Evaluation of Microsatellite Instability Molecular Analysis versus Immuno-Histochemical Interpretation in Malignant Neoplasms with Different Localizations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
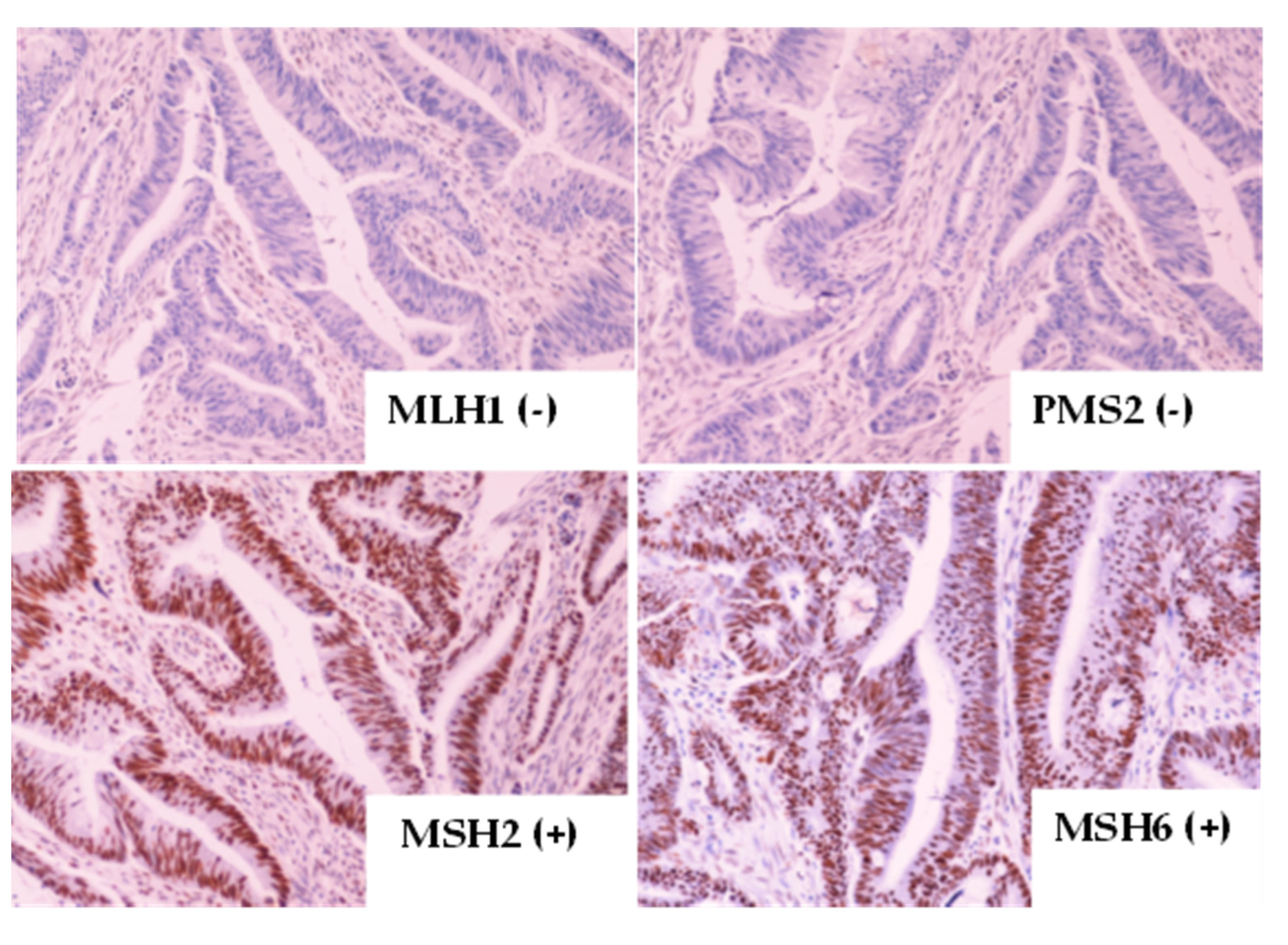
2.1. Immunohistochemistry (IHC) Testing
2.2. PCR with Fragment Analysis
3. Results
3.1. MSI and MMR Status Analysis of Various Malignant Neoplasms
3.2. MSI-H vs. dMMR Status in Different Solid Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
References
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkmann, B.; Klintschar, M.; Neuhuber, F.; Hühne, J.; Rolf, B. Mutation rate in human microsatellites: Influence of the structure and length of the tandem repeat. Am. J. Hum. Genet. 1998, 62, 1408–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008, 18, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Chao, E.C.; Lipkin, S.M. Molecular models for the tissue specificity of DNA mismatch repair-deficient carcinogenesis. Nucleic Acids Res. 2006, 34, 840–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, A.J.; Capo-Chichi, J.M.; Spence, T.; Grenier, S.; Stockley, T.; Kamel-Reid, S.; Serra, S.; Sabatini, P.; Chetty, R. Heterogenous loss of mismatch repair (mmr) protein expression: A challenge for immunohistochemical interpretation and microsatellite instability (msi) evaluation. J. Pathology. Clin. Res. 2019, 5, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Jiricny, J. The multifaceted mismatch-repair system. Nat. Rev. Mol. Cell Biol. 2006, 7, 335–346. [Google Scholar] [CrossRef]
- Ellison, A.R.; Lofing, J.; Bitter, G.A. Human mutl homolog (mlh1) function in DNA mismatch repair: A prospective screen for missense mutations in the atpase domain. Nucleic Acids Res. 2004, 32, 5321–5338. [Google Scholar] [CrossRef] [Green Version]
- Kasela, M.; Nyström, M.; Kansikas, M. Pms2 expression decrease causes severe problems in mismatch repair. Hum. Mutat. 2019, 40, 904–907. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, J.P.; Del Giglio, A.; Achatz, M.I.; Carvalho, F.M. Complete clinical response in stage ivb endometrioid endometrial carcinoma after first-line pembrolizumab therapy: Report of a case with isolated loss of pms2 protein. Case Rep. Oncol. 2020, 13, 1067–1074. [Google Scholar] [CrossRef]
- Bai, W.; Ma, J.; Liu, Y.; Liang, J.; Wu, Y.; Yang, X.; Xu, E.; Li, Y.; Xi, Y. Screening of msi detection loci and their heterogeneity in east asian colorectal cancer patients. Cancer Med. 2019, 8, 2157–2166. [Google Scholar] [CrossRef]
- Saridaki, Z.; Souglakos, J.; Georgoulias, V. Prognostic and predictive significance of msi in stages ii/iii colon cancer. World J. Gastroenterol. 2014, 20, 6809–6814. [Google Scholar] [CrossRef] [PubMed]
- Damilakis, E.; Mavroudis, D.; Sfakianaki, M.; Souglakos, J. Immunotherapy in metastatic colorectal cancer: Could the latest developments hold the key to improving patient survival? Cancers 2020, 12, 889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puccini, A.; Battaglin, F.; Iaia, M.L.; Lenz, H.J.; Salem, M.E. Overcoming resistance to anti-pd1 and anti-pd-l1 treatment in gastrointestinal malignancies. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Kastrinos, F.; Syngal, S. Screening patients with colorectal cancer for lynch syndrome: What are we waiting for? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part ii. The utility of microsatellite instability testing. J. Mol. Diagn. JMD 2008, 10, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Scott, P. A Review of the Current Testing Methodologies for the Detection of Mismatch Repair Defficiency in Tumours; Medex Consulting: Surrey, BC, Canada, 2020; pp. 1–14. [Google Scholar]
- Papadaki, C.; Mavroudis, D.; Trypaki, M.; Koutsopoulos, A.; Stathopoulos, E.; Hatzidaki, D.; Tsakalaki, E.; Georgoulias, V.; Souglakos, J. Tumoral expression of txr1 and tsp1 predicts overall survival of patients with lung adenocarcinoma treated with first-line docetaxel-gemcitabine regimen. Clin. Cancer Res. 2009, 15, 3827–3833. [Google Scholar] [CrossRef] [Green Version]
- Sfakianaki, M.; Papadaki, C.; Tzardi, M.; Trypaki, M.; Manolakou, S.; Messaritakis, I.; Saridaki, Z.; Athanasakis, E.; Mavroudis, D.; Tsiaoussis, J.; et al. Pkm2 expression as biomarker for resistance to oxaliplatin-based chemotherapy in colorectal cancer. Cancers 2020, 12, 2058. [Google Scholar] [CrossRef]
- Gafà, R.; Maestri, I.; Matteuzzi, M.; Santini, A.; Ferretti, S.; Cavazzini, L.; Lanza, G. Sporadic colorectal adenocarcinomas with high-frequency microsatellite instability. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2000, 89, 2025–2037. [Google Scholar] [CrossRef]
- Cushman-Vokoun, A.M.; Stover, D.G.; Zhao, Z.; Koehler, E.A.; Berlin, J.D.; Vnencak-Jones, C.L. Clinical utility of kras and braf mutations in a cohort of patients with colorectal neoplasms submitted for microsatellite instability testing. Clin. Color. Cancer 2013, 12, 168–178. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.M.; Zhang, S.; Geiger, T.; Hafez, M.J.; Bacher, J.; Berg, K.D.; Eshleman, J.R. Comparison of the microsatellite instability analysis system and the bethesda panel for the determination of microsatellite instability in colorectal cancers. J. Mol. Diagn. JMD 2006, 8, 305–311. [Google Scholar] [CrossRef]
- Vilar, E.; Gruber, S.B. Microsatellite instability in colorectal cancer-the stable evidence. Nat. Rev. Clin. Oncol. 2010, 7, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzi, M.; Amonkar, M.; Zhang, J.; Mehta, S.; Liaw, K.-L. Epidemiology of microsatellite instability high (msi-h) and deficient mismatch repair (dmmr) in solid tumors: A structured literature review. J. Oncol. 2020, 2020, 1807929. [Google Scholar] [CrossRef]
- Deshpande, M.; Romanski, P.A.; Rosenwaks, Z.; Gerhardt, J. Gynecological cancers caused by deficient mismatch repair and microsatellite instability. Cancers 2020, 12, 3319. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Ravindranathan, D.; Russler, G.A.; Yantorni, L.; Drusbosky, L.M.; Bilen, M.A. Detection of microsatellite instability via circulating tumor DNA and response to immunotherapy in metastatic castration-resistant prostate cancer: A case series. Case Rep. Oncol. 2021, 14, 190–196. [Google Scholar] [CrossRef]
- Ratti, M.; Lampis, A.; Hahne, J.C.; Passalacqua, R.; Valeri, N. Microsatellite instability in gastric cancer: Molecular bases, clinical perspectives, and new treatment approaches. Cell. Mol. Life Sci. CMLS 2018, 75, 4151–4162. [Google Scholar] [CrossRef]
- Tempero, M.A. Nccn guidelines updates: Pancreatic cancer. J. Natl. Compr. Canc. Netw. 2019, 17, 603–605. [Google Scholar]
- Laghi, L.; Beghelli, S.; Spinelli, A.; Bianchi, P.; Basso, G.; Di Caro, G.; Brecht, A.; Celesti, G.; Turri, G.; Bersani, S.; et al. Irrelevance of microsatellite instability in the epidemiology of sporadic pancreatic ductal adenocarcinoma. PLoS ONE 2012, 7, e46002. [Google Scholar] [CrossRef]
- Nakata, B.; Wang, Y.Q.; Yashiro, M.; Nishioka, N.; Tanaka, H.; Ohira, M.; Ishikawa, T.; Nishino, H.; Hirakawa, K. Prognostic value of microsatellite instability in resectable pancreatic cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 2536–2540. [Google Scholar]
- Riazy, M.; Kalloger, S.E.; Sheffield, B.S.; Peixoto, R.D.; Li-Chang, H.H.; Scudamore, C.H.; Renouf, D.J.; Schaeffer, D.F. Mismatch repair status may predict response to adjuvant chemotherapy in resectable pancreatic ductal adenocarcinoma. Mod. Pathol. 2015, 28, 1383–1389. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.Y.; Haile, R.W.; Templeton, A.; Macrae, F.; Qin, F.; Sundaram, V.; Ladabaum, U. Worldwide practice patterns in lynch syndrome diagnosis and management, based on data from the international mismatch repair consortium. Clin. Gastroenterol. Hepatol. 2018, 16, 1901–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eso, Y.; Shimizu, T.; Takeda, H.; Takai, A.; Marusawa, H. Microsatellite instability and immune checkpoint inhibitors: Toward precision medicine against gastrointestinal and hepatobiliary cancers. J. Gastroenterol. 2020, 55, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Lin-Hurtubise, K.M.; Ishihara, K.; McLaughlin, K.; Morte, D.; Sheffler, R.J.T.G.C. Lynch syndrome: Expanded tumor spectrum, universal screening and multimodal treatment strategies for colon cancer. Transl. Gastrointest Cancer 2015, 4, 367–372. [Google Scholar]
- Loughrey, M.B.; McGrath, J.; Coleman, H.G.; Bankhead, P.; Maxwell, P.; McGready, C.; Bingham, V.; Humphries, M.P.; Craig, S.G.; McQuaid, S.; et al. Identifying mismatch repair-deficient colon cancer: Near-perfect concordance between immunohistochemistry and microsatellite instability testing in a large, population-based series. Histopathology 2021, 78, 401–413. [Google Scholar] [CrossRef]
- Cicek, M.S.; Lindor, N.M.; Gallinger, S.; Bapat, B.; Hopper, J.L.; Jenkins, M.A.; Young, J.; Buchanan, D.; Walsh, M.D.; Le Marchand, L.; et al. Quality assessment and correlation of microsatellite instability and immunohistochemical markers among population- and clinic-based colorectal tumors results from the colon cancer family registry. J. Mol. Diagn. JMD 2011, 13, 271–281. [Google Scholar] [CrossRef]
- Cheah, P.L.; Li, J.; Looi, L.M.; Koh, C.C.; Lau, T.P.; Chang, S.W.; Teoh, K.H.; Mun, K.S.; Nazarina, A.R. Screening for microsatellite instability in colorectal carcinoma: Practical utility of immunohistochemistry and pcr with fragment analysis in a diagnostic histopathology setting. Malays. J. Pathol. 2019, 41, 91–100. [Google Scholar]
- Sugimoto, R.; Endo, M.; Osakabe, M.; Toya, Y.; Yanagawa, N.; Matsumoto, T.; Sugai, T. Immunohistochemical analysis of mismatch repair gene proteins in early gastric cancer based on microsatellite status. Digestion 2020, 102, 691–700. [Google Scholar] [CrossRef]
- Zlobec, I.; Terracciano, L.; Jass, J.R.; Lugli, A. Value of staining intensity in the interpretation of immunohistochemistry for tumor markers in colorectal cancer. Virchows Arch. Int. J. Pathol. 2007, 451, 763–769. [Google Scholar] [CrossRef]
- Ryan, E.; Sheahan, K.; Creavin, B.; Mohan, H.M.; Winter, D.C. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit. Rev. Oncol./Hematol. 2017, 116, 38–57. [Google Scholar] [CrossRef]



| Feature | N | % |
|---|---|---|
| 242 | ||
| Median Age (Range) | 64 (19–89) | |
| <64 years | 158 | 65.3 |
| ≥64 | 79 | 32.6 |
| Gender | ||
| Female | 111 | 4.9 |
| Male | 131 | 54.1 |
| Patients | ||
| Adjuvant | 142 | 58.7 |
| Metastatic | 90 | 37.2 |
| Unknown | 10 | 4.1 |
| BRAFV600E status | ||
| WT | 99 | 40.9 |
| Mutant | 11 | 4.5 |
| Not Done | 132 | 54.5 |
| MMR Status | ||
| dMMR | 21 | 8.7 |
| pMMR | 209 | 86.4 |
| Not Done | 12 | 5.7 |
| MSI Status | ||
| MSI-High | 29 | 12 |
| MSI-Stable | 212 | 87.6 |
| Failed | 1 | 0.4 |
| MMR | ||||
|---|---|---|---|---|
| dMMR | pMMR p Value | |||
| MSI Total | High | 18 (64.3%) | 10 (35.7%) | |
| Stable | 5 (2.5%) 23 | 197 (97.5%) > 0.001 207 | ||
| (a) Analysis of MSI Status in various malignant neoplasms by PCR-Fragment Analysis | ||||
| Neoplasm | N | % | MSI-H Status | % |
| Colorectal | 160 | 66.1 | 23 | 14.46 |
| Stomach | 14 | 5.7 | 1 | 7.1 |
| Pancreas | 13 | 5.3 | 0 | 0 |
| Endometrium | 9 | 3.7 | 2 | 22.2 |
| Biliary | 7 | 2.9 | 0 | 0 |
| Esophageal | 6 | 2.5 | 0 | 0 |
| Cervix | 5 | 2.0 | 0 | 0 |
| Breast | 4 | 1.6 | 1 | 25.0 |
| Unknown primary | 4 | 1.6 | 0 | 0 |
| Lung | 4 | 1.6 | 1 | 25.0 |
| Liver | 3 | 1.2 | 0 | 0 |
| Duodenum | 3 | 1.2 | 0 | 0 |
| Mesothelioma | 2 | 0.8 | 0 | 0 |
| Ovarian | 2 | 0.8 | 0 | 0 |
| Small Intestine | 2 | 0.8 | 0 | 0 |
| Anal | 1 | 0.4 | 0 | 0 |
| Bladder | 1 | 0.4 | 0 | 0 |
| Sarkoma | 1 | 0.4 | 0 | 0 |
| Brain | 1 | 0.4 | 0 | 0 |
| Total | 242 | 100 | 28 | 11.57 |
| (b) Analysis of MMR Status in various malignant neoplasms by ICH. | ||||
| Colorectal Stomach | 151 13 | 65.7 5.7 | 15 1 | 9.93 7.69 |
| Pancreas Endometrial | 13 9 | 5.7 3.9 | 3 | 0 33.0 |
| Biliary | 7 | 2.9 | 0 | 0 |
| Esophageal | 6 | 2.5 | 0 | 0 |
| Cervix | 5 | 2.0 | 0 | 0 |
| Lung | 4 | 1.7 | 1 | 25.0 |
| Liver | 3 | 1.3 | 0 | 0 |
| Duodenum | 3 | 1.2 | 1 | 33.3 |
| Breast | 3 | 1.3 | 0 | 0 |
| Unknown primary | 3 | 1.3 | 0 | 0 |
| Mesothelioma | 2 | 0.9 | 0 | 0 |
| Small Intestine | 2 | 0.9 | 0 | 0 |
| Ovarian | 2 | 0.9 | 0 | 0 |
| Bladder | 1 | 0.4 | 0 | 0 |
| Sarcoma | 1 | 0.4 | 0 | 0 |
| Anal | 1 | 0.4 | 0 | 0 |
| Brain | 1 | 0.4 | 0 | 0 |
| Total | 242 | 100 | 21 | 8.67 |
| (a) | |||||
|---|---|---|---|---|---|
| Stage | N | % | MSI-H (%) | dMMR (%) | |
| IA | 25 | 10.2 | 4 (15.4) | 3 (15) | |
| II | 4 | 1.6 | 1 (25) | 1 (25) | |
| IIA | 36 | 14.8 | 9 (25) | 7 (20.6) | |
| IIB | 7 | 2.9 | 1 (14.3) | 1 (14.3) | |
| III | 6 | 2.5 | 1 (20) | 0 | |
| IIIA | 3 | 1.2 | 1 (33.3) | 0 | |
| IIIB | 28 | 11.5 | 1 (3.6) | 0 | |
| IIIC | 27 | 11.1 | 2 (7.7) | 2 (7.7) | |
| IV | 88 | 36.1 | 6 (6.8) | 5 (5.8) | |
| IVA | 4 | 1.6 | 0 | 0 | |
| Unknown | 16 | 6.6 | 0 | 0 | |
| Total | 242 | 100 | 26 | 19 | |
| (b) | |||||
| I | II | III | IV | pValue | |
| MSI-High | 4 (1.7%) | 12 (5.2%) | 7 (3.0%) | 5 (2.2%) | 0.017 |
| MSS Total | 21 (9.1%) 25 | 40 (17.2%) 52 | 56 (24.1%) 63 | 87 (37.5%) 92 | |
| dMMR | 4 (1.8%) | 9(4.1%) | 2 (1.8%) | 5 (2.3%) | 0.008 |
| pMMR Total | 17 (7.7%) 21 | 39 (17.7%) 48 | 59 (26.8%) 61 | 85 (38.6%) 90 | |
| (c) | |||||
| M0 | M1 | pValue | |||
| MSI-High | 24 (85.7%) | 4 (14.3%) | 0.005 | ||
| MSS Total | 118 (57.8%) 142 | 86 (42.2%) 9 90 | |||
| dMMR | 16 (80%) | 4 (20.0%) | 0.056 | ||
| pMMR | 116 (58%) | 84 (42.0%) | |||
| Total | 132 | 88 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfakianaki, M.; Tzardi, M.; Tsantaki, K.; Koutoulaki, C.; Messaritakis, I.; Datseri, G.; Moustou, E.; Mavroudis, D.; Souglakos, J. Evaluation of Microsatellite Instability Molecular Analysis versus Immuno-Histochemical Interpretation in Malignant Neoplasms with Different Localizations. Cancers 2023, 15, 353. https://doi.org/10.3390/cancers15020353
Sfakianaki M, Tzardi M, Tsantaki K, Koutoulaki C, Messaritakis I, Datseri G, Moustou E, Mavroudis D, Souglakos J. Evaluation of Microsatellite Instability Molecular Analysis versus Immuno-Histochemical Interpretation in Malignant Neoplasms with Different Localizations. Cancers. 2023; 15(2):353. https://doi.org/10.3390/cancers15020353
Chicago/Turabian StyleSfakianaki, Maria, Maria Tzardi, Konstantina Tsantaki, Chara Koutoulaki, Ippokratis Messaritakis, Galateia Datseri, Eleni Moustou, Dimitrios Mavroudis, and John Souglakos. 2023. "Evaluation of Microsatellite Instability Molecular Analysis versus Immuno-Histochemical Interpretation in Malignant Neoplasms with Different Localizations" Cancers 15, no. 2: 353. https://doi.org/10.3390/cancers15020353
APA StyleSfakianaki, M., Tzardi, M., Tsantaki, K., Koutoulaki, C., Messaritakis, I., Datseri, G., Moustou, E., Mavroudis, D., & Souglakos, J. (2023). Evaluation of Microsatellite Instability Molecular Analysis versus Immuno-Histochemical Interpretation in Malignant Neoplasms with Different Localizations. Cancers, 15(2), 353. https://doi.org/10.3390/cancers15020353







