Validation of a Temperature-Feedback Controlled Automated Magnetic Hyperthermia Therapy Device
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Power Supply and Coil
2.2. Optical Fiber Thermometry and Multi-Sensor Probe
2.3. Controller Design
2.3.1. Safety Controls
2.3.2. Hardware and Software
2.4. Calculation and Verification of PID Gains ( for Temperature Feedback Control
2.5. Ex Vivo Validation of Controller Performance for MHT in Bovine Liver Tissue
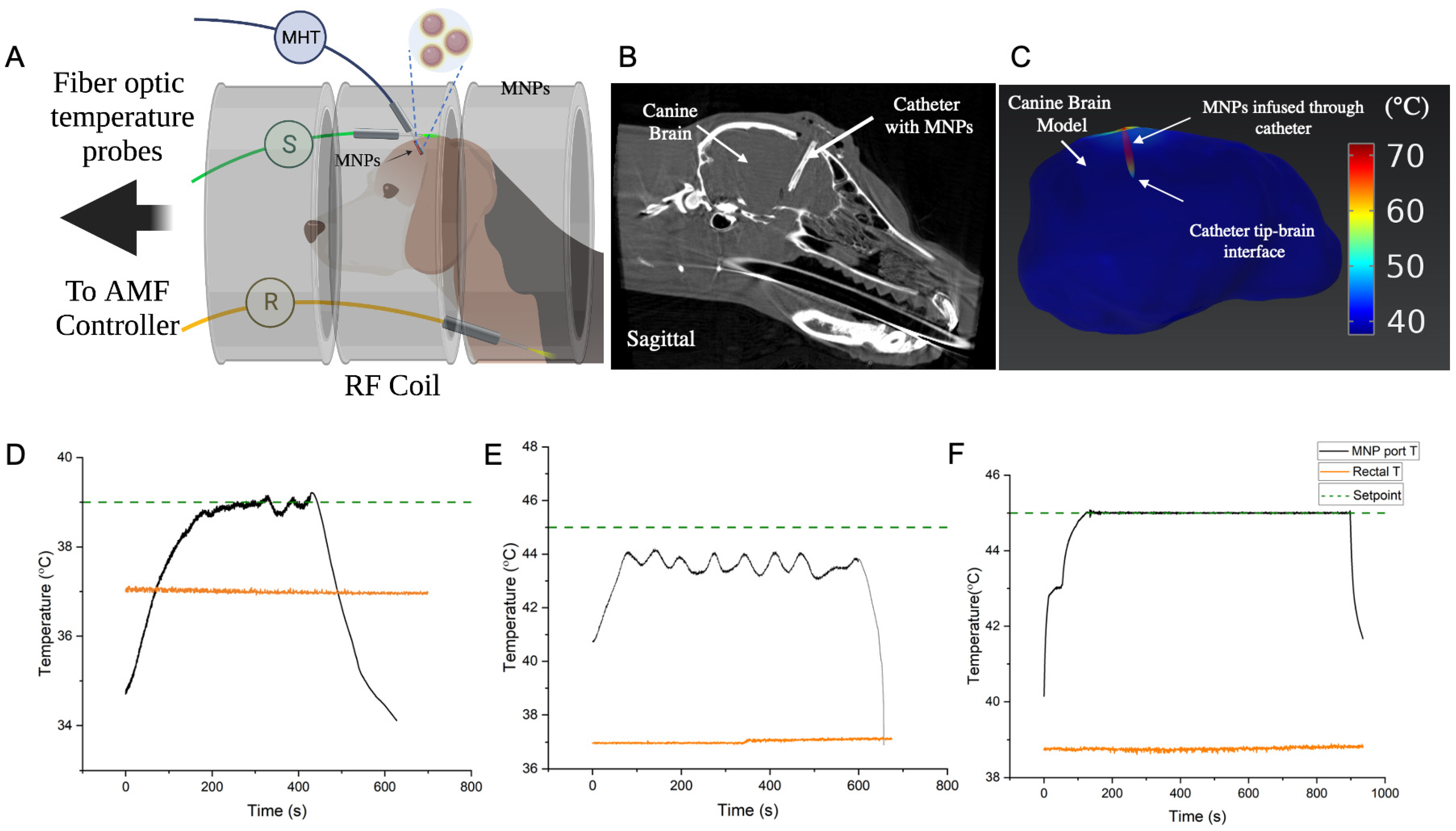
2.6. In Vivo Validation of Controller Performance for MHT in a Canine Research Subject
3. Results
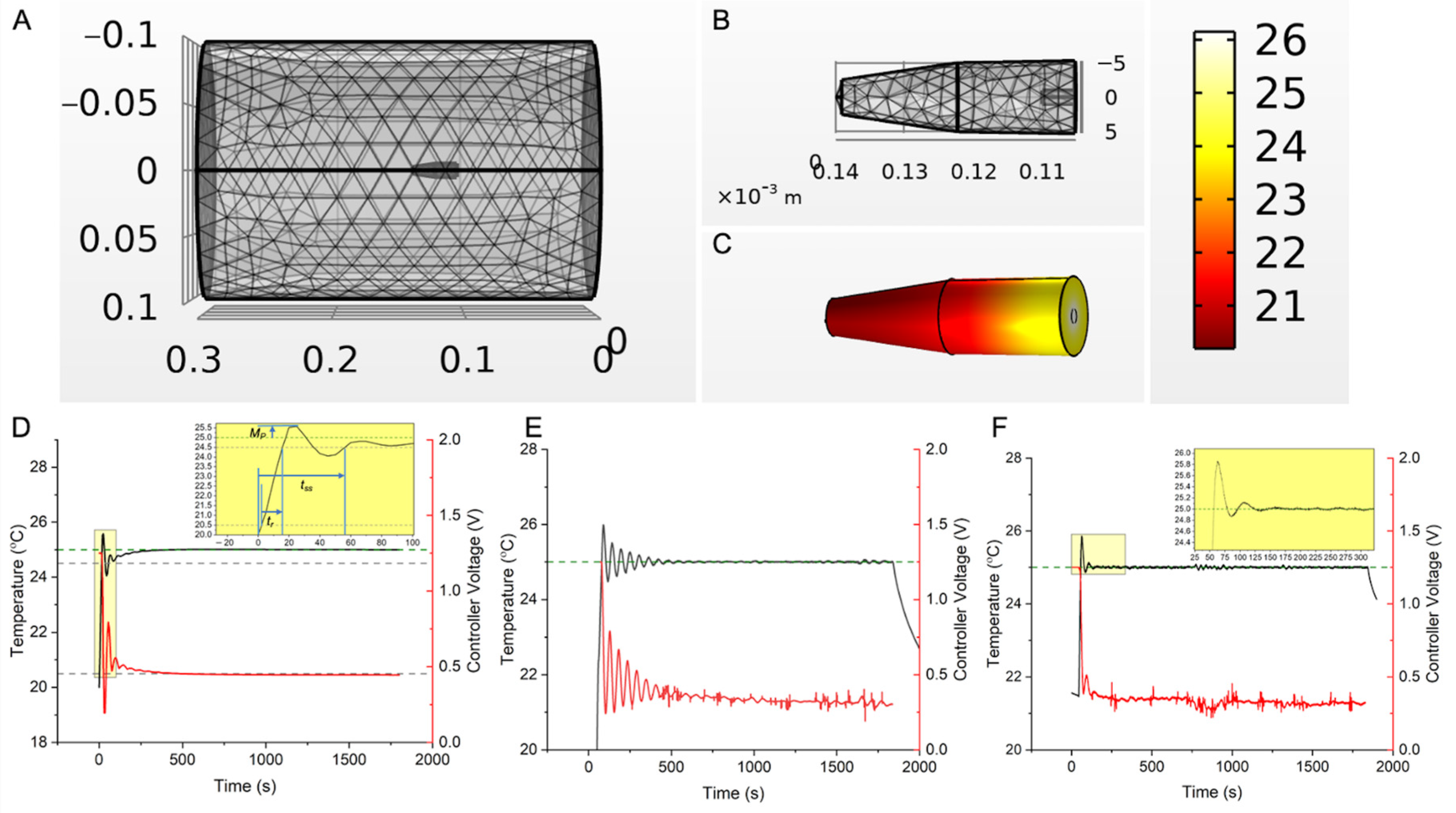
3.1. In Vitro Verification
3.2. Controller MHT Performance Validation in Ex Vivo Bovine Liver Tissue
3.3. Performance of Safety Controls
3.4. In Vivo Validation of Controller for MHT in a Canine Research Subject
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatima, H.; Charinpanitkul, T.; Kim, K.-S. Fundamentals to Apply Magnetic Nanoparticles for Hyperthermia Therapy. Nanomaterials 2021, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and Safety of Intratumoral Thermotherapy Using Magnetic Iron-Oxide Nanoparticles Combined with External Beam Radiotherapy on Patients with Recurrent Glioblastoma Multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial Thermotherapy Using Magnetic Nanoparticles Combined with External Beam Radiotherapy: Results of a Feasibility Study on Patients with Glioblastoma Multiforme. J. Neuro-Oncol. 2007, 81, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Rego, G.N.A.; Nucci, M.P.; Mamani, J.B.; Oliveira, F.A.; Marti, L.C.; Filgueiras, I.S.; Ferreira, J.M.; Real, C.C.; de Paula Faria, D.; Espinha, P.L.; et al. Therapeutic Efficiency of Multiple Applications of Magnetic Hyperthermia Technique in Glioblastoma Using Aminosilane Coated Iron Oxide Nanoparticles: In Vitro and In Vivo Study. Int. J. Mol. Sci. 2020, 21, 958. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Shinkai, M.; Honda, H.; Yoshikawa, K.; Saga, S.; Wakabayashi, T.; Yoshida, J.; Kobayashi, T. Heat Shock Protein 70 Expression Induces Antitumor Immunity during Intracellular Hyperthermia Using Magnetite Nanoparticles. Cancer Immunol. Immunother. 2003, 52, 80–88. [Google Scholar] [CrossRef]
- Yanase, M.; Shinkai, M.; Honda, H.; Wakabayashi, T.; Yoshida, J.; Kobayashi, T. Antitumor Immunity Induction by Intracellular Hyperthermia Using Magnetite Cationic Liposomes. Jpn. J. Cancer Res. Gann 1998, 89, 775–782. [Google Scholar] [CrossRef]
- Meenach, S.A.; Hilt, J.Z.; Anderson, K.W. Poly(Ethylene Glycol)-Based Magnetic Hydrogel Nanocomposites for Hyperthermia Cancer Therapy. Acta Biomater. 2010, 6, 1039–1046. [Google Scholar] [CrossRef]
- Grauer, O.; Jaber, M.; Hess, K.; Weckesser, M.; Schwindt, W.; Maring, S.; Wölfer, J.; Stummer, W. Combined Intracavitary Thermotherapy with Iron Oxide Nanoparticles and Radiotherapy as Local Treatment Modality in Recurrent Glioblastoma Patients. J. Neuro-Oncol. 2019, 141, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.; Kim, S.H.; Oh, Y.T.; Chun, M.; Noh, O.K. Concurrent Hyperthermia and Re-Irradiation for Recurrent High-Grade Gliomas. Neoplasma 2017, 64, 803–808. [Google Scholar] [CrossRef] [Green Version]
- Horsman, M.R.; Overgaard, J. Hyperthermia: A Potent Enhancer of Radiotherapy. Clin. Oncol. 2007, 19, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Triantopoulou, S.; Efstathopoulos, E.; Platoni, K.; Uzunoglou, N.; Kelekis, N.; Kouloulias, V. Radiotherapy in Conjunction with Superficial and Intracavitary Hyperthermia for the Treatment of Solid Tumors: Survival and Thermal Parameters. Clin. Transl. Oncol. 2013, 15, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kroesen, M.; Mulder, H.T.; van Holthe, J.M.L.; Aangeenbrug, A.A.; Mens, J.W.M.; van Doorn, H.C.; Paulides, M.M.; Hoop, E.O.; Vernhout, R.M.; Lutgens, L.C.; et al. The Effect of the Time Interval between Radiation and Hyperthermia on Clinical Outcome in 400 Locally Advanced Cervical Carcinoma Patients. Front. Oncol. 2019, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Elming, P.B.; Sørensen, B.S.; Spejlborg, H.; Overgaard, J.; Horsman, M.R. Does the Combination of Hyperthermia with Low LET (Linear Energy Transfer) Radiation Induce Anti-Tumor Effects Equivalent to Those Seen with High LET Radiation Alone? Int. J. Hyperth. 2021, 38, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Vujaskovic, Z.; Poulson, J.M.; Gaskin, A.A.; Thrall, D.E.; Page, R.L.; Charles, H.C.; MacFall, J.R.; Brizel, D.M.; Meyer, R.E.; Prescott, D.M.; et al. Temperature-Dependent Changes in Physiologic Parameters of Spontaneous Canine Soft Tissue Sarcomas after Combined Radiotherapy and Hyperthermia Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 179–185. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, W.; Che, S.; Zhang, Y.; Meng, D.; Shi, F.; Su, J.; Yang, Y.; Ma, H.; Liu, R.; et al. Outcomes for Hyperthermia Combined with Concurrent Radiochemotherapy for Patients with Cervical Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 499–511. [Google Scholar] [CrossRef]
- Fiorentini, G.; Sarti, D.; Gadaleta, C.D.; Ballerini, M.; Fiorentini, C.; Garfagno, T.; Ranieri, G.; Guadagni, S. A Narrative Review of Regional Hyperthermia: Updates from 2010 to 2019. Integr. Cancer Ther. 2020, 19, 1534735420932648. [Google Scholar] [CrossRef]
- Russell, A.H. Clinical Radiation Oncology. Edited by L. L. Gunderson and J. E. Tepper. Gynecol. Oncol. 2001, 81, 335. [Google Scholar] [CrossRef]
- Kroesen, M.; Mulder, H.T.; van Holthe, J.M.L.; Aangeenbrug, A.A.; Mens, J.W.M.; van Doorn, H.C.; Paulides, M.M.; Hoop, E.O.; Vernhout, R.M.; Lutgens, L.C.; et al. Confirmation of Thermal Dose as a Predictor of Local Control in Cervical Carcinoma Patients Treated with State-of-the-Art Radiation Therapy and Hyperthermia. Radiother. Oncol. 2019, 140, 150–158. [Google Scholar] [CrossRef]
- Franckena, M.; Fatehi, D.; de Bruijne, M.; Canters, R.A.M.; van Norden, Y.; Mens, J.W.; van Rhoon, G.C.; van der Zee, J. Hyperthermia Dose-Effect Relationship in 420 Patients with Cervical Cancer Treated with Combined Radiotherapy and Hyperthermia. Eur. J. Cancer 2009, 45, 1969–1978. [Google Scholar] [CrossRef]
- van Rhoon, G.C. Is CEM43 Still a Relevant Thermal Dose Parameter for Hyperthermia Treatment Monitoring? Int. J. Hyperth. 2016, 32, 50–62. [Google Scholar] [CrossRef]
- Kandala, S.K.; Liapi, E.; Whitcomb, L.L.; Attaluri, A.; Ivkov, R. Temperature-Controlled Power Modulation Compensates for Heterogeneous Nanoparticle Distributions: A Computational Optimization Analysis for Magnetic Hyperthermia. Int. J. Hyperth. 2018, 36, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, V.; Navidbakhsh, M.; Amanpour, S. Heat and Mass Transfer in the Hyperthermia Cancer Treatment by Magnetic Nanoparticles. Heat Mass Transf. 2021, 58, 1029–1039. [Google Scholar] [CrossRef]
- Tansi, F.L.; Maduabuchi, W.O.; Hirsch, M.; Southern, P.; Hattersley, S.; Quaas, R.; Teichgräber, U.; Pankhurst, Q.A.; Hilger, I. Deep-Tissue Localization of Magnetic Field Hyperthermia Using Pulse Sequencing. Int. J. Hyperth. 2021, 38, 743–754. [Google Scholar] [CrossRef]
- Singh, M.; Ma, R.; Zhu, L. Quantitative Evaluation of Effects of Coupled Temperature Elevation, Thermal Damage, and Enlarged Porosity on Nanoparticle Migration in Tumors during Magnetic Nanoparticle Hyperthermia. Int. Commun. Heat Mass 2021, 126, 105393. [Google Scholar] [CrossRef]
- Moroz, P.; Pardoe, H.; Jones, S.K.; Pierre, T.G.S.; Song, S.; Gray, B.N. Arterial Embolization Hyperthermia: Hepatic Iron Particle Distribution and Its Potential Determination by Magnetic Resonance Imaging. Phys. Med. Biol. 2002, 47, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Ivkov, R.; DeNardo, S.J.; Daum, W.; Foreman, A.R.; Goldstein, R.C.; Nemkov, V.S.; DeNardo, G.L. Application of High Amplitude Alternating Magnetic Fields for Heat Induction of Nanoparticles Localized in Cancer. Clin. Cancer Res. 2005, 11, 7093s–7103s. [Google Scholar] [CrossRef] [Green Version]
- Attaluri, A.; Kandala, S.K.; Zhou, H.; Wabler, M.; DeWeese, T.L.; Ivkov, R. Magnetic Nanoparticle Hyperthermia for Treating Locally Advanced Unresectable and Borderline Resectable Pancreatic Cancers: The Role of Tumor Size and Eddy-Current Heating. Int. J. Hyperth. 2021, 37, 108–119. [Google Scholar] [CrossRef]
- Soetaert, F.; Dupré, L.; Ivkov, R.; Crevecoeur, G. Computational Evaluation of Amplitude Modulation for Enhanced Magnetic Nanoparticle Hyperthermia. Biomed. Eng. Biomed. Tech. 2015, 60, 491–504. [Google Scholar] [CrossRef]
- Ahmed, A.; Kim, E.; Jeon, S.; Kim, J.; Choi, H. Closed-Loop Temperature-Controlled Magnetic Hyperthermia Therapy with Magnetic Guidance of Superparamagnetic Iron-Oxide Nanoparticles. Adv. Ther. 2022, 5, 2100237. [Google Scholar] [CrossRef]
- Hedayatnasab, Z.; Saadatabadi, A.R.; Shirgahi, H.; Mozafari, M.R. Heat Induction of Iron Oxide Nanoparticles with Rational Artificial Neural Network Design-Based Particle Swarm Optimization for Magnetic Cancer Hyperthermia. Mater. Res. Bull. 2023, 157, 112035. [Google Scholar] [CrossRef]
- Korganbayev, S.; Orrico, A.; Bianchi, L.; Paloschi, D.; Wolf, A.; Dostovalov, A.; Saccomandi, P. PID Controlling Approach Based on FBG Array Measurements for Laser Ablation of Pancreatic Tissues. IEEE Trans. Instrum. Meas. 2021, 70, 7006409. [Google Scholar] [CrossRef]
- Korganbayev, S.; Orrico, A.; Bianchi, L.; Landro, M.D.; Wolf, A.; Dostovalov, A.; Saccomandi, P. Closed-Loop Temperature Control Based on Fiber Bragg Grating Sensors for Laser Ablation of Hepatic Tissue. Sensors 2020, 20, 6496. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Korganbayev, S.; Orrico, A.; Landro, M.D.; Saccomandi, P. Quasi-Distributed Fiber Optic Sensor-Based Control System for Interstitial Laser Ablation of Tissue: Theoretical and Experimental Investigations. Biomed. Opt. Express 2021, 12, 2841. [Google Scholar] [CrossRef] [PubMed]
- Hartov, A.; Colacchio, T.A.; Strobehn, J.W.; Ryan, T.P.; Hoopes, P.J. Performance of an Adaptive MIMO Controller for a Multiple-Element Ultrasound Hyperthermia System. Int. J. Hyperth. 2009, 9, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Kandala, S.K.; Sharma, A.; Mirpour, S.; Liapi, E.; Ivkov, R.; Attaluri, A. Validation of a Coupled Electromagnetic and Thermal Model for Estimating Temperatures during Magnetic Nanoparticle Hyperthermia. Int. J. Hyperth. 2021, 38, 611–622. [Google Scholar] [CrossRef]
- Kok, H.P.; Crezee, J. Adapt2Heat: Treatment Planning-Assisted Locoregional Hyperthermia by on-Line Visualization, Optimization and Re-Optimization of SAR and Temperature Distributions. Int. J. Hyperth. 2022, 39, 265–277. [Google Scholar] [CrossRef]
- Crezee, H.; van Leeuwen, C.M.; Oei, A.L.; Stalpers, L.J.A.; Bel, A.; Franken, N.A.; Kok, H.P. Thermoradiotherapy Planning: Integration in Routine Clinical Practice. Int. J. Hyperth. 2015, 32, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Design Control Guidance Design Control Guidance for Medical Device Manufacturers. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/design-control-guidance-medical-device-manufacturers (accessed on 19 January 2021).
- Kahne, S. Feedback Control of Dynamic Systems Gene F. Franklin, J. David Powell and Abbas Emami-Naeini. Automatica 1987, 23, 413. [Google Scholar] [CrossRef]
- Di, C.; Mattox, A.K.; Harward, S.; Adamson, C. Emerging Therapeutic Targets and Agents for Glioblastoma Migrating Cells. Anti-Cancer Agents Med. Chem. 2010, 10, 543–555. [Google Scholar] [CrossRef]
- Meir, E.G.V.; Hadjipanayis, C.G.; Norden, A.D.; Shu, H.; Wen, P.Y.; Olson, J.J. Exciting New Advances in Neuro-Oncology: The Avenue to a Cure for Malignant Glioma. CA Cancer J. Clin. 2010, 60, 166–193. [Google Scholar] [CrossRef]
- Meyer, M.A. Malignant Gliomas in Adults. N. Engl. J. Med. 2008, 359, 1850. [Google Scholar] [CrossRef] [PubMed]
- Iacob, G.; Dinca, E.B. Current Data and Strategy in Glioblastoma Multiforme. J. Med. Life 2009, 2, 386–393. [Google Scholar] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. Oncol. Times 2005, 27, 15–16. [Google Scholar] [CrossRef] [Green Version]
- Piroth, M.D.; Gagel, B.; Pinkawa, M.; Stanzel, S.; Asadpour, B.; Eble, M.J. Postoperative Radiotherapy of Glioblastoma Multiforme: Analysis and Critical Assessment of Different Treatment Strategies and Predictive Factors. Strahlenther. Onkol. 2007, 183, 695–702. [Google Scholar] [CrossRef]
- Safari, M.; Khoshnevisan, A. Cancer Stem Cells and Chemoresistance in Glioblastoma Multiform: A Review Article. J. Stem Cells 2015, 10, 271–285. [Google Scholar] [PubMed]
- Ellis, H.P.; Greenslade, M.; Powell, B.; Spiteri, I.; Sottoriva, A.; Kurian, K.M. Current Challenges in Glioblastoma: Intratumour Heterogeneity, Residual Disease, and Models to Predict Disease Recurrence. Front. Oncol. 2015, 5, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma Stem Cells Promote Radioresistance by Preferential Activation of the DNA Damage Response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Bacco, F.D.; D’Ambrosio, A.; Casanova, E.; Orzan, F.; Neggia, R.; Albano, R.; Verginelli, F.; Cominelli, M.; Poliani, P.L.; Luraghi, P.; et al. MET Inhibition Overcomes Radiation Resistance of Glioblastoma Stem-like Cells. EMBO Mol. Med. 2016, 8, 550–568. [Google Scholar] [CrossRef]
- Kampinga, H.H. Cell Biological Effects of Hyperthermia Alone or Combined with Radiation or Drugs: A Short Introduction to Newcomers in the Field. Int. J. Hyperth. 2009, 22, 191–196. [Google Scholar] [CrossRef]
- Iwata, K.; Shakil, A.; Hur, W.J.; Makepeace, C.M.; Griffin, R.J.; Song, C.W. Tumour PO2 Can Be Increased Markedly by Mild Hyperthermia. Br. J. Cancer Suppl. 1996, 27, S217–S221. [Google Scholar] [PubMed]
- Raaphorst, G.P.; Chabot, P.; Doja, S.; Wilkins, D.; Stewart, D.; Ng, C.E. Effect of Hyperthermia on Cisplatin Sensitivity in Human Glioma and Ovarian Carcinoma Cell Lines Resistant and Sensitive to Cisplatin Treatment. Int. J. Hyperth. 2009, 12, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Man, J.; Shoemake, J.D.; Ma, T.; Rizzo, A.E.; Godley, A.R.; Wu, Q.; Mohammadi, A.M.; Bao, S.; Rich, J.N.; Yu, J.S. Hyperthermia Sensitizes Glioma Stem-like Cells to Radiation by Inhibiting AKT Signaling. Cancer Res. 2015, 75, 1760–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; WaldÖFner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.A.; Jordan, A. Clinical Hyperthermia of Prostate Cancer Using Magnetic Nanoparticles: Presentation of a New Interstitial Technique. Int. J. Hyperth. 2009, 21, 637–647. [Google Scholar] [CrossRef] [Green Version]
- Johannsen, M.; Gneveckow, U.; Thiesen, B.; Taymoorian, K.; Cho, C.H.; Waldöfner, N.; Scholz, R.; Jordan, A.; Loening, S.A.; Wust, P.; et al. Thermotherapy of Prostate Cancer Using Magnetic Nanoparticles: Feasibility, Imaging, and Three-Dimensional Temperature Distribution. Eur. Urol. 2007, 52, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Attaluri, A.; Kandala, S.K.; Wabler, M.; Zhou, H.; Cornejo, C.; Armour, M.; Hedayati, M.; Zhang, Y.; DeWeese, T.L.; Herman, C.; et al. Magnetic Nanoparticle Hyperthermia Enhances Radiation Therapy: A Study in Mouse Models of Human Prostate Cancer. Int. J. Hyperth. 2015, 31, 359–374. [Google Scholar] [CrossRef] [Green Version]
- Beola, L.; Grazú, V.; Fernández-Afonso, Y.; Fratila, R.M.; de las Heras, M.; de la Fuente, J.M.; Gutiérrez, L.; Asín, L. Critical Parameters to Improve Pancreatic Cancer Treatment Using Magnetic Hyperthermia: Field Conditions, Immune Response, and Particle Biodistribution. ACS Appl. Mater. Interfaces 2021, 13, 12982–12996. [Google Scholar] [CrossRef]
- Maluta, S.; Schaffer, M.; Pioli, F.; Dall’Oglio, S.; Pasetto, S.; Schaffer, P.M.; Weber, B.; Giri, M.G. Regional Hyperthermia Combined with Chemoradiotherapy in Primary or Recurrent Locally Advanced Pancreatic Cancer. Strahlenther. Onkol. 2011, 187, 619. [Google Scholar] [CrossRef]
- Matsumine, A.; Takegami, K.; Asanuma, K.; Matsubara, T.; Nakamura, T.; Uchida, A.; Sudo, A. A Novel Hyperthermia Treatment for Bone Metastases Using Magnetic Materials. Int. J. Clin. Oncol. 2011, 16, 101–108. [Google Scholar] [CrossRef]
- Attaluri, A.; Seshadri, M.; Mirpour, S.; Wabler, M.; Marinho, T.; Furqan, M.; Zhou, H.; Paoli, S.D.; Gruettner, C.; Gilson, W.; et al. Image-Guided Thermal Therapy with a Dual-Contrast Magnetic Nanoparticle Formulation: A Feasibility Study. Int. J. Hyperth. 2016, 32, 543–557. [Google Scholar] [CrossRef]
- Sun, H.; Xu, L.; Fan, T.; Zhan, H.; Wang, X.; Zhou, Y.; Yang, R. Targeted Hyperthermia after Selective Embolization with Ferromagnetic Nanoparticles in a VX2 Rabbit Liver Tumor Model. Int. J. Nanomed. 2013, 8, 3795–3804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attaluri, A.; Jackowski, J.; Sharma, A.; Kandala, S.K.; Nemkov, V.; Yakey, C.; DeWeese, T.L.; Kumar, A.; Goldstein, R.C.; Ivkov, R. Design and Construction of a Maxwell-Type Induction Coil for Magnetic Nanoparticle Hyperthermia. Int. J. Hyperth. 2020, 37, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attaluri, A.; Nusbaum, C.; Wabler, M.; Ivkov, R. Calibration of a Quasi-Adiabatic Magneto-Thermal Calorimeter Used to Characterize Magnetic Nanoparticle Heating. J. Nanotechnol. Eng. Med. 2013, 4, 011006. [Google Scholar] [CrossRef]
- Jones, E.L.; Oleson, J.R.; Prosnitz, L.R.; Samulski, T.V.; Vujaskovic, Z.; Yu, D.; Sanders, L.L.; Dewhirst, M.W. Randomized Trial of Hyperthermia and Radiation for Superficial Tumors. J. Clin. Oncol. 2005, 23, 3079–3085. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Jangam, A.; Low, J.Y.S.; Ahmed, A.; Arepally, N.; Carlton, H.; Ivkov, R.; Attaluri, A. Design of a Temperature-Feedback Controlled Automated Magnetic Hyperthermia Therapy Device; Department of Radiation Oncology and Molecular Radiation Sciences, The Johns Hopkins University School of Medicine: Baltimore, MD, USA, 2022. [Google Scholar]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-Enhanced Delivery of Macromolecules in the Brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef] [Green Version]
- Lueshen, E.; Tangen, K.; Mehta, A.I.; Linninger, A. Backflow-Free Catheters for Efficient and Safe Convection-Enhanced Delivery of Therapeutics. Med. Eng. Phys. 2017, 45, 15–24. [Google Scholar] [CrossRef]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Sanche, L. Convection-Enhanced Delivery in Malignant Gliomas: A Review of Toxicity and Efficacy. J. Oncol. 2019, 2019, 9342796. [Google Scholar] [CrossRef] [Green Version]
- Freeman, A.C.; Platt, S.R.; Holmes, S.; Kent, M.; Robinson, K.; Howerth, E.; Eagleson, J.; Bouras, A.; Kaluzova, M.; Hadjipanayis, C.G. Convection-Enhanced Delivery of Cetuximab Conjugated Iron-Oxide Nanoparticles for Treatment of Spontaneous Canine Intracranial Gliomas. J. Neuro-Oncol. 2018, 137, 653–663. [Google Scholar] [CrossRef]
- Atkinson, W.J.; Brezovich, I.A.; Chakraborty, D.P. Usable Frequencies in Hyperthermia with Thermal Seeds. IEEE Trans. Biomed. Eng. 1984, 31, 70–75. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Jangam, A.; Shen, J.L.Y.; Ahmad, A.; Arepally, N.; Rodriguez, B.; Borrello, J.; Bouras, A.; Kleinberg, L.; Ding, K.; et al. Validation of a Temperature-Feedback Controlled Automated Magnetic Hyperthermia Therapy Device. Cancers 2023, 15, 327. https://doi.org/10.3390/cancers15020327
Sharma A, Jangam A, Shen JLY, Ahmad A, Arepally N, Rodriguez B, Borrello J, Bouras A, Kleinberg L, Ding K, et al. Validation of a Temperature-Feedback Controlled Automated Magnetic Hyperthermia Therapy Device. Cancers. 2023; 15(2):327. https://doi.org/10.3390/cancers15020327
Chicago/Turabian StyleSharma, Anirudh, Avesh Jangam, Julian Low Yung Shen, Aiman Ahmad, Nageshwar Arepally, Benjamin Rodriguez, Joseph Borrello, Alexandros Bouras, Lawrence Kleinberg, Kai Ding, and et al. 2023. "Validation of a Temperature-Feedback Controlled Automated Magnetic Hyperthermia Therapy Device" Cancers 15, no. 2: 327. https://doi.org/10.3390/cancers15020327





