Role of Clock Genes and Circadian Rhythm in Renal Cell Carcinoma: Recent Evidence and Therapeutic Consequences
Abstract
:Simple Summary
Abstract
1. Introduction
2. Role of Clock Genes in Cancer
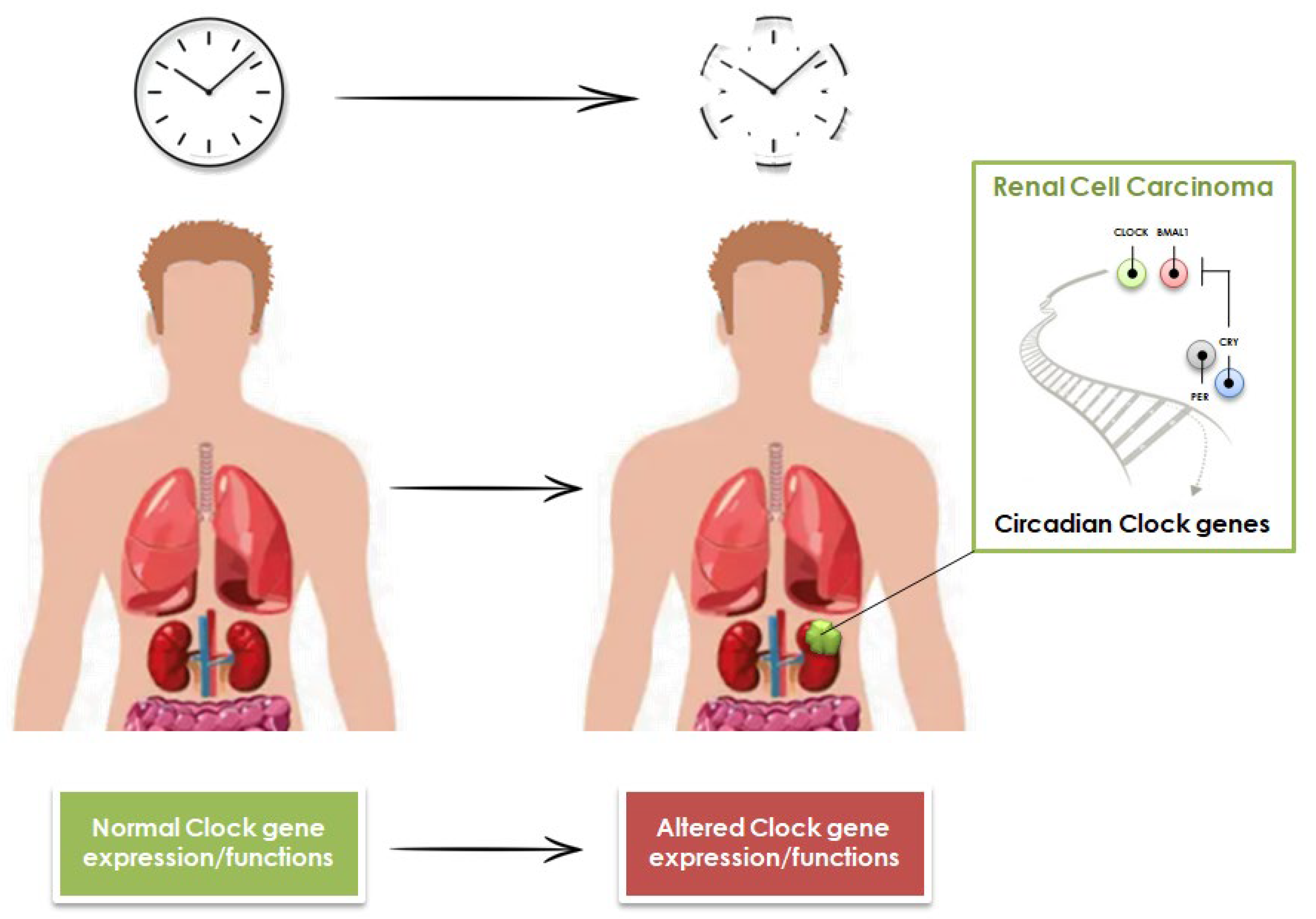
3. Role of Circadian Clock Genes in Renal Cell Carcinoma Tumorigenesis and Prognosis
4. Circadian Variations of Cytokines and Chemokines Involved in Renal Cell Carcinoma
5. Evidence on the Time-of-Day Influence on the Efficacy of Immunotherapy and Targeted Therapy
6. Emerging Strategies to Modulate Clock Genes in Patients with Cancer
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosbash, M. Circadian Rhythms and the Transcriptional Feedback Loop (Nobel Lecture). Angew. Chem. Int. Ed. 2021, 60, 8650–8666. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Zehring, W.A.; Wheeler, D.A.; Pirrotta, V.; Hadfield, C.; Hall, J.C.; Rosbash, M. Molecular Analysis of the Period Locus in Drosophila Melanogaster and Identification of a Transcript Involved in Biological Rhythms. Cell 1984, 38, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, F.; Gomez, E.C.; Chen, J.; Buas, M.F.; Schlecht, N.F.; Hulme, K.; Kulkarni, S.V.; Singh, P.K.; O’Connor, R.; Ambrosone, C.B.; et al. Genes Relevant to Tissue Response to Cancer Therapy Display Diurnal Variation in mRNA Expression in Human Oral Mucosa. J. Circadian Rhythm. 2021, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Piva, F.; Porta, C.; Bracarda, S.; Heng, D.Y.; Matrana, M.R.; Grande, E.; Mollica, V.; Aurilio, G.; Rizzo, M.; et al. Artificial Neural Networks as a Way to Predict Future Kidney Cancer Incidence in the United States. Clin. Genitourin. Cancer 2021, 19, e84–e91. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [Green Version]
- Santoni, M.; Conti, A.; Porta, C.; Procopio, G.; Sternberg, C.N.; Basso, U.; De Giorgi, U.; Bracarda, S.; Rizzo, M.; Ortega, C.; et al. Sunitinib, pazopanib or sorafenib for the treatment of patients with late relapsing metastatic renal cell carcinoma. J. Urol. 2015, 193, 41–47. [Google Scholar] [CrossRef]
- Conti, A.; Santoni, M.; Amantini, C.; Burattini, L.; Berardi, R.; Santoni, G.; Cascinu, S.; Muzzonigro, G. Progress of molecular targeted therapies for advanced renal cell carcinoma. Biomed Res. Int. 2013, 2013, 419176. [Google Scholar] [CrossRef] [Green Version]
- Ciccarese, C.; Alfieri, S.; Santoni, M.; Santini, D.; Brunelli, M.; Bergamini, C.; Licitra, L.; Montironi, R.; Tortora, G.; Massari, F. New toxicity profile for novel immunotherapy agents: Focus on immune-checkpoint inhibitors. Expert Opin. Drug Metab. Toxicol. 2016, 12, 57–75. [Google Scholar] [CrossRef]
- Rizzo, A.; Mollica, V.; Dall’Olio, F.G.; Ricci, A.D.; Maggio, I.; Marchetti, A.; Rosellini, M.; Santoni, M.; Ardizzoni, A.; Massari, F. Quality of life assessment in renal cell carcinoma Phase II and III clinical trials published between 2010 and 2020: A systematic review. Future Oncol. 2021, 17, 2671–2681. [Google Scholar] [CrossRef]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Yuan, X.; Eltzschig, H.K. Circadian Rhythm as a Therapeutic Target. Nat. Rev. Drug Discov. 2021, 20, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular Mechanisms and Physiological Importance of Circadian Rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef]
- Shafi, A.A.; Knudsen, K.E. Cancer and the Circadian Clock. Cancer Res. 2019, 79, 3806–3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.T.; Choo, K.B.; Hou, M.F.; Yeh, K.T.; Kuo, S.J.; Chang, J.-G. Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis 2005, 26, 1241–1246. [Google Scholar] [CrossRef]
- Liu, B.; Xu, K.; Jiang, Y.; Li, X. Aberrant expression of Per1, Per2 and Per3 and their prognostic relevance in non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 7863–7871. [Google Scholar]
- Kang, T.H.; Sancar, A. Circadian regulation of DNA excision repair: Implications for chrono-chemotherapy. Cell Cycle 2009, 8, 1665–1667. [Google Scholar] [CrossRef]
- Xue, X.; Liu, F.; Han, Y.; Li, P.; Yuan, B.; Wang, X.; Chen, Y.; Kuang, Y.; Zhi, Q.; Zhao, H. Silencing NPAS2 promotes cell growth and invasion in DLD-1 cells and correlated with poor prognosis of colorectal cancer. Biochem. Biophys. Res. Commun. 2014, 450, 1058–1062. [Google Scholar] [CrossRef]
- Wang, L.; Chen, B.; Wang, Y.; Sun, N.; Lu, C.; Qian, R.; Hua, L. hClock gene expression in human colorectal carcinoma. Mol. Med. Rep. 2013, 8, 1017–1022. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yan, D.; Teng, M.; Fan, J.; Zhou, C.; Li, D.; Qiu, G.; Sun, X.; Li, T.; Xing, T.; et al. Reduced expression of PER3 is associated with incidence and development of colon cancer. Ann. Surg. Oncol. 2012, 19, 3081–3088. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.; Liu, D.; Jin, S.; Yang, Y.; Tang, W.; Gong, L. Decreased circadian component Bmal1 predicts tumor progression and poor prognosis in human pancreatic ductal adenocarcinoma. Biochem. Biophys. Res. Commun. 2016, 472, 156–162. [Google Scholar] [CrossRef]
- Hsu, C.M.; Lin, S.F.; Lu, C.T.; Lin, P.M.; Yang, M.Y. Altered expression of circadian clock genes in head and neck squamous cell carcinoma. Tumor Biol. 2012, 33, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Hwang-Verslues, W.W.; Chang, P.H.; Jeng, Y.M.; Kuo, W.H.; Chiang, P.H.; Chang, Y.C.; Hsieh, T.H.; Su, F.Y.; Lin, L.C.; Abbondante, S.; et al. Loss of corepressor PER2 under hypoxia up-regulates OCT1-mediated EMT gene expression and enhances tumor malignancy. Proc. Natl. Acad. Sci. USA 2013, 110, 12331–12336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellet, M.M.; Stincardini, C.; Costantini, C.; Gargaro, M.; Pieroni, S.; Castelli, M.; Piobbico, D.; Sassone-corsi, P.; Della-Fazia, M.A.; Romani, L.; et al. The Circadian Protein PER1 Modulates the Cellular Response to Anticancer Treatments. Int. J. Mol. Sci. 2021, 22, 2974. [Google Scholar] [CrossRef] [PubMed]
- de Assis, L.V.M.; Kinker, G.S.; Moraes, M.N.; Markus, R.P.; Fernandes, P.A.; de Lauro Castrucci, A.M. Expression of the circadian clock gene BMAL1 positively correlates with antitumor immunity and patient survival in metastatic melanoma. Front. Oncol. 2018, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Ukai, H.; Ueda, H.R. Systems Biology of Mammalian Circadian Clocks. Annu. Rev. Physiol. 2009, 72, 579–603. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, M.; Umemura, Y.; Koike, N.; Tsuchiya, Y.; Inada, Y.; Watanabe, H.; Tanaka, T.; Minami, Y.; Ukimura, O.; Miki, T.; et al. Disruption of circadian clockwork in in vivo reprogramming-induced mouse kidney tumors. Genes Cells 2018, 23, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Mazzoccoli, G.; Piepoli, A.; Carella, M.; Panza, A.; Pazienza, V.; Benegiamo, G.; Palumbo, O.; Ranieri, E. Altered expression of the clock gene machinery in kidney cancer patients. Biomed. Pharmacother. 2012, 66, 175–179. [Google Scholar] [CrossRef]
- Santoni, M.; Pantano, F.; Amantini, C.; Nabissi, M.; Conti, A.; Burattini, L.; Zoccoli, A.; Berardi, R.; Santoni, G.; Tonini, G.; et al. Emerging strategies to overcome the resistance to current mTOR inhibitors in renal cell carcinoma. Biochim. Biophys. Acta 2014, 1845, 221–231. [Google Scholar] [CrossRef]
- Okazaki, H.; Matsunaga, N.; Fujioka, T.; Okazaki, F.; Akagawa, Y.; Tsurudome, Y.; Ono, M.; Kuwano, M.; Koyanagi, S.; Ohdo, S. Circadian regulation of mTOR by the ubiquitin pathway in renal cell carcinoma. Cancer Res. 2014, 74, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Jonasch, E.; Donskov, F.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.K.; Maughan, B.L.; Oudard, S.; Else, T.; Maranchie, J.K.; Welsh, S.J.; et al. Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. N. Engl. J. Med. 2021, 385, 2036–2046. [Google Scholar] [CrossRef]
- Okabe, T.; Kumagai, M.; Nakajima, Y.; Shirotake, S.; Kodaira, K.; Oyama, M.; Ueno, M.; Ikeda, M. The impact of HIF1α on the Per2 circadian rhythm in renal cancer cell lines. PLoS ONE 2014, 9, e109693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, M.J.; Liu, L.P.; Jin, S.; Fang, X.F.; He, X.X.; Xiong, Z.F.; Yang, S.L. Research on circadian clock genes in common abdominal malignant tumors. Chronobiol. Int. 2019, 36, 906–918. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, Y.; Wang, S.; Liu, H. Circadian Clock Genes Modulate Immune, Cell Cycle and Apoptosis in the Diagnosis and Prognosis of Pan-Renal Cell Carcinoma. Front. Mol. Biosci. 2021, 8, 747629. [Google Scholar] [CrossRef]
- Aggen, D.H.; Ager, C.R.; Obradovic, A.Z.; Chowdhury, N.; Ghasemzadeh, A.; Mao, W.; Chaimowitz, M.G.; Lopez-Bujanda, Z.A.; Spina, C.S.; Hawley, J.E.; et al. Blocking IL1 Beta Promotes Tumor Regression and Remodeling of the Myeloid Compartment in a Renal Cell Carcinoma Model: Multidimensional Analyses. Clin. Cancer Res. 2021, 27, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Koguchi, T.; Matsuoka, K.; Onagi, A.; Tanji, R.; Takinami-Honda, R.; Hoshi, S.; Onoda, M.; Kurimura, Y.; Hata, J.; et al. Interleukin-6 induces drug resistance in renal cell carcinoma. Fukushima J. Med. Sci. 2018, 64, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoni, M.; Bracarda, S.; Nabissi, M.; Massari, F.; Conti, A.; Bria, E.; Tortora, G.; Santoni, G.; Cascinu, S. CXC and CC chemokines as angiogenic modulators in nonhaematological tumors. Biomed Res. Int. 2014, 2014, 768758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoni, M.; Massari, F.; Amantini, C.; Nabissi, M.; Maines, F.; Burattini, L.; Berardi, R.; Santoni, G.; Montironi, R.; Tortora, G.; et al. Emerging role of tumor-associated macrophages as therapeutic targets in patients with metastatic renal cell carcinoma. Cancer Immunol. Immunother. 2013, 62, 1757–1768. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Bixler, E.O.; Lin, H.M.; Prolo, P.; Trakada, G.; Chrousos, G.P. IL-6 and its circadian secretion in humans. Neuroimmunomodulation 2005, 12, 131–140. [Google Scholar] [CrossRef]
- Ertosun, M.G.; Kocak, G.; Ozes, O.N. The regulation of circadian clock by tumor necrosis factor alpha. Cytokine Growth Factor Rev. 2019, 46, 10–16. [Google Scholar] [CrossRef]
- Onoue, T.; Nishi, G.; Hikima, J.I.; Sakai, M.; Kono, T. Circadian oscillation of TNF-α gene expression regulated by clock gene, BMAL1 and CLOCK1, in the Japanese medaka (Oryzias latipes). Int. Immunopharmacol. 2019, 70, 362–371. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Q.; Zhang, K.; Teng, H.; Li, M.; Li, D.; Wang, J.; Du, Q.; Zhao, M. The clock-controlled chemokine contributes to neuroinflammation-induced depression. FASEB J. 2020, 34, 8357–8366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, K.; Zhang, Q.H.; Li, H.C.; Saito, S.; Sato, Y. Timing of cancer chemotherapy based on circadian variations in tumor tissue blood flow. Int. J. Cancer 1996, 65, 360–364. [Google Scholar] [CrossRef]
- Lee, Y.; Lahens, N.F.; Zhang, S.; Bedont, J.; Field, J.M.; Sehgal, A. G1/S Cell Cycle Regulators Mediate Effects of Circadian Dysregulation on Tumor Growth and Provide Targets for Timed Anticancer Treatment. PLoS Biol. 2019, 17, e3000228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deprés-Brummer, P.; Levi, F.; Di Palma, M.; Beliard, A.; Lebon, P.; Marion, S.; Jasmin, C.; Misset, J.L. A phase I trial of 21-day continuous venous infusion of alpha-interferon at circadian rhythm modulated rate in cancer patients. J. Immunother. 1991, 10, 440–447. [Google Scholar] [CrossRef]
- Shiba, M.; Nonomura, N.; Nakai, Y.; Nakayama, M.; Takayama, H.; Inoue, H.; Tsujimura, A.; Nishimura, K.; Okuyama, A. Type-I interferon receptor expression: Its circadian rhythm and downregulation after interferon-alpha administration in peripheral blood cells from renal cancer patients. Int. J. Urol. 2009, 16, 356–359. [Google Scholar] [CrossRef]
- Iacobelli, S.; Garufi, C.; Irtelli, L.; Martino, M.T.; Santobuono, F.; Vicario, G.; Tinari, N.; Fiorentino, B.; Innocenti, P.; Natoli, C. A phase I study of recombinant interferon-alpha administered as a seven-day continuous venous infusion at circadian-rhythm modulated rate in patients with cancer. Am. J. Clin. Oncol. 1995, 18, 27–31. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing Toxicities Associated with Immune Checkpoint Inhibitors: Consensus Recommendations from the Society forImmunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Zhu, S.; Zeng, L.; Liu, J.; Kang, R.; Yang, M.; Cao, L.; Wang, H.; Billiar, T.R.; Jiang, J.; et al. The Circadian Clock Controls Immune Checkpoint Pathway in Sepsis. Cell Rep. 2018, 24, 366–378. [Google Scholar] [CrossRef] [Green Version]
- Qian, D.C.; Kleber, T.; Brammer, B.; Xu, K.M.; Switchenko, J.M.; Janopaul-Naylor, J.R.; Zhong, J.; Yushak, M.L.; Harvey, R.D.; Paulos, C.M.; et al. Effect of immunotherapy time-of-day infusion on overall survival among patients with advanced melanoma in the USA (MEMOIR): A propensity score-matched analysis of a single-centre, longitudinal study. Lancet Oncol. 2021, 22, 1777–1786. [Google Scholar] [CrossRef]
- Santoni, M.; Molina-Cerrillo, J.; Massari, F.; Montironi, R.; Grande, E. Re: Effect of Immunotherapy Time-of-day Infusion on Overall Survival among Patients with Advanced Melanoma in the USA (MEMOIR): A Propensity Score-matched Analysis of a Single-centre, Longitudinal Study. Eur. Urol. 2022, 81, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Molina-Cerrillo, J.; Ortego, I.; Pinto, A.; Alonso-Gordoa, T.; Massari, F.; Aurilio, G.; Buti, S.; Santoni, M.; Grande, E. Does timing of Immune checkpoint inhibitors (ICIs) administration in first line Metastatic Renal Cell Carcinoma (mRCC) have impact in survival outcomes? J. Clin. Oncol. 2022, 40, e16512. [Google Scholar] [CrossRef]
- Oshima, T.; Niwa, Y.; Kuwata, K.; Srivastava, A.; Hyoda, T.; Tsuchiya, Y.; Kumagai, M.; Tsuyuguchi, M.; Tamaru, T.; Sugiyama, A.; et al. Cell-based screen identifies a new potent and highly selective CK2 inhibitor for modulation of circadian rhythms and cancer cell growth. Sci. Adv. 2019, 5, eaau9060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borgo, C.; Cesaro, L.; Hirota, T.; Kuwata, K.; D’Amore, C.; Ruppert, T.; Blatnik, R.; Salvi, M.; Pinna, L.A. Comparing the efficacy and selectivity of Ck2 inhibitors. A phosphoproteomics approach. Eur. J. Med. Chem. 2021, 214, 113217. [Google Scholar] [CrossRef]
- Rahman, S.; Wittine, K.; Sedić, M.; Markova-Car, E.P. Small Molecules Targeting Biological Clock; A Novel Prospective for Anti-Cancer Drugs. Molecules 2020, 25, 4937. [Google Scholar] [CrossRef] [PubMed]
- Greathouse, K.L.; Wyatt, M.; Johnson, A.J.; Toy, E.P.; Khan, J.M.; Dunn, K.; Clegg, D.J.; Reddy, S. Diet-microbiome interactions in cancer treatment: Opportunities and challenges for precision nutrition in cancer. Neoplasia 2022, 29, 100800. [Google Scholar] [CrossRef]
- Westheim, A.J.F.; Stoffels, L.M.; Dubois, L.J.; van Bergenhenegouwen, J.; van Helvoort, A.; Langen, R.C.J.; Shiri-Sverdlov, R.; Theys, J. Fatty Acids as a Tool to Boost Cancer Immunotherapy Efficacy. Front. Nutr. 2022, 9, 868436. [Google Scholar] [CrossRef]
- Cortellino, S.; Raveane, A.; Chiodoni, C.; Delfanti, G.; Pisati, F.; Spagnolo, V.; Visco, E.; Fragale, G.; Ferrante, F.; Magni, S.; et al. Fasting renders immunotherapy effective against low-immunogenic breast cancer while reducing side effects. Cell Rep. 2022, 40, 111256. [Google Scholar] [CrossRef]
- Santoni, M.; Massari, F.; Matrana, M.R.; Basso, U.; De Giorgi, U.; Aurilio, G.; Buti, S.; Incorvaia, L.; Rizzo, M.; Martignetti, A.; et al. Statin use improves the efficacy of nivolumab in patients with advanced renal cell carcinoma. Eur. J. Cancer 2022, 172, 191–198. [Google Scholar] [CrossRef]
- Santoni, M.; Molina-Cerrillo, J.; Myint, Z.W.; Massari, F.; Buchler, T.; Buti, S.; Matrana, M.R.; De Giorgi, U.; Rizzo, M.; Zabalza, I.O.; et al. Concomitant Use of Statins, Metformin, or Proton Pump Inhibitors in Patients with Advanced Renal Cell Carcinoma Treated with First-Line Combination Therapies. Target. Oncol. 2022, 17, 571–581. [Google Scholar] [CrossRef]
- Bersanelli, M.; Giannarelli, D.; De Giorgi, U.; Pignata, S.; Di Maio, M.; Clemente, A.; Verzoni, E.; Giusti, R.; Di Napoli, M.; Aprile, G.; et al. INfluenza Vaccine Indication During therapy with Immune checkpoint inhibitors: A multicenter prospective observational study (INVIDIa-2). J. Immunother. Cancer 2021, 9, e002619. [Google Scholar] [CrossRef] [PubMed]
- Buti, S.; Bersanelli, M.; Perrone, F.; Bracarda, S.; Di Maio, M.; Giusti, R.; Nigro, O.; Cortinovis, D.L.; Aerts, J.G.J.V.; Guaitoli, G.; et al. Predictive ability of a drug-based score in patients with advanced non-small-cell lung cancer receiving first-line immunotherapy. Eur. J. Cancer 2021, 150, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Shaver, A.L.; Sharma, S.; Nikita, N.; Lefler, D.S.; Basu-Mallick, A.; Johnson, J.M.; Butryn, M.; Lu-Yao, G. The Effects of Physical Activity on Cancer Patients Undergoing Treatment with Immune Checkpoint Inhibitors: A Scoping Review. Cancers 2021, 13, 6364. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, N.; Ozturk, D.; Kavakli, I.H.; Okyar, A. Molecular Aspects of Circadian Pharmacology and Relevance for Cancer Chronotherapy. Int. J. Mol. Sci. 2017, 18, 2168. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoni, M.; Molina-Cerrillo, J.; Santoni, G.; Lam, E.T.; Massari, F.; Mollica, V.; Mazzaschi, G.; Rapoport, B.L.; Grande, E.; Buti, S. Role of Clock Genes and Circadian Rhythm in Renal Cell Carcinoma: Recent Evidence and Therapeutic Consequences. Cancers 2023, 15, 408. https://doi.org/10.3390/cancers15020408
Santoni M, Molina-Cerrillo J, Santoni G, Lam ET, Massari F, Mollica V, Mazzaschi G, Rapoport BL, Grande E, Buti S. Role of Clock Genes and Circadian Rhythm in Renal Cell Carcinoma: Recent Evidence and Therapeutic Consequences. Cancers. 2023; 15(2):408. https://doi.org/10.3390/cancers15020408
Chicago/Turabian StyleSantoni, Matteo, Javier Molina-Cerrillo, Giorgio Santoni, Elaine T. Lam, Francesco Massari, Veronica Mollica, Giulia Mazzaschi, Bernardo L. Rapoport, Enrique Grande, and Sebastiano Buti. 2023. "Role of Clock Genes and Circadian Rhythm in Renal Cell Carcinoma: Recent Evidence and Therapeutic Consequences" Cancers 15, no. 2: 408. https://doi.org/10.3390/cancers15020408








