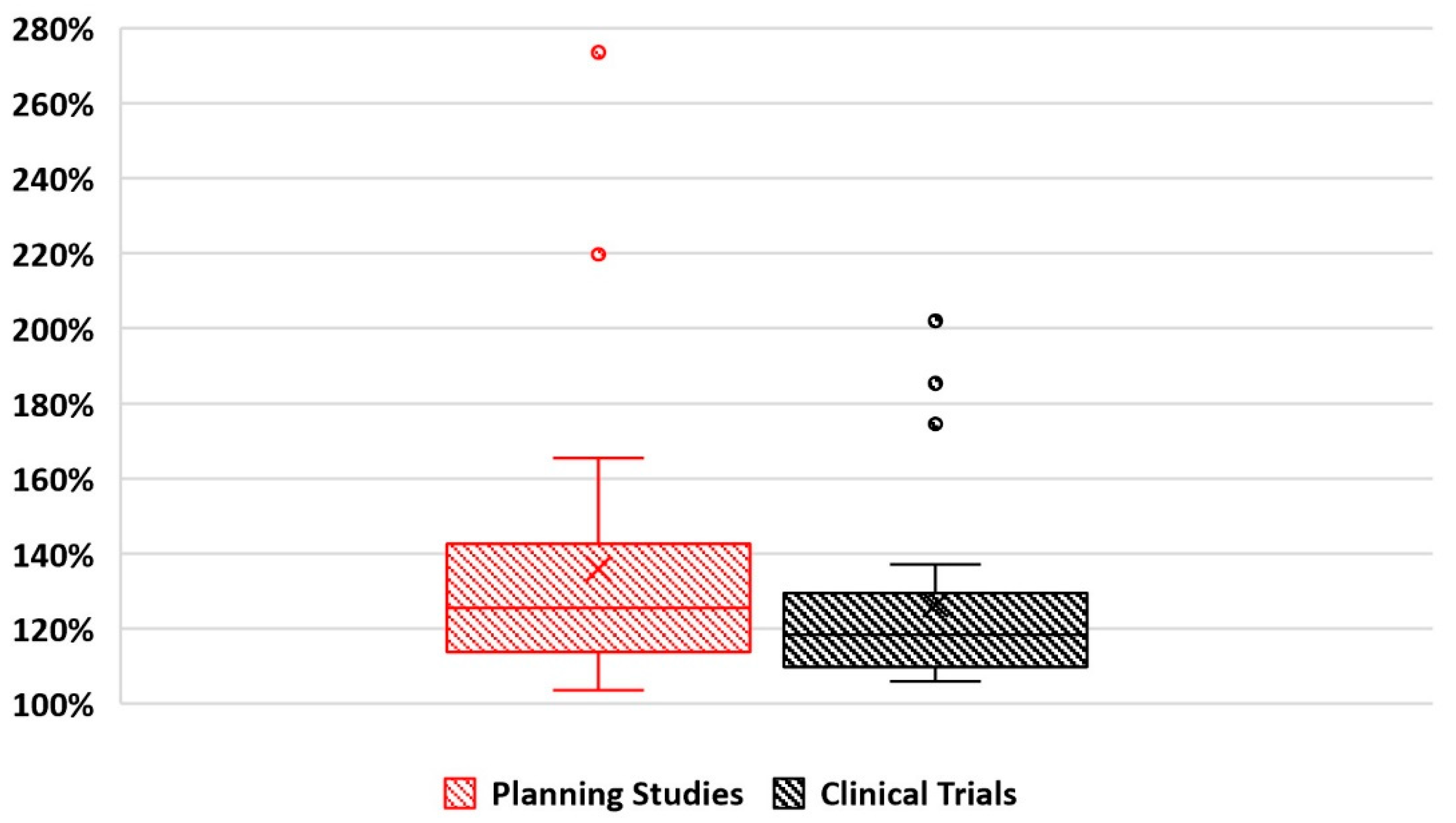
5.1. Dosimetric Outcomes
5.2. Dose-Limiting Factors
5.3. Treatment Modality Comparison
5.4. Toxicity
5.5. Clinical Efficacy
5.5.1. Conventional Fractionation Focal Boost Trials
5.5.2. Extreme Hypo-Fractionation Focal Boost Trials
5.5.3. Brachytherapy Focal Boost Trials
5.6. Challenges in Focal Boost Studies
5.7. Future Opportunities
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADC | Apparent diffusion coefficient |
| ADT | Androgen deprivation therapy |
| bDFS | Biochemical disease-free survival |
| BEDs | Biologically effective doses |
| BF | Biochemical failures |
| bRFS | Biochemical relapse-free survival |
| BTV | Biological target volume |
| CC | Corpora cavernosa |
| DCE | Dynamic contrast-enhanced MR perfusion |
| DFS | Disease-free survival |
| DLT | Dose-limiting toxicities |
| DWI | diffusion-weighted MRI |
| EBRT | External beam radiotherapy |
| ED | Erectile dysfunction |
| ERBs | Endorectal balloons |
| GI | Gastrointestinal |
| GU | Genitourinary |
| GTV | Gross tumor volume |
| HDR | High-dose-rate |
| IGRT | Image-guided radiation therapy |
| IMRT | Intensity modulated radiotherapy |
| IMPT | Intensity modulated proton therapy |
| IPL | Intra-prostatic lesion |
| IPAs | Internal pudendal arteries |
| Ktrans | Volume transfer constant |
| LDR | Low-dose-rate |
| mpMRI | Multi-parametric MRI |
| MRSI | MR spectroscopic imaging |
| NTCP | Normal tissue complication probability |
| NVB | Neurovascular bundles |
| OARs | Organs at risk |
| OS | Overall survival |
| PB | Penile bulb |
| PBS | Pencil beam scanning |
| PCa | Prostate cancer |
| PET-CT | Positron emission tomography |
| PFS | Progression-free survival |
| ProxSV | Proximal seminal vesicles |
| PSA | Prostate-specific antigen |
| PS | Passive scattering |
| PUC | Probability of uncomplicated control |
| PVE | Partial-volume effect |
| PZ | Peripheral zone |
| RCT | Randomized controlled trial |
| ROI | Region of interest |
| SOBP | Spread-out proton Bragg peak |
| SUV | Standardized uptake value |
| SVs | Seminal vesicles |
| T2w/T1w | T2/T1 weighted MRI |
| TBR | Tumor-to-background ratio |
| TRUS | Transrectal ultrasound |
| TURP | Transurethral resection of the prostate |
| VMAT | Volumetric modulated arc therapy |
References
- International Agency for Research on Cancer 2020. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today (accessed on 1 November 2020).
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent global patterns in prostate cancer incidence and mortality rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar]
- Opell, M.B.; Zeng, J.; Bauer, J.J.; Connelly, R.R.; Zhang, W.; Sesterhenn, I.A.; Lynch, J.H. Investigating the distribution of prostate cancer using three-dimensional computer simulation. Prostate Cancer Prostatic Dis. 2002, 5, 204–208. [Google Scholar]
- Greene, D.R.; Fitzpatrick, J.M.; Scardino, P.T. Anatomy of the prostate and distribution of early prostate cancer. In Seminars in Surgical Oncology; Wiley Online Library: Hoboken, NJ, USA, 1995. [Google Scholar]
- Cheng, L.; Jones, T.D.; Pan, C.X.; Barbarin, A.; Eble, J.N.; Koch, M.O. Anatomic distribution and pathologic characterization of small-volume prostate cancer (<0.5 mL) in whole-mount prostatectomy specimens. Mod. Pathol. 2005, 18, 1022–1026. [Google Scholar] [PubMed]
- Pucar, D.; Hricak, H.; Shukla-Dave, A.; Kuroiwa, K.; Drobnjak, M.; Eastham, J.; Scardino, P.T.; Zelefsky, M.J. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: Magnetic resonance imaging and step-section pathology evidence. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Cellini, N.; Morganti, A.G.; Mattiucci, G.C.; Valentini, V.; Leone, M.; Luzi, S.; Manfredi, R.; Dinapoli, N.; Digesu, C.; Smaniotto, D. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: Implications for conformal therapy planning. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Her, E.; Haworth, A.; Reynolds, H.; Sun, Y.; Kennedy, A.; Panettieri, V.; Bangert, M.; Williams, S.; Ebert, M. Voxel-level biological optimisation of prostate IMRT using patient-specific tumour location and clonogen density derived from mpMRI. Radiat. Oncol. 2020, 15, 172. [Google Scholar]
- Ling, C.C.; Humm, J.; Larson, S.; Amols, H.; Fuks, Z.; Leibel, S.; Koutcher, J.A. Towards multidimensional radiotherapy (MD-CRT): Biological imaging and biological conformality. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 551–560. [Google Scholar] [PubMed]
- De Meerleer, G.; Villeirs, G.; Bral, S.; Paelinck, L.; De Gersem, W.; Dekuyper, P.; De Neve, W. The magnetic resonance detected intraprostatic lesion in prostate cancer: Planning and delivery of intensity-modulated radiotherapy. Radiother. Oncol. 2005, 75, 325–333. [Google Scholar]
- Pinkawa, M.; Attieh, C.; Piroth, M.D.; Holy, R.; Nussen, S.; Klotz, J.; Hawickhorst, R.; Schäfer, W.; Eble, M.J. Dose-escalation using intensity-modulated radiotherapy for prostate cancer—Evaluation of the dose distribution with and without18 F-choline PET-CT detected simultaneous integrated boost. Radiother. Oncol. 2009, 93, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, J.; Seppänen, M.; Arponen, E.; Lindholm, P.; Minn, H. Carbon-11 acetate PET/CT based dose escalated IMRT in prostate cancer. Radiother. Oncol. 2009, 93, 234–240. [Google Scholar]
- Søvik, Å.; Malinen, E.; Olsen, D.R. Strategies for biologic image-guided dose escalation: A review. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 650–658. [Google Scholar] [PubMed]
- Hanks, G.E.; Hanlon, A.L.; Schultheiss, T.E.; Pinover, W.H.; Movsas, B.; Epstein, B.E.; Hunt, M.A. Dose escalation with 3D conformal treatment: Five year outcomes, treatment optimization, and future directions. Int. J. Radiat. Oncol. Biol. Phys. 1998, 41, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A.; Zagars, G.K.; Smith, L.G.; Lee, J.J.; von Eschenbach, A.C.; Antolak, J.A.; Starkschall, G.; Rosen, I. Preliminary results of a randomized radiotherapy dose-escalation study comparing 70 Gy with 78 Gy for prostate cancer. J. Clin. Oncol. 2000, 18, 3904–3911. [Google Scholar] [CrossRef] [PubMed]
- Zelefsky, M.J.; Fuks, Z.; Wolfe, T.; Kutcher, G.J.; Burman, C.; Ling, C.C.; Venkatraman, E.S.; Leibel, S.A. Locally advanced prostatic cancer: Long-term toxicity outcome after three-dimensional conformal radiation therapy—A dose-escalation study. Radiology 1998, 209, 169–174. [Google Scholar] [CrossRef]
- Pickett, B.; Vigneault, E.; Kurhanewicz, J.; Verhey, L.; Roach, M. Static field intensity modulation to treat a dominant intra-prostatic lesion to 90 Gy compared to seven field 3-dimensional radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 921–929. [Google Scholar]
- van Lin, E.N.; Fütterer, J.J.; Heijmink, S.W.; van der Vight, L.P.; Hoffmann, A.L.; van Kollenburg, P.; Huisman, H.J.; Scheenen, T.W.; Witjes, J.A.; Leer, J.W. IMRT boost dose planning on dominant intraprostatic lesions: Gold marker-based three-dimensional fusion of CT with dynamic contrast-enhanced and 1H-spectroscopic MRI. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 291–303. [Google Scholar]
- Singh, A.K.; Guion, P.; Sears-Crouse, N.; Ullman, K.; Smith, S.; Albert, P.S.; Fichtinger, G.; Choyke, P.L.; Xu, S.; Kruecker, J. Simultaneous integrated boost of biopsy proven, MRI defined dominant intra-prostatic lesions to 95 Gray with IMRT: Early results of a phase I NCI study. Radiat. Oncol. 2007, 2, 36. [Google Scholar]
- Fonteyne, V.; Villeirs, G.; Speleers, B.; De Neve, W.; De Wagter, C.; Lumen, N.; De Meerleer, G. Intensity-modulated radiotherapy as primary therapy for prostate cancer: Report on acute toxicity after dose escalation with simultaneous integrated boost to intraprostatic lesion. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 799–807. [Google Scholar]
- Buwenge, M.; Alitto, A.R.; Cilla, S.; Capocaccia, I.; Mazzeo, E.; Ippolito, E.; Mantini, G.; Siepe, G.; Cavallini, L.; Valentini, V. Simultaneous integrated radiotherapy boost to the dominant intraprostatic lesion: Final results of a phase I/II trial. Anticancer Res. 2020, 40, 6499–6503. [Google Scholar] [CrossRef]
- Ippolito, E.; Mantini, G.; Morganti, A.G.; Mazzeo, E.; Padula, G.D.; Digesù, C.; Cilla, S.; Frascino, V.; Luzi, S.; Massaccesi, M. Intensity-modulated radiotherapy with simultaneous integrated boost to dominant intraprostatic lesion: Preliminary report on toxicity. Am. J. Clin. Oncol. 2012, 35, 158–162. [Google Scholar]
- Murray, J.R.; Tree, A.C.; Alexander, E.J.; Sohaib, A.; Hazell, S.; Thomas, K.; Gunapala, R.; Parker, C.C.; Huddart, R.A.; Gao, A. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Efficacy and toxicity in the delineate trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 715–724. [Google Scholar] [PubMed]
- Tree, A.C.; Satchwell, L.; Alexander, E.; Blasiak-Wal, I.; deSouza, N.M.; Gao, A.; Greenlay, E.; McNair, H.; Parker, C.; Talbot, J.; et al. Standard and Hypofractionated Dose Escalation to Intraprostatic Tumor Nodules in Localized Prostate Cancer: 5-Year Efficacy and Toxicity in the DELINEATE Trial. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 305–316. [Google Scholar] [CrossRef]
- Kerkmeijer, L.G.; Groen, V.H.; Pos, F.J.; Haustermans, K.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; de Boer, J.C.; van der Voort van Zijp, J.; van Vulpen, M. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients with Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 787–796. [Google Scholar]
- King, M.T.; Nasser, N.J.; Mathur, N.; Cohen, G.a.N.; Kollmeier, M.A.; Yuen, J.; Vargas, H.A.; Pei, X.; Yamada, Y.; Zakian, K.L.; et al. Long-term outcome of magnetic resonance spectroscopic image–directed dose escalation for prostate brachytherapy. Brachytherapy 2016, 15, 266–273. [Google Scholar] [CrossRef]
- Onjukka, E.; Uzan, J.; Baker, C.; Howard, L.; Nahum, A.; Syndikus, I. Twenty fraction prostate radiotherapy with intra-prostatic boost: Results of a pilot study. Clin. Oncol. 2017, 29, 6–14. [Google Scholar]
- McDonald, A.M.; Dobelbower, M.C.; Yang, E.S.; Clark, G.M.; Jacob, R.; Kim, R.Y.; Cardan, R.A.; Popple, R.; Nix, J.W.; Rais-Bahrami, S. Prostate stereotactic body radiation therapy with a focal simultaneous integrated boost: Acute toxicity and dosimetry results from a prospective trial. Adv. Radiat. Oncol. 2019, 4, 90–95. [Google Scholar]
- Pollack, A.; Chinea, F.M.; Bossart, E.; Kwon, D.; Abramowitz, M.C.; Lynne, C.; Jorda, M.; Marples, B.; Patel, V.N.; Wu, X. Phase I trial of MRI-guided prostate cancer lattice extreme ablative dose (LEAD) boost radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 305–315. [Google Scholar] [PubMed]
- Herrera, F.G.; Valerio, M.; Berthold, D.; Tawadros, T.; Meuwly, J.-Y.; Vallet, V.; Baumgartner, P.; Thierry, A.-C.; De Bari, B.; Jichlinski, P. 50-Gy stereotactic body radiation therapy to the dominant intraprostatic nodule: Results from a phase 1a/b trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 320–334. [Google Scholar] [PubMed]
- Hannan, R.; Salamekh, S.; Desai, N.B.; Garant, A.; Folkert, M.R.; Costa, D.N.; Mannala, S.; Ahn, C.; Mohamad, O.; Laine, A. SABR for high-risk prostate cancer: A prospective multilevel MRI-based dose escalation trial. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 290–301. [Google Scholar] [PubMed]
- Aluwini, S.; van Rooij, P.; Hoogeman, M.; Kirkels, W.; Kolkman-Deurloo, I.-K.; Bangma, C. Stereotactic body radiotherapy with a focal boost to the MRI-visible tumor as monotherapy for low-and intermediate-risk prostate cancer: Early results. Radiat. Oncol. 2013, 8, 84. [Google Scholar]
- Marvaso, G.; Gugliandolo, S.G.; Bellerba, F.; Gandini, S.; Corrao, G.; Volpe, S.; Rojas, D.P.; Riva, G.; Zerini, D.; Pepa, M.; et al. Phase II prospective trial “Give Me Five” short-term high precision radiotherapy for early prostate cancer with simultaneous boost to the dominant intraprostatic lesion: The impact of toxicity on quality of life (AIRC IG-13218). Med. Oncol. 2020, 37, 74. [Google Scholar] [CrossRef]
- Sanmamed, N.; Lee, J.; Berlin, A.; Craig, T.; Lao, B.; Rink, A.; Bayley, A.; Catton, C.; Sundaramurthy, A.; Foltz, W. Tumor-targeted dose escalation for localized prostate cancer using MR-guided HDR brachytherapy (HDR) or integrated VMAT (IB-VMAT) boost: Dosimetry, toxicity and health related quality of life. Radiother. Oncol. 2020, 149, 240–245. [Google Scholar] [PubMed]
- Zapatero, A.; Roch, M.; Castro Tejero, P.; Büchser, D.; Martin de Vidales, C.; González, S.; Rodríguez, P.; San Jose, L.A.; Celada, G.; Murillo, M.T. MRI-guided focal boost to dominant intraprostatic lesion using volumetric modulated arc therapy in prostate cancer. Results of a phase II trial. Br. J. Radiol. 2021, 95, 20210683. [Google Scholar] [CrossRef]
- Rezaeijo, S.M.; Hashemi, B.; Mofid, B.; Bakhshandeh, M.; Mahdavi, A.; Hashemi, M.S. The feasibility of a dose painting procedure to treat prostate cancer based on mpMR images and hierarchical clustering. Radiat. Oncol. 2021, 16, 182. [Google Scholar] [CrossRef]
- Armstrong, S.; Brown, S.; Stancliffe, M.; Ostler, P.; Hughes, R.; Hoskin, P.; Alonzi, R. Single dose high-dose-rate brachytherapy with focal dose escalation for prostate cancer: Mature results of a phase 2 clinical trial. Radiother. Oncol. 2021, 159, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, L.; Suh, Y.-E.; Chapman, E.; Henderson, D.; Jones, C.; Morrison, K.; Sohaib, A.; Taylor, H.; Tree, A.; van As, N. Stereotactic radiotherapy with focal boost for intermediate and high-risk prostate cancer: Initial results of the SPARC trial. Clin. Transl. Radiat. Oncol. 2020, 25, 88–93. [Google Scholar]
- Draulans, C.; van der Heide, U.A.; Haustermans, K.; Pos, F.J.; van Zyp, J.v.d.V.; De Boer, H.; Groen, V.H.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M. Primary endpoint analysis of the multicentre phase II hypo-FLAME trial for intermediate and high risk prostate cancer. Radiother. Oncol. 2020, 147, 92–98. [Google Scholar]
- Alayed, Y.; D’Alimonte, L.; Helou, J.; Ravi, A.; Morton, G.; Chung, H.T.; Haider, M.; McGuffin, M.; Zhang, L.; Loblaw, A. MRI assisted focal boost integrated with HDR monotherapy study in low and intermediate risk prostate cancer (MARS): Results from a phase II clinical trial. Radiother. Oncol. 2019, 141, 144–148. [Google Scholar] [CrossRef]
- Alayed, Y.; Davidson, M.; Liu, S.; Chu, W.; Tseng, E.; Cheung, P.; Vesprini, D.; Cheung, H.; Morton, G.; Musunuru, H.B. Evaluating the tolerability of a simultaneous focal boost to the gross tumor in prostate SABR: A toxicity and quality-of-life comparison of two prospective trials. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 136–142. [Google Scholar]
- Timon, G.; Ciardo, D.; Bazani, A.; Marvaso, G.; Riva, G.; Volpe, S.; Rojas, D.P.; Renne, G.; Petralia, G.; Zerini, D.; et al. Short-term high precision radiotherapy for early prostate cancer with concomitant boost to the dominant lesion: Ad interim analysis and preliminary results of Phase II trial AIRC-IG-13218. Br. J. Radiol. 2018, 91, 20160725. [Google Scholar] [CrossRef]
- Dankulchai, P.; Sittiwong, W.; Teerasamit, W. Feasibility and safety of definite volumetric modulated arc therapy with simultaneous integrated boost to the dominant intraprostatic lesion in patients with unfavorable intermediate to high-risk prostate cancer. Rep. Pract. Oncol. Radiother. 2022, 27, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Schild, M.H.; Schild, S.E.; Wong, W.W.; Vora, S.A.; Silva, A.C.; Silva, A.M.; Daniels, T.B.; Keole, S.R. Early outcome of prostate intensity modulated radiation therapy (IMRT) incorporating a simultaneous intra-prostatic MRI directed boost. OMICS J. Radiol. 2014, 3, 170. [Google Scholar]
- Crook, J.; Ots, A.; Gaztañaga, M.; Schmid, M.; Araujo, C.; Hilts, M.; Batchelar, D.; Parker, B.; Bachand, F.; Milette, M.-P. Ultrasound-planned high-dose-rate prostate brachytherapy: Dose painting to the dominant intraprostatic lesion. Brachytherapy 2014, 13, 433–441. [Google Scholar] [PubMed]
- Sundahl, N.; De Meerleer, G.; Villeirs, G.; Ost, P.; De Neve, W.; Lumen, N.; De Visschere, P.; Van Eijkeren, M.; Fonteyne, V. Combining high dose external beam radiotherapy with a simultaneous integrated boost to the dominant intraprostatic lesion: Analysis of genito-urinary and rectal toxicityToxicity after integrated boost for prostate cancer. Radiother. Oncol. 2016, 119, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Iturriaga, A.; Casquero, F.; Urresola, A.; Ezquerro, A.; Lopez, J.I.; Espinosa, J.M.; Minguez, P.; Llarena, R.; Irasarri, A.; Bilbao, P.; et al. Dose escalation to dominant intraprostatic lesions with MRI-transrectal ultrasound fusion High-Dose-Rate prostate brachytherapy. Prospective phase II trial. Radiother. Oncol. 2016, 119, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Uzan, J.; Nahum, A.; Syndikus, I. Prostate dose-painting radiotherapy and radiobiological guided optimisation enhances the therapeutic ratio. Clin. Oncol. 2016, 28, 165–170. [Google Scholar]
- Kuisma, A.; Wright, P.; Suilamo, S.; Seppälä, J.; Koivisto, M.; Lindholm, P.; Minn, H. Long-term outcome of biologically guided dose-escalated radiotherapy of localized prostate cancer. Acta Oncol. 2021, 61, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Schlenter, M.; Berneking, V.; Krenkel, B.; Mottaghy, F.M.; Vögeli, T.-A.; Eble, M.J.; Pinkawa, M. Intensity-modulated radiotherapy of prostate cancer with simultaneous integrated boost after molecular imaging with 18F-choline-PET/CT: Clinical results and quality of life. Strahlenther. Onkol. 2018, 194, 638–645. [Google Scholar] [CrossRef]
- Eade, T.; Kneebone, A.; Hruby, G.; Booth, J.; Hsiao, E.; Le, A.; Kwong, C.; Atyeo, J.; Brown, C.; Hunter, J.; et al. Early Outcomes and Decision Regret Using PSMA/MRI-Guided Focal Boost for Prostate Cancer SBRT. Pract. Radiat. Oncol. 2022, 12, e201–e206. [Google Scholar] [CrossRef]
- Zamboglou, C.; Spohn, S.K.; Ruf, J.; Benndorf, M.; Gainey, M.; Kamps, M.; Jilg, C.; Gratzke, C.; Adebahr, S.; Schmidtmayer-Zamboglou, B. PSMA-PET-and MRI-Based Focal Dose Escalated Radiation Therapy of Primary Prostate Cancer: Planned Safety Analysis of a Nonrandomized 2-Armed Phase 2 Trial (ARO2020-01). Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 1025–1035. [Google Scholar]
- Guimond, E.; Lavallée, M.-C.; Foster, W.; Vigneault, É.; Guay, K.; Martin, A.-G. Impact of a dominant intraprostatic lesion (DIL) boost defined by sextant biopsy in permanent I-125 prostate implants on biochemical disease free survival (bDFS) and toxicity outcomes. Radiother. Oncol. 2019, 133, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Strnad, V.; Lotter, M.; Kreppner, S.; Fietkau, R. Brachytherapy focal dose escalation using ultrasound based tissue characterization by patients with non-metastatic prostate cancer: Five-year results from single-center phase 2 trial. Brachytherapy 2022, 21, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Ennis, R.D.; Quinn, S.A.; Trichter, F.; Ryemon, S.; Jain, A.; Saigal, K.; Chandrashekhar, S.; Romas, N.A.; Feleppa, E.J. Phase I/II prospective trial of cancer-specific imaging using ultrasound spectrum analysis tissue-type imaging to guide dose-painting prostate brachytherapy. Brachytherapy 2015, 14, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Zamboglou, C.; Spohn, S.K.B.; Adebahr, S.; Huber, M.; Kirste, S.; Sprave, T.; Gratzke, C.; Chen, R.C.; Carl, E.G.; Weber, W.A.; et al. Psma-pet/mri-based focal dose escalation in patients with primary prostate cancer treated with stereotactic body radiation therapy (Hypofocal-sbrt): Study protocol of a randomized, multicentric phase iii trial. Cancers 2021, 13, 5795. [Google Scholar] [CrossRef]
- Cambria, R.; Ciardo, D.; Bazani, A.; Pansini, F.; Rondi, E.; Maestri, D.; Zerini, D.; Marvaso, G.; Romanelli, P.; Timon, G.; et al. Ultrahypofractionated radiotherapy for localized prostate cancer with simultaneous boost to the dominant intraprostatic lesion: A plan comparison. Tumori 2021, 108, 263–269. [Google Scholar] [CrossRef]
- Murray, L.J.F.; Lilley, J.M.; Thompson, C.M.M.; Cosgrove, V.P.; Mason, J.M.; Sykes, J.P.; Franks, K.F.; Sebag-Montefiore, D.F.; Henry, A.M.F. Prostate Stereotactic Ablative Radiation Therapy Using Volumetric Modulated Arc Therapy to Dominant Intraprostatic Lesions. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 406–415. [Google Scholar] [CrossRef]
- Ciabatti, S.; Ntreta, M.; Buwenge, M.; Gaudiano, C.; Sessagesimi, E.; Romani, F.; Angelini, A.L.; Cammelli, S.; Macchia, G.; Deodato, F.; et al. Dominant intraprostatic lesion boosting in sexual-sparing radiotherapy of prostate cancer: A planning feasibility study. Med. Dosim. Off. J. Am. Assoc. Med. Dosim. 2019, 44, 356–364. [Google Scholar] [CrossRef]
- Blake, S.W.; Stapleton, A.; Brown, A.; Curtis, S.; Ash-Miles, J.; Dennis, E.; Masson, S.; Bowers, D.; Hilman, S. A study of the clinical, treatment planning and dosimetric feasibility of dose painting in external beam radiotherapy of prostate cancer. Phys. Imaging Radiat. Oncol. 2020, 15, 66–71. [Google Scholar]
- Mason, J.; Al-Qaisieh, B.; Bownes, P.; Wilson, D.; Buckley, D.L.; Thwaites, D.; Carey, B.; Henry, A. Multi-parametric MRI-guided focal tumor boost using HDR prostate brachytherapy: A feasibility study. Brachytherapy 2014, 13, 137–145. [Google Scholar] [CrossRef]
- Ost, P.; Speleers, B.; De Meerleer, G.; De Neve, W.; Fonteyne, V.; Villeirs, G.; De Gersem, W. Volumetric arc therapy and intensity-modulated radiotherapy for primary prostate radiotherapy with simultaneous integrated boost to intraprostatic lesion with 6 and 18 MV: A planning comparison study. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 920–926. [Google Scholar]
- Housri, N.; Ning, H.; Ondos, J.; Choyke, P.; Camphausen, K.; Citrin, D.; Arora, B.; Shankavaram, U.; Kaushal, A. Parameters favorable to intraprostatic radiation dose escalation in men with localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 614–620. [Google Scholar]
- Dankulchai, P.; Alonzi, R.; Lowe, G.J.; Burnley, J.; Padhani, A.R.; Hoskin, P.J. Optimal source distribution for focal boosts using high dose rate (HDR) brachytherapy alone in prostate cancer. Radiother. Oncol. 2014, 113, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Bossart, E.L.P.; Stoyanova, R.P.; Sandler, K.M.D.; Studenski, M.P.; Orman, A.M.D.; Abramowitz, M.M.D.; Pollack, A.M.D.P. Feasibility and Initial Dosimetric Findings for a Randomized Trial Using Dose-Painted Multiparametric Magnetic Resonance Imaging–Defined Targets in Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 827–834. [Google Scholar] [CrossRef]
- Kim, Y.; Hsu, I.-C.J.; Lessard, E.; Kurhanewicz, J.; Noworolski, S.M.; Pouliot, J. Class solution in inverse planned HDR prostate brachytherapy for dose escalation of DIL defined by combined MRI/MRSI. Radiother. Oncol. 2008, 88, 148–155. [Google Scholar]
- Kazi, A.; Godwin, G.; Simpson, J.; Sasso, G. MRS-guided HDR brachytherapy boost to the dominant intraprostatic lesion in high risk localised prostate cancer. BMC Cancer 2010, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- DiBiase, S.J.; Hosseinzadeh, K.; Gullapalli, R.P.; Jacobs, S.C.; Naslund, M.J.; Sklar, G.N.; Alexander, R.B.; Yu, C. Magnetic resonance spectroscopic imaging-guided brachytherapy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 429–438. [Google Scholar] [PubMed]
- Pouliot, J.; Kim, Y.; Lessard, E.; Hsu, I.-C.; Vigneron, D.B.; Kurhanewicz, J. Inverse planning for HDR prostate brachytherapy used to boost dominant intraprostatic lesions defined by magnetic resonance spectroscopy imaging. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 1196–1207. [Google Scholar] [PubMed]
- Udrescu, C.; Rouvière, O.; Enachescu, C.; Sotton, M.P.; Bouffard-Vercelli, J.; Jalade, P.; Chapet, O. Potential interest of developing an integrated boost dose escalation for stereotactic irradiation of primary prostate cancer. Phys. Medica 2013, 30, 320–325. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoon, K.J.; Kim, Y.S. Simultaneous integrated boost with stereotactic radiotherapy for dominant intraprostatic lesion of localized prostate cancer: A dosimetric planning study. Sci. Rep. 2020, 10, 14713. [Google Scholar] [CrossRef]
- Tree, A.; Jones, C.; Sohaib, A.; Khoo, V.; van As, N. Prostate stereotactic body radiotherapy with simultaneous integrated boost: Which is the best planning method? Radiat. Oncol. 2013, 8, 228. [Google Scholar] [CrossRef]
- Ashida, R.; Nakamura, K.; Aizawa, R.; Iramina, H.; Takayama, K.; Nakamura, M.; Mizowaki, T. Highly hypofractionated intensity-modulated radiation therapy for nonmetastatic prostate cancer with a simultaneous integrated boost to intraprostatic lesions: A planning study. Jpn. J. Radiol. 2022, 40, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Ting, W.-C.; Chang, Y.-C.; Yang, C.-C.; Lin, L.-C.; Ho, H.-W.; Chu, S.-S.; Lin, Y.-W. Whole Pelvic Radiotherapy with Stereotactic Body Radiotherapy Boost vs. Conventionally Fractionated Radiotherapy for Patients with High or Very High-Risk Prostate Cancer. Front. Oncol. 2020, 10, 814. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Fiorino, C.; Mangili, P.; Cozzarini, C.; de Cobelli, F.; Cattaneo, G.M.; Rancati, T.; Maschio, A.D.; Muzio, N.D.; Calandrino, R. Feasibility of safe ultra-high (EQD2>100 Gy) dose escalation on dominant intra-prostatic lesions (DILs) by Helical Tomotheraphy. Acta Oncol. 2011, 50, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Kantz, S.; Hung, A.; Monaco, D.; Gaertner, F.C.; Essler, M.; Strunk, H.; Laub, W.; Bundschuh, R.A. 68Ga-PSMA-PET/CT imaging of localized primary prostate cancer patients for intensity modulated radiation therapy treatment planning with integrated boost. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Pinkawa, M.; Holy, R.; Piroth, M.D.; Klotz, J.; Nussen, S.; Krohn, T.; Mottaghy, F.M.; Weibrecht, M.; Eble, M.J. Intensity-Modulated Radiotherapy for Prostate Cancer Implementing Molecular Imaging with 18F-Choline PET-CT to Define a Simultaneous Integrated Boost. Strahlenther. Und Onkol. 2010, 186, 600–606. [Google Scholar] [CrossRef]
- Kuang, Y.P.; Wu, L.M.S.; Hirata, E.M.S.; Miyazaki, K.B.S.; Sato, M.M.S.M.P.H.; Kwee, S.A.M.D.P. Volumetric Modulated Arc Therapy Planning for Primary Prostate Cancer with Selective Intraprostatic Boost Determined by18 F-Choline PET/CT. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 1017–1025. [Google Scholar] [CrossRef]
- Chang, J.H.; Lim Joon, D.; Lee, S.T.; Gong, S.J.; Anderson, N.J.; Scott, A.M.; Davis, I.D.; Clouston, D.; Bolton, D.; Hamilton, C.S.; et al. Intensity Modulated Radiation Therapy Dose Painting for Localized Prostate Cancer Using 11C-choline Positron Emission Tomography Scans. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e691–e696. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Joon, D.L.; Lee, S.T.; Gong, S.J.; Scott, A.M.; Davis, I.D.; Clouston, D.; Bolton, D.; Hamilton, C.S.; Khoo, V. Histopathological correlation of 11C-choline PET scans for target volume definition in radical prostate radiotherapy. Radiother. Oncol. 2011, 99, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Westerly, D.C.; Waxweiler, T.V.; Ryan, N.; Raben, D. Dose painting to treat single-lobe prostate cancer with hypofractionated high-dose radiation using targeted external beam radiation: Is it feasible? Med. Dosim. 2015, 40, 256–261. [Google Scholar] [PubMed]
- Nutting, C.; Corbishley, C.; Sanchez-Nieto, B.; Cosgrove, V.; Webb, S.; Dearnaley, D. Potential improvements in the therapeutic ratio of prostate cancer irradiation: Dose escalation of pathologically identified tumour nodules using intensity modulated radiotherapy. Br. J. Radiol. 2002, 75, 151–161. [Google Scholar]
- Zamboglou, C.; Thomann, B.; Koubar, K.; Bronsert, P.; Krauss, T.; Rischke, H.C.; Sachpazidis, I.; Drendel, V.; Salman, N.; Reichel, K.; et al. Focal dose escalation for prostate cancer using 68Ga-HBED-CC PSMA PET/CT and MRI: A planning study based on histology reference. Radiat. Oncol. 2018, 13, 81. [Google Scholar] [CrossRef]
- Yeo, I.; Nookala, P.; Gordon, I.; Schulte, R.; Barnes, S.; Ghebremedhin, A.; Wang, N.; Yang, G.; Ling, T.; Bush, D.; et al. Passive proton therapy vs. IMRT planning study with focal boost for prostate cancer. Radiat. Oncol. 2015, 10, 213. [Google Scholar] [CrossRef]
- Kajihara, H.; Hayashida, Y.; Murakami, R.; Katahira, K.; Nishimura, R.; Hamada, Y.; Kitani, K.; Kitaoka, M.; Suzuki, Y.; Kitajima, M. Usefulness of diffusion-weighted imaging in the localization of prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 399–403. [Google Scholar] [PubMed]
- Groenendaal, G.; van den Berg, C.A.; Korporaal, J.G.; Philippens, M.E.; Luijten, P.R.; van Vulpen, M.; van der Heide, U.A. Simultaneous MRI diffusion and perfusion imaging for tumor delineation in prostate cancer patients. Radiother. Oncol. 2010, 95, 185–190. [Google Scholar] [PubMed]
- Kozlowski, P.; Chang, S.D.; Jones, E.C.; Berean, K.W.; Chen, H.; Goldenberg, S.L. Combined diffusion-weighted and dynamic contrast-enhanced MRI for prostate cancer diagnosis—Correlation with biopsy and histopathology. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2006, 24, 108–113. [Google Scholar]
- Tan, C.H.; Wei, W.; Johnson, V.; Kundra, V. Diffusion weighted magnetic resonance imaging in prostate cancer: Meta-analysis. AJR. Am. J. Roentgenol. 2012, 199, 822. [Google Scholar]
- Reynolds, H.M.; Tadimalla, S.; Wang, Y.-F.; Montazerolghaem, M.; Sun, Y.; Williams, S.; Mitchell, C.; Finnegan, M.E.; Murphy, D.G.; Haworth, A. Semi-quantitative and quantitative dynamic contrast-enhanced (DCE) MRI parameters as prostate cancer imaging biomarkers for biologically targeted radiation therapy. Cancer Imaging 2022, 22, 71. [Google Scholar]
- Selnæs, K.M.; Heerschap, A.; Jensen, L.R.; Tessem, M.-B.; Jarosch-Von Schweder, G.; Goa, P.E.; Viset, T.; Angelsen, A.; Gribbestad, I.S. Peripheral zone prostate cancer localization by multiparametric magnetic resonance at 3 T: Unbiased cancer identification by matching to histopathology. Investig. Radiol. 2012, 47, 624–633. [Google Scholar]
- Jung, J.A.; Coakley, F.V.; Vigneron, D.B.; Swanson, M.G.; Qayyum, A.; Weinberg, V.; Jones, K.D.; Carroll, P.R.; Kurhanewicz, J. Prostate depiction at endorectal MR spectroscopic imaging: Investigation of a standardized evaluation system. Radiology 2004, 233, 701–708. [Google Scholar]
- van Dorsten, F.A.; van der Graaf, M.; Engelbrecht, M.R.; van Leenders, G.J.; Verhofstad, A.; Rijpkema, M.; de la Rosette, J.J.; Barentsz, J.O.; Heerschap, A. Combined quantitative dynamic contrast-enhanced MR imaging and 1H MR spectroscopic imaging of human prostate cancer. J. Magn. Reson. Imaging: Off. J. Int. Soc. Magn. Reson. Med. 2004, 20, 279–287. [Google Scholar]
- Riches, S.F.; Payne, G.S.; Morgan, V.A.; Sandhu, S.; Fisher, C.; Germuska, M.; Collins, D.J.; Thompson, A.; Desouza, N.M. MRI in the detection of prostate cancer: Combined apparent diffusion coefficient, metabolite ratio, and vascular parameters. Am. J. Roentgenol. 2009, 193, 1583. [Google Scholar]
- Zamboglou, C.; Schiller, F.; Fechter, T.; Wieser, G.; Jilg, C.A.; Chirindel, A.; Salman, N.; Drendel, V.; Werner, M.; Mix, M. 68Ga-HBED-CC-PSMA PET/CT versus histopathology in primary localized prostate cancer: A voxel-wise comparison. Theranostics 2016, 6, 1619. [Google Scholar] [PubMed]
- Spohn, S.K.; Kramer, M.; Kiefer, S.; Bronsert, P.; Sigle, A.; Schultze-Seemann, W.; Jilg, C.A.; Sprave, T.; Ceci, L.; Fassbender, T.F. Comparison of manual and semi-automatic [18F] PSMA-1007 PET based contouring techniques for intraprostatic tumor delineation in patients with primary prostate cancer and validation with histopathology as standard of reference. Front. Oncol. 2020, 10, 600690. [Google Scholar] [PubMed]
- Kwee, S.A.; Thibault, G.P.; Stack, R.S.; Coel, M.N.; Furusato, B.; Sesterhenn, I.A. Use of step-section histopathology to evaluate 18F-fluorocholine PET sextant localization of prostate cancer. Mol. Imaging 2008, 7, 7290-2008. [Google Scholar]
- Kwee, S.A.; Wei, H.; Sesterhenn, I.; Yun, D.; Coel, M.N. Localization of primary prostate cancer with dual-phase 18F-fluorocholine PET. J. Nucl. Med. 2006, 47, 262–269. [Google Scholar]
- Reske, S.N.; Blumstein, N.M.; Neumaier, B.; Gottfried, H.-W.; Finsterbusch, F.; Kocot, D.; Möller, P.; Glatting, G.; Perner, S. Imaging prostate cancer with 11C-choline PET/CT. J. Nucl. Med. 2006, 47, 1249–1254. [Google Scholar]
- Farsad, M.; Schiavina, R.; Castellucci, P.; Nanni, C.; Corti, B.; Martorana, G.; Canini, R.; Grigioni, W.; Boschi, S.; Marengo, M. Detection and localization of prostate cancer: Correlation of 11C-choline PET/CT with histopathologic step-section analysis. J. Nucl. Med. 2005, 46, 1642–1649. [Google Scholar]
- Thie, J.A. Understanding the standardized uptake value, its methods, and implications for usage. J. Nucl. Med. 2004, 45, 1431–1434. [Google Scholar]
- Sutinen, E.; Nurmi, M.; Roivainen, A.; Varpula, M.; Tolvanen, T.; Lehikoinen, P.; Minn, H. Kinetics of [11 C] choline uptake in prostate cancer: A PET stydy. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 317–324. [Google Scholar]
- Zamboglou, C.; Drendel, V.; Jilg, C.A.; Rischke, H.C.; Beck, T.I.; Schultze-Seemann, W.; Krauss, T.; Mix, M.; Schiller, F.; Wetterauer, U. Comparison of 68Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics 2017, 7, 228. [Google Scholar]
- Eiber, M.; Weirich, G.; Holzapfel, K.; Souvatzoglou, M.; Haller, B.; Rauscher, I.; Beer, A.J.; Wester, H.-J.; Gschwend, J.; Schwaiger, M. Simultaneous 68Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur. Urol. 2016, 70, 829–836. [Google Scholar]
- Abedi, I.; Tavakkoli, M.B.; Jabbari, K.; Amouheidari, A.; Yadegarfard, G. Dosimetric and Radiobiological Evaluation of Multiparametric MRI-Guided Dose Painting in Radiotherapy of Prostate Cancer. J. Med. Signals Sens. 2017, 7, 114–121. [Google Scholar] [CrossRef]
- Mouraviev, V.; Villers, A.; Bostwick, D.G.; Wheeler, T.M.; Montironi, R.; Polascik, T.J. Understanding the pathological features of focality, grade and tumour volume of early-stage prostate cancer as a foundation for parenchyma-sparing prostate cancer therapies: Active surveillance and focal targeted therapy. BJU Int. 2011, 108, 1074–1085. [Google Scholar] [PubMed]
- Pommer, T.; Falk, M.; Poulsen, P.R.; Keall, P.J.; O’Brien, R.T.; Petersen, P.M.; af Rosenschöld, P.M. Dosimetric benefit of DMLC tracking for conventional and sub-volume boosted prostate intensity-modulated arc radiotherapy. Phys. Med. Biol. 2013, 58, 2349. [Google Scholar]
- Choi, C.; Cho, C.; Kim, G.; Park, K.; Jo, M.; Lee, C.; Yoo, S.; Kim, M.; Yang, K.; Yoo, H. Stereotactic radiation therapy of localized prostate cancer using cyberknife. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, S375. [Google Scholar]
- Tang, C.; Loblaw, D.; Cheung, P.; Holden, L.; Morton, G.; Basran, P.; Tirona, R.; Cardoso, M.; Pang, G.; Gardner, S. Phase I/II study of a five-fraction hypofractionated accelerated radiotherapy treatment for low-risk localised prostate cancer: Early results of pHART3. Clin. Oncol. 2008, 20, 729–737. [Google Scholar]
- Franiel, T.; Lüdemann, L.; Taupitz, M.; Böhmer, D.; Beyersdorff, D. MRI before and after external beam intensity-modulated radiotherapy of patients with prostate cancer: The feasibility of monitoring of radiation-induced tissue changes using a dynamic contrast-enhanced inversion-prepared dual-contrast gradient echo sequence. Radiother. Oncol. 2009, 93, 241–245. [Google Scholar]
- Bauman, G.; Haider, M.; Van der Heide, U.A.; Ménard, C. Boosting imaging defined dominant prostatic tumors: A systematic review. Radiother. Oncol. 2013, 107, 274–281. [Google Scholar]
- Niyazi, M.; Bartenstein, P.; Belka, C.; Ganswindt, U. Choline PET based dose-painting in prostate cancer-Modelling of dose effects. Radiat. Oncol. 2010, 5, 23. [Google Scholar] [PubMed]
- Ghilezan, M.J.; Jaffray, D.A.; Siewerdsen, J.H.; Van Herk, M.; Shetty, A.; Sharpe, M.B.; Jafri, S.Z.; Vicini, F.A.; Matter, R.C.; Brabbins, D.S. Prostate gland motion assessed with cine-magnetic resonance imaging (cine-MRI). Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 406–417. [Google Scholar]
- Pinkawa, M.; Pursch-Lee, M.; Asadpour, B.; Gagel, B.; Piroth, M.D.; Klotz, J.; Nussen, S.; Eble, M.J. Image-guided radiotherapy for prostate cancer. Strahlenther. Und Onkol. 2008, 184, 679–685. [Google Scholar]
- Boda-Heggemann, J.; Köhler, F.M.; Wertz, H.; Ehmann, M.; Hermann, B.; Riesenacker, N.; Küpper, B.; Lohr, F.; Wenz, F. Intrafraction motion of the prostate during an IMRT session: A fiducial-based 3D measurement with Cone-beam CT. Radiat. Oncol. 2008, 3, 37. [Google Scholar] [PubMed]
- Ullman, K.L.; Ning, H.; Susil, R.C.; Ayele, A.; Jocelyn, L.; Havelos, J.; Guion, P.; Xie, H.; Li, G.; Arora, B.C. Intra-and inter-radiation therapist reproducibility of daily isocenter verification using prostatic fiducial markers. Radiat. Oncol. 2006, 1, 2. [Google Scholar]
- Fiorino, C.; Di Muzio, N.; Broggi, S.; Cozzarini, C.; Maggiulli, E.; Alongi, F.; Valdagni, R.; Fazio, F.; Calandrino, R. Evidence of limited motion of the prostate by carefully emptying the rectum as assessed by daily MVCT image guidance with helical tomotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 611–617. [Google Scholar] [PubMed]
- Fiorino, C.; Alongi, F.; Broggi, S.; Mauro Cattaneo, G.; Cozzarini, C.; Di Muzio, N.; Maggiulli, E.; Mangili, P.; Perna, L.; Valdagni, R. Physics aspects of prostate tomotherapy: Planning optimization and image-guidance issues. Acta Oncol. 2008, 47, 1309–1316. [Google Scholar]
- Dearnaley, D.P.; Jovic, G.; Syndikus, I.; Khoo, V.; Cowan, R.A.; Graham, J.D.; Aird, E.G.; Bottomley, D.; Huddart, R.A.; Jose, C.C. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: Long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014, 15, 464–473. [Google Scholar]
- Fowler, J.; Chappell, R.; Ritter, M. Is α/β for prostate tumors really low? Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 1021–1031. [Google Scholar]
- Wang, J.Z.; Guerrero, M.; Li, X.A. How low is the α/β ratio for prostate cancer? Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 194–203. [Google Scholar]
- Azzeroni, R.; Maggio, A.; Fiorino, C.; Mangili, P.; Cozzarini, C.; De Cobelli, F.; Di Muzio, N.G.; Calandrino, R. Biological optimization of simultaneous boost on intra-prostatic lesions (DILs): Sensitivity to TCP parameters. Phys. Medica 2012, 29, 592–598. [Google Scholar] [CrossRef]
- Agren, A.; Brahme, A.; Turesson, I. Optimization of uncomplicated control for head and neck tumors. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, J.; Tian, S.; Wang, Y.; Patel, P.; Jani, A.B.; Langen, K.M.; Curran, W.J.; Liu, T.; Yang, X. A planning study of focal dose escalations to multiparametric MRI-defined dominant intraprostatic lesions in prostate proton radiation therapy. Br. J. Radiol. 2020, 93, 20190845. [Google Scholar] [CrossRef]
- Van der Wielen, G.J.; Mulhall, J.P.; Incrocci, L. Erectile dysfunction after radiotherapy for prostate cancer and radiation dose to the penile structures: A critical review. Radiother. Oncol. 2007, 84, 107–113. [Google Scholar]
- Steenbakkers, R.J.; Deurloo, K.E.; Nowak, P.J.; Lebesque, J.V.; van Herk, M.; Rasch, C.R. Reduction of dose delivered to the rectum and bulb of the penis using MRI delineation for radiotherapy of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 1269–1279. [Google Scholar]
- Perna, L.; Fiorino, C.; Cozzarini, C.; Broggi, S.; Cattaneo, G.M.; De Cobelli, F.; Mangili, P.; Di Muzio, N.; Calandrino, R. Sparing the penile bulb in the radical irradiation of clinically localised prostate carcinoma: A comparison between MRI and CT prostatic apex definition in 3DCRT, Linac-IMRT and Helical Tomotherapy. Radiother. Oncol. 2009, 93, 57–63. [Google Scholar]
- Khor, R.; Duchesne, G.; Tai, K.-H.; Foroudi, F.; Chander, S.; Van Dyk, S.; Garth, M.; Williams, S. Direct 2-arm comparison shows benefit of high-dose-rate brachytherapy boost vs external beam radiation therapy alone for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 679–685. [Google Scholar]
- Lips, I.M.; van der Heide, U.A.; Haustermans, K.; van Lin, E.N.; Pos, F.; Franken, S.P.; Kotte, A.N.; van Gils, C.H.; van Vulpen, M. Single blind randomized phase III trial to investigate the benefit of a focal lesion ablative microboost in prostate cancer (FLAME-trial): Study protocol for a randomized controlled trial. Trials 2011, 12, 255. [Google Scholar] [PubMed]
- Hoskin, P.J.; Colombo, A.; Henry, A.; Niehoff, P.; Hellebust, T.P.; Siebert, F.-A.; Kovacs, G. GEC/ESTRO recommendations on high dose rate afterloading brachytherapy for localised prostate cancer: An update. Radiother. Oncol. 2013, 107, 325–332. [Google Scholar]
- Yamada, Y.; Rogers, L.; Demanes, D.J.; Morton, G.; Prestidge, B.R.; Pouliot, J.; Gil’ad, N.C.; Zaider, M.; Ghilezan, M.; Hsu, I.-C. American Brachytherapy Society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy 2012, 11, 20–32. [Google Scholar]
- Tree, A.; Khoo, V.; van As, N. ep-1300 To Deliver A Focal Boost During Whole Prostate Gland Irradiation Using Cyberknife. Radiother. Oncol. 2012, 103, S494. [Google Scholar]
- Tamihardja, J.; Zenk, M.; Flentje, M. MRI-guided localization of the dominant intraprostatic lesion and dose analysis of volumetric modulated arc therapy planning for prostate cancer. Strahlenther. Und Onkol. 2018, 195, 145–152. [Google Scholar] [CrossRef]
- Xia, P.; Pickett, B.; Vigneault, E.; Verhey, L.J.; Roach III, M. Forward or inversely planned segmental multileaf collimator IMRT and sequential tomotherapy to treat multiple dominant intraprostatic lesions of prostate cancer to 90 Gy. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 244–254. [Google Scholar]
- Chuong, M.; Badiyan, S.N.; Yam, M.; Li, Z.; Langen, K.; Regine, W.; Morris, C.; Snider III, J.; Mehta, M.; Huh, S. Pencil beam scanning versus passively scattered proton therapy for unresectable pancreatic cancer. J. Gastrointest. Oncol. 2018, 9, 687. [Google Scholar] [PubMed]
- Quan, E.M.; Li, X.; Li, Y.; Wang, X.; Kudchadker, R.J.; Johnson, J.L.; Kuban, D.A.; Lee, A.K.; Zhang, X. A comprehensive comparison of IMRT and VMAT plan quality for prostate cancer treatment. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1169–1178. [Google Scholar] [PubMed]
- Aoyama, H.; Westerly, D.C.; Mackie, T.R.; Olivera, G.H.; Bentzen, S.M.; Patel, R.R.; Jaradat, H.; Tome, W.A.; Ritter, M.A.; Mehta, M.P. Integral radiation dose to normal structures with conformal external beam radiation. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 962–967. [Google Scholar]
- Peeters, S.; Heemsbergen, W.D.; Koper, P.; Van Putten, W.; Slot, A.; Dielwart, M.; Bonfrer, J.; Incrocci, L.; Lebesque, J.V. Dose-response in radiotherapy for localized prostate cancer: Results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J. Clin. Oncol. 2006, 24, 1990–1996. [Google Scholar]
- Beckendorf, V.; Guerif, S.; Le Prisé, E.; Cosset, J.-M.; Bougnoux, A.; Chauvet, B.; Salem, N.; Chapet, O.; Bourdain, S.; Bachaud, J.-M. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1056–1063. [Google Scholar]
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT Trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high-and intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285. [Google Scholar] [PubMed]
- Dearnaley, D.; Syndikus, I.; Mossop, H.; Khoo, V.; Birtle, A.; Bloomfield, D.; Graham, J.; Kirkbride, P.; Logue, J.; Malik, Z. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016, 17, 1047–1060. [Google Scholar]
- Incrocci, L.; Wortel, R.C.; Alemayehu, W.G.; Aluwini, S.; Schimmel, E.; Krol, S.; van der Toorn, P.-P.; de Jager, H.; Heemsbergen, W.; Heijmen, B. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): Final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 1061–1069. [Google Scholar]
- Monninkhof, E.M.; Van Loon, J.W.; van Vulpen, M.; Kerkmeijer, L.G.; Pos, F.J.; Haustermans, K.; van den Bergh, L.; Isebaert, S.; McColl, G.M.; Smeenk, R.J. Standard whole prostate gland radiotherapy with and without lesion boost in prostate cancer: Toxicity in the FLAME randomized controlled trial. Radiother. Oncol. 2018, 127, 74–80. [Google Scholar]
- Zapatero, A.; Roch, M.; Büchser, D.; Castro, P.; Banda, L.F.; Pozo, G.; Martin de Vidales, C.; Conde, A.C.; Murillo, M.T.; Garcia-Vicente, F. Improved Late Rectal and Urinary Toxicity with High-Dose Intensity Modulated Radiation Therapy Using Intraprostate Fiducial Markers for Localized Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, E241. [Google Scholar] [CrossRef]
- Franzese, E.; Falco, S.D.; Laterza, M.M.; Montella, L.; Facchini, S.; Liguori, C.; Coppola, P.; Diessa, Y.; Berretta, M.; Pisconti, S. The use of 68Ga prostate-specific membrane antigen PET-CT in prostate cancer: Diagnostic challenges and therapeutic opportunities. Future Sci. 2021, 7, FSO705. [Google Scholar]
- Hope, T.A.; Goodman, J.Z.; Allen, I.E.; Calais, J.; Fendler, W.P.; Carroll, P.R. Metaanalysis of 68Ga-PSMA-11 PET accuracy for the detection of prostate cancer validated by histopathology. J. Nucl. Med. 2019, 60, 786–793. [Google Scholar]
- Hara, T.; Bansal, A.; DeGrado, T.R. Effect of hypoxia on the uptake of [ methyl-3H]choline, [1-14C] acetate and [18F]FDG in cultured prostate cancer cells. Nucl. Med. Biol. 2006, 33, 977–984. [Google Scholar] [CrossRef]
- Murthy, V.; Maitre, P.; Kannan, S.; Panigrahi, G.; Krishnatry, R.; Bakshi, G.; Prakash, G.; Pal, M.; Menon, S.; Phurailatpam, R.; et al. Prostate-Only Versus Whole-Pelvic Radiation Therapy in High-Risk and Very High-Risk Prostate Cancer (POP-RT): Outcomes From Phase III Randomized Controlled Trial. J. Clin. Oncol. 2021, 39, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Pommier, P.M.D.P.; Chabaud, S.P.; Lagrange, J.-L.M.D.P.; Richaud, P.M.D.; Le Prise, E.M.D.; Wagner, J.-P.M.D.; Azria, D.M.D.P.; Beckendorf, V.M.D.; Suchaud, J.-P.M.D.; Bernier, V.M.D.; et al. Is There a Role for Pelvic Irradiation in Localized Prostate Adenocarcinoma? Update of the Long-Term Survival Results of the GETUG-01 Randomized Study. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 759–769. [Google Scholar] [CrossRef]
- Roach, M.; Moughan, J.; Lawton, C.A.F.; Dicker, A.P.; Zeitzer, K.L.; Gore, E.M.; Kwok, Y.; Seider, M.J.; Hsu, I.C.; Hartford, A.C.; et al. Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): Long-term results of a randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Brand, D.H.; Tree, A.C.; Ostler, P.; van der Voet, H.; Loblaw, A.; Chu, W.; Ford, D.; Tolan, S.; Jain, S.; Martin, A.; et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): Acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019, 20, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Widmark, A.; Gunnlaugsson, A.; Beckman, L.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; Ginman, C.; Johansson, B.; Björnlinger, K.; et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet (Br. Ed.) 2019, 394, 385–395. [Google Scholar] [CrossRef]
- Lips, I.M.; Dehnad, H.; van Gils, C.H.; Kruger, A.E.B.; van der Heide, U.A.; van Vulpen, M. High-dose intensity-modulated radiotherapy for prostate cancer using daily fiducial marker-based position verification: Acute and late toxicity in 331 patients. Radiat. Oncol. 2008, 3, 15. [Google Scholar]
- Morton, G.; Loblaw, A.; Chung, H.T.; McGuffin, M.; Tseng, E.; Mendez, L.; Ravi, A.; Zhang, L.; Mamedov, A.; Hasan, Y. Efficacy Results of a Randomized Trial of Prostate HDR Monotherapy in Either One or Two Fractions for Low and Intermediate Risk Disease. Brachytherapy 2019, 18, S52–S53. [Google Scholar] [CrossRef]
- Gaudet, M.; Vigneault, É.; Aubin, S.; Varfalvy, N.; Harel, F.; Beaulieu, L.; Martin, A.-G. Dose escalation to the dominant intraprostatic lesion defined by sextant biopsy in a permanent prostate I-125 implant: A prospective comparative toxicity analysis. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 153–159. [Google Scholar]
- Huisman, H.J.; Fütterer, J.J.; van Lin, E.N.; Welmers, A.; Scheenen, T.W.; van Dalen, J.A.; Visser, A.G.; Witjes, J.; Barentsz, J.O. Prostate cancer: Precision of integrating functional MR imaging with radiation therapy treatment by using fiducial gold markers. Radiology 2005, 236, 311–317. [Google Scholar]
- Mizowaki, T.; Gil’ad, N.C.; Fung, A.Y.; Zaider, M. Towards integrating functional imaging in the treatment of prostate cancer with radiation: The registration of the MR spectroscopy imaging to ultrasound/CT images and its implementation in treatment planning. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1558–1564. [Google Scholar]
- Rivest-Hénault, D.; Dowson, N.; Greer, P.B.; Fripp, J.; Dowling, J.A. Robust inverse-consistent affine CT–MR registration in MRI-assisted and MRI-alone prostate radiation therapy. Med. Image Anal. 2015, 23, 56–69. [Google Scholar]
- Yang, X.; Jani, A.B.; Rossi, P.J.; Mao, H.; Curran, W.J.; Liu, T. A MRI-CT prostate registration using sparse representation technique. In Proceedings of the Medical Imaging 2016: Image-Guided Procedures, Robotic Interventions, and Modeling, San Diego, CA, USA, 27 February–3 March 2016; pp. 663–670. [Google Scholar]
- Paganelli, C.; Meschini, G.; Molinelli, S.; Riboldi, M.; Baroni, G. Patient-specific validation of deformable image registration in radiation therapy: Overview and caveats. Med. Phys. 2018, 45, e908–e922. [Google Scholar] [PubMed]
- Barrett, T.; Gill, A.; Kataoka, M.; Priest, A.; Joubert, I.; McLean, M.; Graves, M.; Stearn, S.; Lomas, D.; Griffiths, J. DCE and DW MRI in monitoring response to androgen deprivation therapy in patients with prostate cancer: A feasibility study. Magn. Reson. Med. 2012, 67, 778–785. [Google Scholar]
- Alonzi, R.; Padhani, A.R.; Taylor, N.J.; Collins, D.J.; D’Arcy, J.A.; Stirling, J.J.; Saunders, M.I.; Hoskin, P.J. Antivascular effects of neoadjuvant androgen deprivation for prostate cancer: An in vivo human study using susceptibility and relaxivity dynamic MRI. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 721–727. [Google Scholar]
- Groenendaal, G.; van Vulpen, M.; Pereboom, S.R.; Poelma-Tap, D.; Korporaal, J.G.; Monninkhof, E.; van der Heide, U.A. The effect of hormonal treatment on conspicuity of prostate cancer: Implications for focal boosting radiotherapy. Radiother. Oncol. 2012, 103, 233–238. [Google Scholar]
- Padhani, A.R.; MacVicar, A.D.; Gapinski, C.J.; Dearnaley, D.P.; Parker, G.J.; Suckling, J.; Leach, M.O.; Husband, J.E. Effects of androgen deprivation on prostatic morphology and vascular permeability evaluated with MR imaging. Radiology 2001, 218, 365–374. [Google Scholar]
- Soret, M.; Bacharach, S.L.; Buvat, I. Partial-volume effect in PET tumor imaging. J. Nucl. Med. 2007, 48, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.; Raymond, Y.; Salhani, D.; Yang, H.; Esche, B. Prostate motion during standard radiotherapy as assessed by fiducial markers. Radiother. Oncol. 1995, 37, 35–42. [Google Scholar] [PubMed]
- Bergström, P.; Löfroth, P.-O.; Widmark, A. High-precision conformal radiotherapy (HPCRT) of prostate cancer—A new technique for exact positioning of the prostate at the time of treatment. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 305–311. [Google Scholar] [PubMed]
- van Lin, E.N.T.; Hoffmann, A.L.; van Kollenburg, P.; Leer, J.W.; Visser, A.G. Rectal wall sparing effect of three different endorectal balloons in 3D conformal and IMRT prostate radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 565–576. [Google Scholar] [PubMed]
- Woel, R.; Beard, C.; Chen, M.-H.; Hurwitz, M.; Loffredo, M.; McMahon, E.; Ching, J.; Lopes, L.; D’Amico, A.V. Acute gastrointestinal, genitourinary, and dermatological toxicity during dose-escalated 3D-conformal radiation therapy (3DCRT) using an intrarectal balloon for prostate gland localization and immobilization. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 392–396. [Google Scholar]
- Van Lin, E.N.T.; van der Vight, L.P.; Witjes, J.A.; Huisman, H.J.; Leer, J.W.; Visser, A.G. The effect of an endorectal balloon and off-line correction on the interfraction systematic and random prostate position variations: A comparative study. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 278–288. [Google Scholar]
- Vigneault, E.; Pouliot, J.; Laverdière, J.; Roy, J.; Dorion, M. Electronic portal imaging device detection of radioopaque markers for the evaluation of prostate position during megavoltage irradiation: A clinical study. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 205–212. [Google Scholar]
- Litzenberg, D.; Dawson, L.A.; Sandler, H.; Sanda, M.G.; McShan, D.L.; Ten Haken, R.K.; Lam, K.L.; Brock, K.K.; Balter, J.M. Daily prostate targeting using implanted radiopaque markers. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 699–703. [Google Scholar]
- Herman, M.G.; Abrams, R.A.; Mayer, R.R. Clinical use of on-line portal imaging for daily patient treatment verification. Int. J. Radiat. Oncol. Biol. Phys. 1994, 28, 1017–1023. [Google Scholar]
- Langen, K.M.; Willoughby, T.R.; Meeks, S.L.; Santhanam, A.; Cunningham, A.; Levine, L.; Kupelian, P.A. Observations on real-time prostate gland motion using electromagnetic tracking. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1084–1090. [Google Scholar]
- Rose, C.; Ebert, M.A.; Mukwada, G.; Skorska, M.; Gill, S. Intrafraction motion during CyberKnife® prostate SBRT: Impact of imaging frequency and patient factors. Phys. Eng. Sci. Med. 2023, 46, 1–17. [Google Scholar]
- Reynolds, H.M.; Williams, S.; Zhang, A.M.; Ong, C.S.; Rawlinson, D.; Chakravorty, R.; Mitchell, C.; Haworth, A. Cell density in prostate histopathology images as a measure of tumor distribution. In Proceedings of the Medical Imaging 2014: Digital Pathology, San Diego, CA, USA, 16–17 February 2014; p. 90410S. [Google Scholar]
- Reynolds, H.; Williams, S.; Zhang, A.; Chakravorty, R.; Rawlinson, D.; Ong, C.; Esteva, M.; Mitchell, C.; Parameswaran, B.; Finnegan, M. Development of a registration framework to validate MRI with histology for prostate focal therapy. Med. Phys. 2015, 42, 7078–7089. [Google Scholar]
- Zhao, Y.; Haworth, A.; Reynolds, H.M.; Her, E.J.; Sun, Y.; Finnegan, R.; Rowshanfarzad, P.; Ebert, M.A. Patient-specific voxel-level dose prescription for prostate cancer radiotherapy considering tumour cell density and grade distribution. Med. Phys. 2023, 50, 3746–3761. [Google Scholar] [PubMed]
- Finnegan, R.N.; Reynolds, H.M.; Ebert, M.A.; Sun, Y.; Holloway, L.; Sykes, J.R.; Dowling, J.; Mitchell, C.; Williams, S.G.; Murphy, D.G. A statistical, voxelised model of prostate cancer for biologically optimised radiotherapy. Phys. Imaging Radiat. Oncol. 2022, 21, 136–145. [Google Scholar] [PubMed]
- Sun, Y.; Williams, S.; Byrne, D.; Keam, S.; Reynolds, H.M.; Mitchell, C.; Wraith, D.; Murphy, D.; Haworth, A. Association analysis between quantitative MRI features and hypoxia-related genetic profiles in prostate cancer: A pilot study. Br. J. Radiol. 2019, 92, 20190373. [Google Scholar]
- Sun, Y.; Reynolds, H.M.; Wraith, D.; Williams, S.; Finnegan, M.E.; Mitchell, C.; Murphy, D.; Haworth, A. Voxel-wise prostate cell density prediction using multiparametric magnetic resonance imaging and machine learning. Acta Oncol. 2018, 57, 1540–1546. [Google Scholar]
- Turchan, W.T.; Kauffmann, G.; Patel, P.; Oto, A.; Liauw, S.L. PI-RADS score is associated with biochemical control and distant metastasis in men with intermediate-risk and high-risk prostate cancer treated with radiation therapy. Urol. Oncol. 2020, 38, 600.e1–600.e8. [Google Scholar] [CrossRef]
- Karzai, F.; Walker, S.M.; Wilkinson, S.; Madan, R.A.; Shih, J.H.; Merino, M.J.; Harmon, S.A.; VanderWeele, D.J.; Cordes, L.M.; Carrabba, N.V.; et al. Sequential Prostate Magnetic Resonance Imaging in Newly Diagnosed High-risk Prostate Cancer Treated with Neoadjuvant Enzalutamide is Predictive of Therapeutic Response. Clin. Cancer Res. 2021, 27, 429–437. [Google Scholar] [CrossRef]
- Peng, X.; Chen, Z.; Farshidfar, F.; Xu, X.; Lorenzi, P.L.; Wang, Y.; Cheng, F.; Tan, L.; Mojumdar, K.; Du, D.; et al. Molecular Characterization and Clinical Relevance of Metabolic Expression Subtypes in Human Cancers. Cell Rep. 2018, 23, 255–269.e254. [Google Scholar] [CrossRef]
- Chan, T.H.; Haworth, A.; Wang, A.; Osanlouy, M.; Williams, S.; Mitchell, C.; Hofman, M.S.; Hicks, R.J.; Murphy, D.G.; Reynolds, H.M. Detecting localised prostate cancer using radiomic features in PSMA PET and multiparametric MRI for biologically targeted radiation therapy. EJNMMI Res. 2023, 13, 34. [Google Scholar]
- Ristau, J.; Hörner-Rieber, J.; Buchele, C.; Klüter, S.; Jäkel, C.; Baumann, L.; Andratschke, N.; Garcia Schüler, H.; Guckenberger, M.; Li, M.; et al. Stereotactic MRI-guided radiation therapy for localized prostate cancer (SMILE): A prospective, multicentric phase-II-trial. Radiat. Oncol. 2022, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.M.; Lamb, J.M.; Casado, M.; Wang, X.; Basehart, T.V.; Yang, Y.; Low, D.; Sheng, K.; Agazaryan, N.; Nickols, N.G.; et al. Magnetic resonance imaging-guided stereotactic body radiotherapy for prostate cancer (mirage): A phase iii randomized trial. BMC Cancer 2021, 21, 538. [Google Scholar] [CrossRef]
- Bruynzeel, A.M.E.; Tetar, S.U.; Oei, S.S.; Senan, S.; Haasbeek, C.J.A.; Spoelstra, F.O.B.; Piet, A.H.M.; Meijnen, P.; Bakker van der Jagt, M.A.B.; Fraikin, T.; et al. A Prospective Single-Arm Phase 2 Study of Stereotactic Magnetic Resonance Guided Adaptive Radiation Therapy for Prostate Cancer: Early Toxicity Results. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Zapatero, A.; Guerrero, A.; Maldonado, X.; Alvarez, A.; Gonzalez San Segundo, C.; Cabeza Rodríguez, M.A.; Macias, V.; Pedro Olive, A.; Casas, F.; Boladeras, A.; et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): A randomised, controlled, phase 3 trial. Lancet Oncol. 2015, 16, 320–327. [Google Scholar] [CrossRef]
- Craft, D.; Khan, F.; Young, M.; Bortfeld, T. The price of target dose uniformity. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 913–914. [Google Scholar]
- Sun, L.; Smith, W.; Ghose, A.; Kirkby, C. A quantitative assessment of the consequences of allowing dose heterogeneity in prostate radiation therapy planning. J. Appl. Clin. Med. Phys. 2018, 19, 580–590. [Google Scholar]
- Tamihardja, J.; Cirsi, S.; Kessler, P.; Razinskas, G.; Exner, F.; Richter, A.; Polat, B.; Flentje, M. Cone beam CT-based dose accumulation and analysis of delivered dose to the dominant intraprostatic lesion in primary radiotherapy of prostate cancer. Radiat. Oncol. 2021, 16, 205. [Google Scholar] [CrossRef]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M. PI-RADS prostate imaging–reporting and data system: 2015, version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar]
| Number of Trial Studies Included | N = 34 | |
|---|---|---|
| Risk Group (patient number—proportion %) | ||
| Total included number of participants | n = 2919 | 100% |
| Low-risk | 240 | 8.2% |
| Intermediate-risk | 1196 | 41.0% |
| High-risk | 1361 | 46.6% |
| Not reported | 122 | 4.2% |
| Median PSA | ||
| Average reported median PSA (ng/mL) | 9.08 | |
| Modality for GTV Identification (number of trials (%)—number of patients (%)) | ||
| N | 34 (100%) | 2175 (100%) |
| mpMRI | 27 (79.4%) | 1953 (89.8%) |
| DWI | 1 | 28 |
| T2w | 3 | 119 |
| T2w + DWI | 2 | 361 |
| T1w + T2w | 1 | 26 |
| T2w + DWI + DCE | 13 | 820 |
| T2w + T1w + MRSI | 1 | 47 |
| T1w + T2w + DWI + DCE | 1 | 25 |
| T2w + T1w +DWI + MRSI | 1 | 15 |
| T2w + DWI + DCE + MRSI | 1 | 225 |
| Sequence not reported | 3 | 125 |
| PET-CT | 2 (5.9%) | 97 (5.0%) |
| PET and mpMRI | 2 (5.9%) | 162 |
| Others | 3 (8.8%) | 125 (5.7%) |
| Treatment modality | Number of Trials | Patients Treated/Boosted |
| IMRT and/or VMAT | 21 | 2277/1755 |
| CyberKnife | 3 | 78/78 |
| CyberKnife + IMRT | 1 | 25/25 |
| HDR Brachytherapy | 7 | 313/313 |
| LDR Brachytherapy | 3 | 226/116 |
| Plan Studies Included. | 34 | T2w + DCE + MRSI | 1 |
|---|---|---|---|
| MRI | 24 | T2w + DWI + DCE + MRSI | 1 |
| T1w + T2w | 1 | T1w + T2w + DWI + DCE + MRSI | 1 |
| T2w + DCE | 2 | MRSI | 1 |
| T2w + DWI | 4 | PET-CT | 5 |
| T2w + DCE + DWI | 6 | 68Ga | 1 |
| T2w + T1w + DWI | 3 | 18F | 2 |
| T2w + T1w + DWI + DCE | 1 | 11C | 2 |
| T2w + MRSI | 3 | PET and mpMRI | 1 |
| T1w + T2w + MRSI | 1 | Others | 4 |
| 0 mm | 2 mm | 3 mm | 4 mm | 5 mm | 6 mm | >6 mm | |
|---|---|---|---|---|---|---|---|
| Planning | 6 | 0 | 5 | 4 | 7 | 4 | 0 |
| Trial | 9 | 3 | 5 | 3 | 5 | 1 | 1 |
| Toxicity | n | % | |
|---|---|---|---|
| Acute G2 GU | Total | 1079 | |
| Positive | 354 | 32.8% | |
| Acute G2 GI | Total | 1409 | |
| Positive | 203 | 14.4% | |
| Late G2 GU | Total | 1196 | |
| Positive | 231 | 19.3% | |
| Late G2 GI | Total | 1421 | |
| Positive | 148 | 10.5% |
| G2 GU | G2 GI | G2 GU | G2 GI | |
|---|---|---|---|---|
| Low-risk % | −0.40 | −0.16 | −0.34 | −0.43 |
| Intermediate-risk % | −0.20 | −0.20 | −0.24 | −0.16 |
| High-risk % | 0.25 | 0.24 | 0.31 | 0.24 |
| Hormone therapy % | 0.09 | 0.22 | 0.02 | 0.01 |
| Median PSA | 0.10 | −0.01 | 0.17 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Haworth, A.; Rowshanfarzad, P.; Ebert, M.A. Focal Boost in Prostate Cancer Radiotherapy: A Review of Planning Studies and Clinical Trials. Cancers 2023, 15, 4888. https://doi.org/10.3390/cancers15194888
Zhao Y, Haworth A, Rowshanfarzad P, Ebert MA. Focal Boost in Prostate Cancer Radiotherapy: A Review of Planning Studies and Clinical Trials. Cancers. 2023; 15(19):4888. https://doi.org/10.3390/cancers15194888
Chicago/Turabian StyleZhao, Yutong, Annette Haworth, Pejman Rowshanfarzad, and Martin A. Ebert. 2023. "Focal Boost in Prostate Cancer Radiotherapy: A Review of Planning Studies and Clinical Trials" Cancers 15, no. 19: 4888. https://doi.org/10.3390/cancers15194888
APA StyleZhao, Y., Haworth, A., Rowshanfarzad, P., & Ebert, M. A. (2023). Focal Boost in Prostate Cancer Radiotherapy: A Review of Planning Studies and Clinical Trials. Cancers, 15(19), 4888. https://doi.org/10.3390/cancers15194888






