Risk Factor and Replacement Therapy Analysis of Pre- and Postoperative Endocrine Deficiencies for Craniopharyngioma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data
2.2. Endocrinological Evaluation
2.3. Neuroradiological Evaluation
2.4. Treatment
2.5. Follow-Up
2.6. Statistical Methods
3. Results
3.1. Patient Characteristics and Symptoms
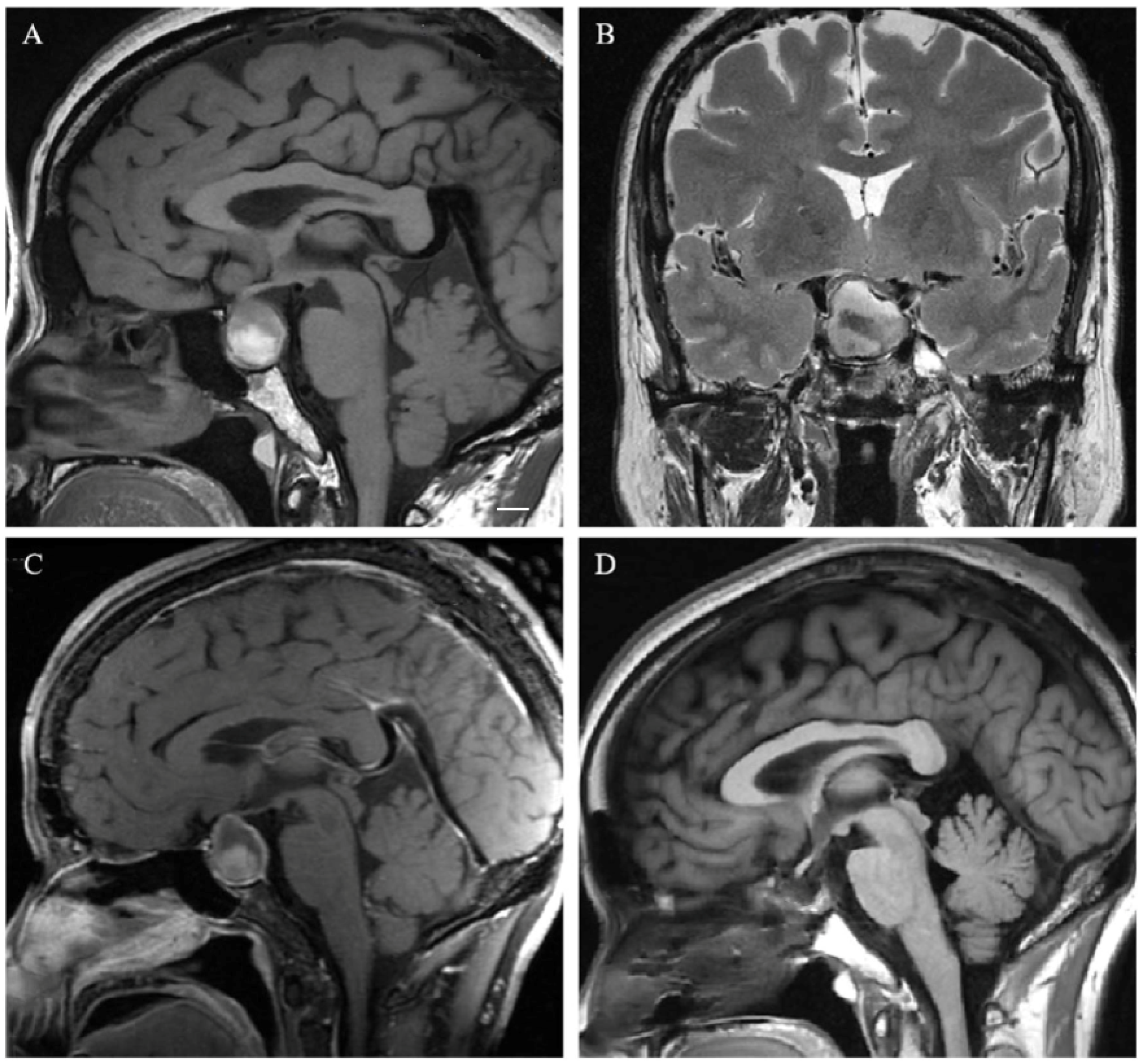
3.2. Neuroradiological Features
3.3. Endocrinological Function
3.4. Surgical Strategies and Outcomes
3.5. The Difference in Clinical Presentation and Results between Children and Adults
3.6. Risk Factors for Endocrine Dysfunction in Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zacharia, B.E.; Bruce, S.S.; Goldstein, H.; Malone, H.R.; Neugut, A.I.; Bruce, J.N. Incidence, treatment and survival of patients with craniopharyngioma in the surveillance, epidemiology and end results program. Neuro-Oncology 2012, 14, 1070–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.M.; Schmid, E.M.; Schutte, P.J.; Voormolen, J.H.; Biermasz, N.R.; van Thiel, S.W.; Corssmit, E.P.; Smit, J.W.; Roelfsema, F.; Romijn, J.A. High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin. Endocrinol. 2005, 62, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Dho, Y.S.; Kim, J.H.; Kim, J.W.; Park, C.K.; Kim, Y.H. Recurrence Rate and Prognostic Factors for the Adult Craniopharyngiomas in Long-Term Follow-Up. World Neurosurg. 2020, 133, e211–e217. [Google Scholar] [CrossRef] [PubMed]
- Mortini, P.; Losa, M.; Pozzobon, G.; Barzaghi, R.; Riva, M.; Acerno, S.; Angius, D.; Weber, G.; Chiumello, G.; Giovanelli, M. Neurosurgical treatment of craniopharyngioma in adults and children: Early and long-term results in a large case series. J. Neurosurg. 2011, 114, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, A.; Muller, H.L. Childhood-onset craniopharyngioma: Latest insights into pathology, diagnostics, treatment, and follow-up. Expert Rev. Neurother. 2018, 18, 793–806. [Google Scholar] [CrossRef]
- Karavitaki, N.; Cudlip, S.; Adams, C.B.; Wass, J.A. Craniopharyngiomas. Endocr. Rev. 2006, 27, 371–397. [Google Scholar] [CrossRef]
- Bates, A.S.; Van’t Hoff, W.; Jones, P.J.; Clayton, R.N. The effect of hypopituitarism on life expectancy. J. Clin. Endocrinol. Metab. 1996, 81, 1169–1172. [Google Scholar] [CrossRef]
- Ho, K.K.; Participants, G.H.D.C.W. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: A statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur. J. Endocrinol. 2007, 157, 695–700. [Google Scholar] [CrossRef] [Green Version]
- Koulouri, O.; Auldin, M.A.; Agarwal, R.; Kieffer, V.; Robertson, C.; Falconer Smith, J.; Levy, M.J.; Howlett, T.A. Diagnosis and treatment of hypothyroidism in TSH deficiency compared to primary thyroid disease: Pituitary patients are at risk of under-replacement with levothyroxine. Clin. Endocrinol. 2011, 74, 744–749. [Google Scholar] [CrossRef]
- Burke, W.T.; Cote, D.J.; Penn, D.L.; Iuliano, S.; McMillen, K.; Laws, E.R. Diabetes Insipidus After Endoscopic Transsphenoidal Surgery. Neurosurgery 2020, 87, 949–955. [Google Scholar] [CrossRef]
- Puget, S.; Garnett, M.; Wray, A.; Grill, J.; Habrand, J.L.; Bodaert, N.; Zerah, M.; Bezerra, M.; Renier, D.; Pierre-Kahn, A.; et al. Pediatric craniopharyngiomas: Classification and treatment according to the degree of hypothalamic involvement. J. Neurosurg. 2007, 106, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Sadhasivam, S.; Menon, G.; Abraham, M.; Nair, S.N. The implication of giant tumor size on surgical resection, oncological, and functional outcomes in craniopharyngioma. Pituitary 2020, 23, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L.; Merchant, T.E.; Warmuth-Metz, M.; Martinez-Barbera, J.P.; Puget, S. Craniopharyngioma. Nat. Rev. Dis. Primers 2019, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Bereket, A. Postoperative and Long-Term Endocrinologic Complications of Craniopharyngioma. Horm. Res. Paediatr. 2020, 93, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L. Craniopharyngioma. Endocr. Rev. 2014, 35, 513–543. [Google Scholar] [CrossRef]
- Muller, H.L. Childhood craniopharyngioma. Recent advances in diagnosis, treatment and follow-up. Horm. Res. 2008, 69, 193–202. [Google Scholar] [CrossRef]
- Karavitaki, N.; Brufani, C.; Warner, J.T.; Adams, C.B.; Richards, P.; Ansorge, O.; Shine, B.; Turner, H.E.; Wass, J.A. Craniopharyngiomas in children and adults: Systematic analysis of 121 cases with long-term follow-up. Clin. Endocrinol. 2005, 62, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Capatina, C.; Vintila, M.; Gherlan, I.; Dumitrascu, A.; Caragheorgheopol, A.; Procopiuc, C.; Ciubotaru, V.; Poiana, C. Craniopharyngioma—Clinical and Therapeutic Outcome Data in a Mixed Cohort of Adult and Paediatric Cases. Acta Endocrinol. 2018, 14, 549–555. [Google Scholar] [CrossRef]
- Gautier, A.; Godbout, A.; Grosheny, C.; Tejedor, I.; Coudert, M.; Courtillot, C.; Jublanc, C.; De Kerdanet, M.; Poirier, J.Y.; Riffaud, L.; et al. Markers of recurrence and long-term morbidity in craniopharyngioma: A systematic analysis of 171 patients. J. Clin. Endocrinol. Metab. 2012, 97, 1258–1267. [Google Scholar] [CrossRef]
- Erfurth, E.M.; Holmer, H.; Fjalldal, S.B. Mortality and morbidity in adult craniopharyngioma. Pituitary 2013, 16, 46–55. [Google Scholar] [CrossRef]
- Komotar, R.J.; Roguski, M.; Bruce, J.N. Surgical management of craniopharyngiomas. J. Neurooncol. 2009, 92, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Elsheikh, M.; Stratton, I.M.; Page, R.C.; Adams, C.B.; Wass, J.A. Outcome of transphenoidal surgery for acromegaly and its relationship to surgical experience. Clin. Endocrinol. 1999, 50, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Honegger, J.; Buchfelder, M.; Fahlbusch, R. Surgical treatment of craniopharyngiomas: Endocrinological results. J. Neurosurg. 1999, 90, 251–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, R.P.; Babu, C.S.; Schwartz, T.H. Endoscopic endonasal approach for craniopharyngiomas. J. Neurosurg. Sci 2021, 65, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Mou, J.; Wang, X.; Huo, G.; Ruan, L.; Jin, K.; Tan, S.; Wang, F.; Hua, H.; Yang, G. Endoscopic Endonasal Surgery for Craniopharyngiomas: A Series of 60 Patients. World Neurosurg. 2019, 124, e424–e430. [Google Scholar] [CrossRef]
- Dho, Y.S.; Kim, Y.H.; Se, Y.B.; Han, D.H.; Kim, J.H.; Park, C.K.; Wang, K.C.; Kim, D.G. Endoscopic endonasal approach for craniopharyngioma: The importance of the relationship between pituitary stalk and tumor. J. Neurosurg. 2018, 129, 611–619. [Google Scholar] [CrossRef]
- Henderson, F., Jr.; Schwartz, T.H. Update on management of craniopharyngiomas. J. Neurooncol. 2022, 156, 97–108. [Google Scholar] [CrossRef]
- Yano, S.; Hide, T.; Shinojima, N. Surgical Outcomes of Endoscopic Endonasal Skull Base Surgery of Craniopharyngiomas Evaluated According to the Degree of Hypothalamic Extension. World Neurosurg. 2017, 100, 288–296. [Google Scholar] [CrossRef]
- Lei, C.; Chuzhong, L.; Chunhui, L.; Peng, Z.; Jiwei, B.; Xinsheng, W.; Yazhuo, Z.; Songbai, G. Approach selection and outcomes of craniopharyngioma resection: A single-institute study. Neurosurg. Rev. 2021, 44, 1737–1746. [Google Scholar] [CrossRef]
- Oldfield, E.H. Transnasal endoscopic surgery for craniopharyngiomas. Neurosurg. Focus 2010, 28, E8a. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Yuan, X.; Yuan, J.; Krumtally, N.A.; Li, Y.; Feng, C.; Liu, Q.; Peng, Z.; Li, X.; Ding, X. Pituitary stalk management during the microsurgery of craniopharyngiomas. Exp. Ther. Med. 2014, 7, 1055–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Lu, X.; Yang, N.; Zheng, J.; Huang, B.; Li, L. Association of pituitary stalk management with endocrine outcomes and recurrence in microsurgery of craniopharyngiomas: A meta-analysis. Clin. Neurol. Neurosurg. 2015, 136, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Otte, A.; Muller, H.L. Childhood-onset Craniopharyngioma. J. Clin. Endocrinol. Metab. 2021, 106, e3820–e3836. [Google Scholar] [CrossRef] [PubMed]
- Dandurand, C.; Sepehry, A.A.; Asadi Lari, M.H.; Akagami, R.; Gooderham, P. Adult Craniopharyngioma: Case Series, Systematic Review, and Meta-Analysis. Neurosurgery 2018, 83, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, R.B.; Ahmed, S.; Johnson, A.; Thomas, H.; Depauw, N.; Horick, N.; Tansky, J.; Evans, C.L.; Pulsifer, M.; Ebb, D.; et al. Proton Radiation Therapy for Pediatric Craniopharyngioma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1480–1487. [Google Scholar] [CrossRef]
- Mortini, P. Craniopharyngiomas: A life-changing tumor. Endocrine 2017, 57, 191–192. [Google Scholar] [CrossRef] [Green Version]
- Yaxian, D.; Chunmei, Y.; Juanyu, X.; Lei, W.; Jian, G.; Chengsong, Z. An analysis of clinical characteristics and postoperative complications in children craniopharyngioma. Child’s Nerv. Syst. 2021, 37, 3033–3040. [Google Scholar] [CrossRef]
- Molitch, M.E.; Clemmons, D.R.; Malozowski, S.; Merriam, G.R.; Vance, M.L.; Endocrine, S. Evaluation and treatment of adult growth hormone deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1587–1609. [Google Scholar] [CrossRef]
- Holmer, H.; Ekman, B.; Bjork, J.; Nordstom, C.H.; Popovic, V.; Siversson, A.; Erfurth, E.M. Hypothalamic involvement predicts cardiovascular risk in adults with childhood onset craniopharyngioma on long-term GH therapy. Eur. J. Endocrinol. 2009, 161, 671–679. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Zhang, S.; Hu, F. Endocrine Disorder in Patients With Craniopharyngioma. Front. Neurol. 2021, 12, 737743. [Google Scholar] [CrossRef]
- Maison, P.; Griffin, S.; Nicoue-Beglah, M.; Haddad, N.; Balkau, B.; Chanson, P.; Metaanalysis of Blinded, R.P.-C.T. Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: A Metaanalysis of Blinded, Randomized, Placebo-Controlled Trials. J. Clin. Endocrinol. Metab. 2004, 89, 2192–2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinks, K.; Boekhoff, S.; Hoffmann, A.; Warmuth-Metz, M.; Eveslage, M.; Peng, J.; Calaminus, G.; Muller, H.L. Quality of life and growth after childhood craniopharyngioma: Results of the multinational trial KRANIOPHARYNGEOM 2007. Endocrine 2018, 59, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, R.; Tsermoulas, G.; Karavitaki, N. Craniopharyngiomas. Presse Med. 2021, 50, 104078. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Total (n = 126), (%) | Children (n = 29) | Adults (n = 97) | χ2/τ | p Value |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean ± SD | 35.4 ± 19.3 | 13.4 ± 3.4 | 41.9 ± 14.0 | ||
| Sex | |||||
| Male | 47 (37.3%) | 21 (72.4%) | 58 (59.8%) | 1.57 | 0.21 |
| Female | 79 (62.7%) | 8 (27.6%) | 39 (40.2%) | ||
| Clinical manifestation | |||||
| Intracranial hypertension | 64 (50.8%) | 13 (44.8%) | 51 (52.6%) | 0.537 | 0.464 |
| visual change | 58 (46%) | 7 (24.1%) | 51 (52.6%) | 7.611 | 0.006 *** |
| Endocrine symptoms | 19 (15.1%) | 3 (10.3%) | 16 (16.5%) | 0.654 | 0.56 |
| Hypothalamic symptom | 11 (8.7%) | 2 (6.9%) | 9 (9.3%) | 0.158 | 1 |
| Others | 15 (11.9%) | 7 (24.1%) | 8 (8.2%) | 4.686 | 0.030 ** |
| Operation frequency | |||||
| Initial operation | 109 (86.5%) | 26 (89.7%) | 83 (85.6%) | 0.317 | 0.76 |
| Reoperation | 17 (13.5%) | 3 (10.3%) | 14 (14.4%) | ||
| Size of lesion | |||||
| ≤3 cm | 46 (36.5%) | 16 (55.2%) | 30 (30.9%) | 5.012 | 0.025 ** |
| >3 cm | 80 (63.5%) | 13 (44.8%) | 67 (69.1%) | ||
| Tumor texture | |||||
| solid | 51 (40.5%) | 11 (37.9%) | 40 (41.2%) | 2.416 | 0.189 |
| cystic-solid | 49 (38.9%) | 13 (44.9%) | 36 (37.1%) | 0.553 | 0.457 |
| cystic | 26 (20.6%) | 5 (17.2%) | 21 (21.7%) | 0.294 | 0.588 |
| Puget hypothalamic involvement | |||||
| Grade 0 | 21 (16.6%) | 2 (6.9%) | 19 (19.6%) | 2.568 | 0.156 |
| Grade 1 | 55 (43.7%) | 14 (48.3%) | 41 (42.3%) | 0.326 | 0.569 |
| Grade 2 | 50 (39.7%) | 13 (44.8%) | 37 (38.1%) | 0.413 | 0.52 |
| Location of tumor | |||||
| Intrasellar | 16 (12.7%) | 1 (3.4%) | 15 (15.5%) | 2.884 | 0.116 |
| Suprasellar-extraventricular | 64 (50.8%) | 12 (41.4%) | 52 (53.6%) | 1.34 | 0.247 |
| Suprasellar-intraventricular | 46 (36.5%) | 16 (55.2%) | 30 (30.9%) | 5.499 | 0.019 ** |
| Anterior pituitary hormone deficiencies | |||||
| Pre-operation | 83 (65.9%) | ||||
| HPAD | 65 (51.6%) | 21 (72.4%) | 44 (45.4%) | 6.75 | 0.009 *** |
| HPTD | 49 (38.9%) | 16 (55.2%) | 33 (34.0%) | 4.12 | 0.042 |
| GHD | 69 (54.8%) | 20 (69.0%) | 49 (50.5%) | 3.144 | 0.076 |
| GD(Male > 14 years, Female > 13 years) | 56 (44.4%) | 19 (65.5%) | 37 (38.1%) | 6.572 | 0.010 ** |
| CH | 36 (28.6%) | 13 (44.8%) | 23 (23.7%) | 4.613 | 0.032 ** |
| Post-operation | 106 (84.1%) | ||||
| HPAD | 100 (79.4%) | 28 (96.6%) | 72 (74.2%) | 6.74 | 0.008 *** |
| HPTD | 90 (71.4%) | 24 (82.8%) | 66 (68.0%) | 2.369 | 0.11 |
| GHD | 103 (81.7%) | 28 (96.6%) | 75 (77.3%) | 5.545 | 0.063 |
| GD | 81(64.3%) | 25 (86.2%) | 56 (57.7%) | 7.822 | 0.007 |
| CH | 85 (67.5%) | 25 (86.2%) | 60 (61.9%) | 5.983 | 0.014 ** |
| Diabetes insipidus | |||||
| Pre-operation | 14 (11.1%) | 2 (6.9%) | 12 (12.4%) | 0.672 | 0.52 |
| Post-operation | |||||
| Transient | 118 (93.6%) | 29 (100%) | 89 (91.8%) | 4.671 | 0.038 ** |
| Permanent | 29 (23%) | 8 (27.6%) | 21 (21.6%) | 0.444 | 0.505 |
| Operation method | |||||
| Transsphenoidal | 22 (17.5%) | 4 (13.8%) | 18 (18.6%) | 0.349 | 0.781 |
| Craniotomy | 104 (82.5%) | 25 (86.2%) | 79 (81.4%) | ||
| Preservation of pituitary stalk | |||||
| Yes | 74 (58.7%) | 16 (55.2%) | 58 (59.8%) | 0.197 | 0.658 |
| No | 52 (41.3%) | 13 (44.8%) | 39 (40.2%) | ||
| Degree of resection | |||||
| Total | 80 (63.5%) | 11 (37.9%) | 69 (71.2%) | 9.88 | 0.002 *** |
| Subtotal | 29 (36.5%) | 15 (51.7%) | 14 (14.4%) | 16.181 | 0.000 *** |
| Partial | 17 (13.5%) | 3 (10.4%) | 14 (14.4%) | 0.048 | 1 |
| Pathological subtype | |||||
| Adamantinomatous | 93 (73.8%) | 26 (89.7%) | 67 (69.1%) | 4.854 | 0.030 ** |
| Papillary | 33 (26.2%) | 3 (10.3%) | 30 (30.9%) | ||
| Hormone alternative therapy | |||||
| Yes | 80 (63.5%) | 24 (82.8%) | 56 (57.7%) | 6.033 | 0.014 ** |
| No | 46 (36.5%) | 5 (17.2%) | 41 (42.3%) | ||
| follow-up period (Mean ± SD, month) | 54.1 ± 33 | 52.7 ± 34.8 | 54.5 ± 32.7 | −0.248 | 0.439 |
| Recurrence | |||||
| Yes | 31 (24.6%) | 8 (27.6%) | 23 (23.7%) | 0.181 | 0.674 |
| No | 95 (75.4%) | 21 (72.4%) | 74 (76.3%) |
| Variable | Pre-Operation (%) | χ2 | p Value | With Age Stratification | ||
|---|---|---|---|---|---|---|
| With Hypopituitarism | Without Hypopituitarism | HR 95 % CI | p Value | |||
| Sex | ||||||
| Male | 56 (70.9%) | 23 (29.1%) | ||||
| Female | 27 (57.4%) | 20 (42.6%) | 2.368 | 0.124 | ||
| Age (years) | ||||||
| Children (<18) | 25 (86.2%) | 4 (13.8%) | 6.928 | 0.008 *** | ||
| Adults (≥18) | 58 (59.8%) | 39 (40.2%) | ||||
| Clinical manifestation | ||||||
| Intracranial hypertension | 40 (62.5%) | 24 (37.5%) | 0.658 | 0.417 | ||
| visual change | 34 (58.6%) | 24 (41.4%) | 2.514 | 0.113 | ||
| Endocrine symptoms | 19 (100%) | 0 (0%) | 11.591 | 0.000 *** | 1.3 (1.1–1.5) | 0.001 *** |
| Hypothalamic symptom | 11 (100%) | 0 (0%) | 6.244 | 0.016 ** | 1.2 (1.1–1.3) | 0.013 ** |
| Others | 12 (80%) | 3 (20%) | 1.512 | 0.261 | ||
| Location of tumor | ||||||
| Intrasellar | 4 (25%) | 12 (75%) | 13.916 | 0.001 *** | ||
| Suprasellar-extraventricular | 42 (65.6%) | 22 (34.4%) | 1.0 (0.5–2.0) | 0.953 | ||
| Suprasellar-intraventricular | 37 (92.5%) | 9 (7.5%) | 3.0 (1.3–7.1) | 0.009 *** | ||
| Size of lesion | ||||||
| ≤3 cm | 24 (%) | 22 (%) | 6.048 | 0.014 ** | 0.4 (0.2–0.8) | 0.014 ** |
| >3 cm | 59 (%) | 21 (%) | ||||
| Tumor texture | ||||||
| solid | 36 (70.6%) | 15 (29.4%) | 0.847 | 0.357 | ||
| cystic-solid | 32 (65.3%) | 17 (34.7%) | ||||
| cystic | 15 (57.7%) | 11 (42.3%) | ||||
| Puget hypothalamic involvement | ||||||
| Grade 0 | 4 (19.5%) | 17 (81.0%) | 22.04 | 0.000 *** | ||
| Grade 1 | 38 (69.1%) | 17 (30.9%) | 1.3 (1.1–2.7) | 0.031 ** | ||
| Grade 2 | 41 (82%) | 9 (18%) | 3.7 (1.6–8.6) | 0.002 *** | ||
| Pathological subtype | ||||||
| Adamantinomatous | 63 (67.7%) | 30 (32.3%) | 0.552 | 0.458 | ||
| Papillary | 20 (60.6%) | 13 (39.4%) | ||||
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| Age (years) | |||
| Children (<18) | 3.024 | 1.21–13.44 | 0.024 |
| Clinical manifestation | |||
| Endocrine symptoms | NS | ||
| Hypothalamic symptom | NA | ||
| Location of tumor | NA | ||
| Size of lesion | NA | ||
| Puget hypothalamic involvement | |||
| Grade 1 vs. Grade 0 | 4.974 | 1.39–17.81 | 0.014 |
| Grade 2 vs. Grade 0 | 11.452 | 2.98–43.97 | 0 |
| Variable | Post-Operation (%) | χ2 | p Value | With Age Stratification | ||
|---|---|---|---|---|---|---|
| With Hypopituitarism | Without Hypopituitarism | HR 95 % CI | p Value | |||
| Sex | ||||||
| Male | 34 (81%) | 8 (19%) | 3.136 | 0.077 * | 0.4 (0.1–1.1) | 0.077 |
| Female | 20 (62.5%) | 12 (37.5%) | ||||
| Age (years) | ||||||
| Children (<18) | 15 (93.8%) | 1 (6.2%) | 4.468 | 0.054 * | ||
| Adults (≥18) | 39 (67.2%) | 19 (32.8%) | ||||
| Clinical manifestation | ||||||
| Intracranial hypertension | 28 (73.7%) | 10 (26.3%) | 0.02 | 0.887 | ||
| visual change | 27 (65.9%) | 14 (34.1%) | 2.363 | 0.124 | ||
| Endocrine symptoms | 10 (100%) | 0 (0%) | 4.282 | 0.053 * | 1.2 (1.1–1.4) | 0.040 ** |
| Hypothalamic symptom | 5 (100%) | 0 (0%) | 1.986 | 0.159 | ||
| Others | 5 (83.3%) | 1 (16.7%) | 0.355 | 1 | ||
| Location of tumor | ||||||
| Intrasellar | 3 (18.8%) | 13 (81.2%) | 26.781 | 0.000 *** | ||
| Suprasellar-extraventricular | 36 (87.8%) | 5 (12.2%) | 6.0 (1.9–19.1) | 0.001 *** | ||
| Suprasellar-intraventricular | 15 (88.2%) | 2 (11.8%) | 3.5 (1.7–16.8) | 0.003 *** | ||
| Size of lesion | ||||||
| ≤3 cm | 23 (62.2%) | 14 (37.8%) | 4.385 | 0.036 ** | 0.3 (0.1–0.9) | 0.036 ** |
| >3 cm | 31 (83.8%) | 6 (16.2%) | ||||
| Tumor texture | ||||||
| solid | 27 (75%) | 9 (25%) | 1.585 | 0.453 | ||
| cystic-solid | 20 (76.9%) | 6 (23.1%) | ||||
| cystic | 7 (58.3%) | 5 (41.7%) | ||||
| Puget hypothalamic involvement | ||||||
| Grade 0 | 6 (28.6%) | 15 (71.4%) | 29.359 | 0.000 *** | ||
| Grade 1 | 24 (80.6%) | 3 (19.4%) | 4.5 (1.2–17.3) | 0.020 ** | ||
| Grade 2 | 24 (92.3%) | 2 (7.7%) | 7.2 (1.5–34.1) | 0.006 *** | ||
| Preoperative hormonal status | ||||||
| With hypopituitarism | 41 (95.3%) | 2 (4.7%) | 26.058 | 0.000 *** | 28.4 (5.8–138.9) | 0.000 *** |
| Without hypopituitarism | 13 (41.9%) | 18 (58.1%) | ||||
| Operation frequency | ||||||
| Initial operation | 6 (85.7%) | 1 (14.3%) | 0.636 | 0.666 | ||
| Reoperation | 48 (71.6%) | 19 (28.4%) | ||||
| Operation method | ||||||
| Transsphenoidal | 8 (36.4%) | 14 (63.6%) | 21.275 | 0.000 *** | 0.1 (0.0–0.3) | 0.000 *** |
| Craniotomy | 46 (88.5%) | 6 (11.5%) | ||||
| Degree of resection | ||||||
| Total | 35 (71.4%) | 14 (28.6%) | 0.675 | 0.175 | ||
| Subtotal | 14 (87.5%) | 2 (12.5%) | 2.184 | 0.207 | ||
| Partial | 5 (55.6%) | 4 (44.4%) | 1.576 | 0.241 | ||
| Pathological subtype | ||||||
| Adamantinomatous | 14 (66.7%) | 7 (33.3%) | 0.591 | 0.442 | ||
| Papillary | 40 (75.5%) | 13 (24.5%) | ||||
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| Sex | NA | ||
| Age (years) | NA | ||
| Clinical manifestation | |||
| Endocrine symptoms | NA | ||
| Hypothalamic symptom | NA | ||
| Location of tumor | |||
| Suprasellar-extraventricular vs. Intrasellar | 39.427 | 3.79–410.22 | 0.002 |
| Suprasellar-intraventricular vs. Intrasellar | 40.14 | 2.67–602.66 | 0.008 |
| Size of lesion | NS | ||
| Puget hypothalamic involvement | NS | ||
| Preoperative hormonal status | |||
| With hypopituitarism | 41.384 | 4.50–380.60 | 0.001 |
| Operation method | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Zhu, H.; Wang, J.; Wu, S.; Zhang, S.; Wang, J.; Shu, K. Risk Factor and Replacement Therapy Analysis of Pre- and Postoperative Endocrine Deficiencies for Craniopharyngioma. Cancers 2023, 15, 340. https://doi.org/10.3390/cancers15020340
Cheng L, Zhu H, Wang J, Wu S, Zhang S, Wang J, Shu K. Risk Factor and Replacement Therapy Analysis of Pre- and Postoperative Endocrine Deficiencies for Craniopharyngioma. Cancers. 2023; 15(2):340. https://doi.org/10.3390/cancers15020340
Chicago/Turabian StyleCheng, Lidong, Hongtao Zhu, Jing Wang, Sisi Wu, Suojun Zhang, Junwen Wang, and Kai Shu. 2023. "Risk Factor and Replacement Therapy Analysis of Pre- and Postoperative Endocrine Deficiencies for Craniopharyngioma" Cancers 15, no. 2: 340. https://doi.org/10.3390/cancers15020340






