Al[18F]F-NOTA-Octreotide Is Comparable to [68Ga]Ga-DOTA-TATE for PET/CT Imaging of Neuroendocrine Tumours in the Latin-American Population
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Radiochemistry
2.2. PET/CT Imaging
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
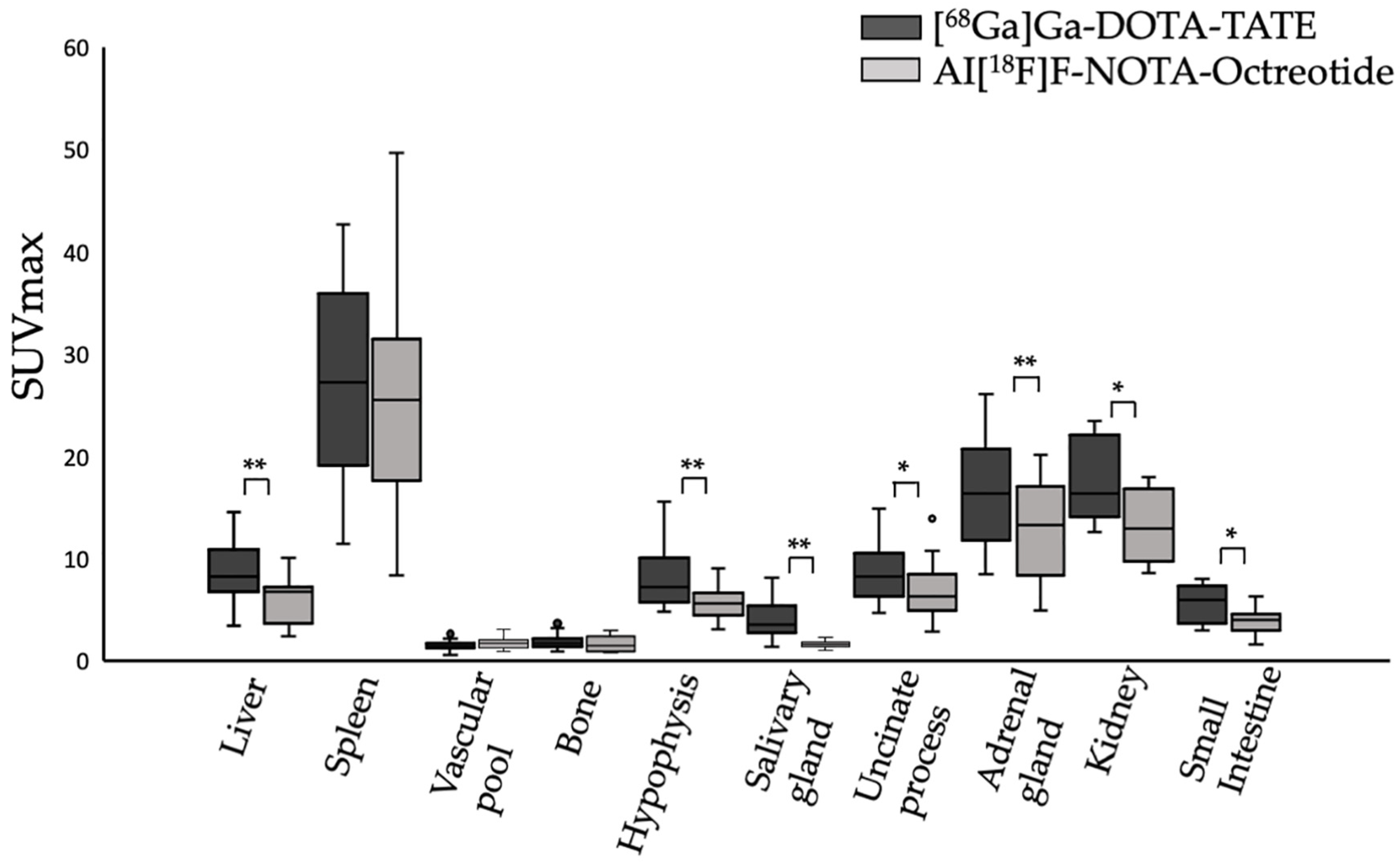
3.1. Biodistribution of [68Ga]Ga-DOTA-TATE Compared to Al[18F]F-NOTA-Octreotide
3.2. Tumoral Lesion Detection of [68Ga]Ga-DOTA-TATE Compared to Al[18F]F-NOTA-Octreotide
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barakat, M.T.; Meeran, K.; Bloom, S.R. Neuroendocrine tumours. Endocr. Relat. Cancer 2004, 11, 1–18. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.V.; Caplin, M.; Delle Fave, G.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Law, C.H.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef]
- Sackstein, P.E.; O’Neil, D.S.; Neugut, A.I.; Chabot, J.; Fojo, T. Epidemiologic trends in neuroendocrine tumors: An examination of incidence rates and survival of specific patient subgroups over the past 20 years. Semin. Oncol. 2018, 45, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Mittra, E.S. Neuroendocrine Tumor Therapy: (177)Lu-DOTATATE. AJR Am. J. Roentgenol. 2018, 211, 278–285. [Google Scholar] [CrossRef]
- Tomita, T. Significance of chromogranin A and synaptophysin in pancreatic neuroendocrine tumors. Bosn. J. Basic Med. Sci. 2020, 20, 336–346. [Google Scholar] [CrossRef]
- Hofland, L.J.; Lamberts, S.W.; van Hagen, P.M.; Reubi, J.C.; Schaeffer, J.; Waaijers, M.; van Koetsveld, P.M.; Srinivasan, A.; Krenning, E.P.; Breeman, W.A. Crucial role for somatostatin receptor subtype 2 in determining the uptake of [111In-DTPA-D-Phe1]octreotide in somatostatin receptor-positive organs. J. Nucl. Med. 2003, 44, 1315–1321. [Google Scholar]
- Banerjee, S.R.; Pomper, M.G. Clinical applications of Gallium-68. Appl. Radiat. Isot. 2013, 76, 2–13. [Google Scholar] [CrossRef]
- Poeppel, T.D.; Binse, I.; Petersenn, S.; Lahner, H.; Schott, M.; Antoch, G.; Brandau, W.; Bockisch, A.; Boy, C. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J. Nucl. Med. 2011, 52, 1864–1870. [Google Scholar] [CrossRef]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with (68)Ga-DOTA-conjugated somatostatin receptor targeting peptides and (18)F-DOPA. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601. [Google Scholar] [CrossRef]
- Hope, T.A.; Bergsland, E.K.; Bozkurt, M.F.; Graham, M.; Heaney, A.P.; Herrmann, K.; Howe, J.R.; Kulke, M.H.; Kunz, P.L.; Mailman, J.; et al. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J. Nucl. Med. 2018, 59, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Amaral, H.; Pruzzo, R.; Fernández, R.; Kramer, V.; Soza-Ried, C.; Coudeu, I. Chilean experience using “Theranostics” for treating metastatic neuroendocrine tumors with [177Lu]Lu DOTA-TATE. Arch. Clin. Gastroenterol. 2020, 6, 5. [Google Scholar]
- Hou, J.; Long, T.; He, Z.; Zhou, M.; Yang, N.; Chen, D.; Zeng, S.; Hu, S. Evaluation of (18)F-AlF-NOTA-octreotide for imaging neuroendocrine neoplasms: Comparison with (68)Ga-DOTATATE PET/CT. EJNMMI Res. 2021, 11, 55. [Google Scholar] [CrossRef]
- Long, T.; Yang, N.; Zhou, M.; Chen, D.; Li, Y.; Li, J.; Tang, Y.; Liu, Z.; Li, Z.; Hu, S. Clinical Application of 18F-AlF-NOTA-Octreotide PET/CT in Combination With 18F-FDG PET/CT for Imaging Neuroendocrine Neoplasms. Clin. Nucl. Med. 2019, 44, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, C.M.; Stuparu, A.D.; van Dam, R.M.; Slavik, R. The Search for an Alternative to [(68)Ga]Ga-DOTA-TATE in Neuroendocrine Tumor Theranostics: Current State of (18)F-labeled Somatostatin Analog Development. Theranostics 2019, 9, 1336–1347. [Google Scholar] [CrossRef] [PubMed]
- Laverman, P.; McBride, W.J.; Sharkey, R.M.; Eek, A.; Joosten, L.; Oyen, W.J.; Goldenberg, D.M.; Boerman, O.C. A novel facile method of labeling octreotide with (18)F-fluorine. J. Nucl. Med. 2010, 51, 454–461. [Google Scholar] [CrossRef]
- Laverman, P.; D’Souza, C.A.; Eek, A.; McBride, W.J.; Sharkey, R.M.; Oyen, W.J.; Goldenberg, D.M.; Boerman, O.C. Optimized labeling of NOTA-conjugated octreotide with F-18. Tumor Biol. 2012, 33, 427–434. [Google Scholar] [CrossRef]
- Leyton, J.; Iddon, L.; Perumal, M.; Indrevoll, B.; Glaser, M.; Robins, E.; George, A.J.; Cuthbertson, A.; Luthra, S.K.; Aboagye, E.O. Targeting somatostatin receptors: Preclinical evaluation of novel 18F-fluoroethyltriazole-Tyr3-octreotate analogs for PET. J. Nucl. Med. 2011, 52, 1441–1448. [Google Scholar] [CrossRef]
- Pauwels, E.; Cleeren, F.; Tshibangu, T.; Koole, M.; Serdons, K.; Dekervel, J.; Van Cutsem, E.; Verslype, C.; Van Laere, K.; Bormans, G.; et al. Al(18)F-NOTA-octreotide: First comparison with (68)Ga-DOTATATE in a neuroendocrine tumour patient. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2398–2399. [Google Scholar] [CrossRef]
- Pauwels, E.; Cleeren, F.; Tshibangu, T.; Koole, M.; Serdons, K.; Dekervel, J.; Van Cutsem, E.; Verslype, C.; Van Laere, K.; Bormans, G.; et al. [(18)F]AlF-NOTA-octreotide PET imaging: Biodistribution, dosimetry and first comparison with [(68)Ga]Ga-DOTATATE in neuroendocrine tumour patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.; Cleeren, F.; Tshibangu, T.; Koole, M.; Serdons, K.; Boeckxstaens, L.; Dekervel, J.; Vandamme, T.; Lybaert, W.; Van den Broeck, B.; et al. (18)F-AlF-NOTA-octreotide outperforms (68)Ga-DOTA-TATE/-NOC PET in neuroendocrine tumor patients: Results from a prospective, multicenter study. J. Nucl. Med. 2022, 64. [Google Scholar] [CrossRef]
- Tshibangu, T.; Cawthorne, C.; Serdons, K.; Pauwels, E.; Gsell, W.; Bormans, G.; Deroose, C.M.; Cleeren, F. Automated GMP compliant production of [(18)F]AlF-NOTA-octreotide. EJNMMI Radiopharm. Chem. 2020, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Mascha, E.J. Equivalence and noninferiority testing in anesthesiology research. Anesthesiology 2010, 113, 779–781. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.R-project.org/ (accessed on 10 August 2022).
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Phys. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Goss, P.E.; Lee, B.L.; Badovinac-Crnjevic, T.; Strasser-Weippl, K.; Chavarri-Guerra, Y.; St Louis, J.; Villarreal-Garza, C.; Unger-Saldana, K.; Ferreyra, M.; Debiasi, M.; et al. Planning cancer control in Latin America and the Caribbean. Lancet Oncol. 2013, 14, 391–436. [Google Scholar] [CrossRef]



| Patient ID | Age (y) | Gender | Primary Tumour | Tumour Grade | Ki-67 Index | [68Ga]Ga-DOTA-TATE Activity (MBq) | Al[18F]F-NOTA-Octreotide Activity (MBq) | Delay (Days) |
|---|---|---|---|---|---|---|---|---|
| 1 | 73 | M | Small Intestine | G2 | 6% | 156.1 | 262.3 | 7 |
| 2 | 45 | F | Bronchial | G1 | 1% | 152.1 | 185.0 | 7 |
| 3 | 49 | M | Pancreas | G2 | 8% | 126.2 | 272.7 | 2 |
| 4 | 44 | M | Pancreas | G2 | 5% | 149.5 | 282.7 | 2 |
| 5 | 73 | M | Apendice cecal | G1 | 1% | 141.3 | 301.9 | 23 |
| 6 | 69 | M | Small Intestine | G2 | 5% | 165.8 | 265.3 | 23 |
| 7 | 60 | M | Small Intestine | G1 | 1% | 159.8 | 276.0 | 30 |
| 8 | 58 | M | Small Intestine | G1 | 1% | 95.8 | 284.9 | 23 |
| 9 | 40 | M | Small Intestine | G2 | 12% | 179.8 | 214.6 | 6 |
| 10 | 42 | M | Liver | G3 | >20% | 143.2 | 236.8 | 22 |
| 11 | 57 | F | Unknown | G3 | 30% | 146.2 | 251.6 | 8 |
| 12 | 57 | F | Colon | G2 | 15% | 172.4 | 254.2 | 7 |
| 13 | 60 | F | Small Intestine | G1 | 2% | 172.1 | 232.3 | 7 |
| 14 | 54 | F | Small Intestine | G2 | NA | 97.7 | 256.8 | 13 |
| 15 | 63 | F | Small Intestine | G2 | 5% | 165.8 | 163.9 | 8 |
| 16 | 60 | M | Unknown | G2 | NA | 131.0 | 237.1 | 8 |
| 17 | 52 | M | Small Intestine | G1 | 1% | 175.0 | 254.9 | 13 |
| 18 | 55 | M | Small Intestine | G2 | 5% | 145.0 | 245.6 | 15 |
| 19 | 83 | F | Small Intestine | G2 | 3% | 152.8 | 252.0 | 16 |
| 20 | 52 | M | Small Intestine | G2 | 3% | 153.5 | 205.7 | 15 |
| Organ | [68Ga]Ga-DOTA-TATE SUVmax | Al[18F]F-NOTA-Octreotide SUVmax | p-Value |
|---|---|---|---|
| Liver | 8.76 ± 2.83 | 6.11 ± 2.23 | <0.001 |
| Spleen | 27.18 ± 9.91 | 25 ± 10.91 | 0.247 |
| Vascular pool | 1.543 ± 0.62 | 1.71 ± 0.54 | 0.07 |
| Bone | 1.86 ± 0.68 | 1.66 ± 0.74 | 0.262 |
| Hypophysis | 8.11 ± 3.05 | 5.75 ± 1.68 | <0.001 |
| Salivary gland | 4.1 ± 1.83 | 1.61 ± 0.29 | <0.001 |
| Uncinate process | 8.58 ± 2.91 | 6.82 ± 2.73 | <0.05 |
| Adrenal gland | 16.42 ± 4.8 | 12.75 ± 4.65 | <0.001 |
| Kidney | 17.64 ± 4 | 13.4 ± 3.63 | <0.05 |
| Small intestine | 5.73 ± 1.88 | 3.9 ± 1.33 | <0.05 |
| Patients | Primary Tumour | Liver Metastases ** | Bone Metastases | LN Metastases | Other Sites Metastases | Total Lesions | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tracer * | GAD | FOC | GAD | FOC | GAD | FOC | GAD | FOC | GAD | FOC | GAD | FOC |
| 1 | 0 | 0 | 1 | 1 | 15 | 15 | 10 | 10 | 0 | 0 | 26 | 26 |
| 2 | 1 | 1 | 10 | 9 | 6 | 6 | 1 | 1 | 0 | 0 | 18 | 17 |
| 3 | 1 | 1 | 10 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 11 |
| 4 | 1 | 1 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 6 |
| 5 | 0 | 0 | 27 | 27 | 3 | 3 | 25 | 25 | 1 | 1 | ≥50 | ≥50 |
| 6 | 1 | 1 | 0 | 0 | 0 | 0 | 11 | 11 | 1 | 1 | 13 | 13 |
| 7 | 0 | 0 | ≥50 | ≥50 | 0 | 0 | 3 | 3 | 0 | 0 | ≥50 | ≥50 |
| 8 | 0 | 0 | 2 | 2 | 0 | 0 | 4 | 4 | 1 | 1 | 7 | 7 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4 | 0 | 0 | 4 | 4 |
| 10 | 0 | 0 | 2 | 3 | 16 | 16 | 2 | 2 | 3 | 3 | 23 | 24 |
| 11 | 0 | 0 | ≥50 | ≥50 | ≥50 | ≥50 | 0 | 0 | 0 | 0 | ≥50 | ≥50 |
| 12 | 0 | 0 | 8 | 8 | 0 | 0 | 0 | 0 | 3 | 3 | 11 | 11 |
| 13 | 0 | 0 | >50 | >50 | ≥50 | ≥50 | 13 | 13 | 2 | 2 | ≥50 | ≥50 |
| 14 | 0 | 0 | 20 | 19 | 0 | 0 | 1 | 1 | 3 | 3 | 24 | 23 |
| 15 | 0 | 0 | 16 | 15 | 0 | 0 | 1 | 1 | 1 | 1 | 18 | 17 |
| 16 | 0 | 0 | 0 | 0 | 11 | 11 | 4 | 4 | 2 | 2 | 17 | 17 |
| 17 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 3 |
| 18 | 0 | 0 | ≥50 | ≥50 | 0 | 0 | 31 | 31 | ≥50 | ≥50 | ≥50 | ≥50 |
| 19 | 0 | 0 | 13 | 11 | 1 | 1 | 1 | 1 | ≥50 | ≥50 | ≥50 | ≥50 |
| 20 | 0 | 0 | ≥50 | ≥50 | 0 | 0 | 0 | 0 | 0 | 0 | ≥50 | ≥50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haeger, A.; Soza-Ried, C.; Kramer, V.; Hurtado de Mendoza, A.; Eppard, E.; Emmanuel, N.; Wettlin, J.; Amaral, H.; Fernández, R. Al[18F]F-NOTA-Octreotide Is Comparable to [68Ga]Ga-DOTA-TATE for PET/CT Imaging of Neuroendocrine Tumours in the Latin-American Population. Cancers 2023, 15, 439. https://doi.org/10.3390/cancers15020439
Haeger A, Soza-Ried C, Kramer V, Hurtado de Mendoza A, Eppard E, Emmanuel N, Wettlin J, Amaral H, Fernández R. Al[18F]F-NOTA-Octreotide Is Comparable to [68Ga]Ga-DOTA-TATE for PET/CT Imaging of Neuroendocrine Tumours in the Latin-American Population. Cancers. 2023; 15(2):439. https://doi.org/10.3390/cancers15020439
Chicago/Turabian StyleHaeger, Arlette, Cristian Soza-Ried, Vasko Kramer, Ana Hurtado de Mendoza, Elisabeth Eppard, Noémie Emmanuel, Johanna Wettlin, Horacio Amaral, and René Fernández. 2023. "Al[18F]F-NOTA-Octreotide Is Comparable to [68Ga]Ga-DOTA-TATE for PET/CT Imaging of Neuroendocrine Tumours in the Latin-American Population" Cancers 15, no. 2: 439. https://doi.org/10.3390/cancers15020439
APA StyleHaeger, A., Soza-Ried, C., Kramer, V., Hurtado de Mendoza, A., Eppard, E., Emmanuel, N., Wettlin, J., Amaral, H., & Fernández, R. (2023). Al[18F]F-NOTA-Octreotide Is Comparable to [68Ga]Ga-DOTA-TATE for PET/CT Imaging of Neuroendocrine Tumours in the Latin-American Population. Cancers, 15(2), 439. https://doi.org/10.3390/cancers15020439






