Gut Microbiota and Breast Cancer: The Dual Role of Microbes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Beneficial Effects Exerted by the Gut Microbiota
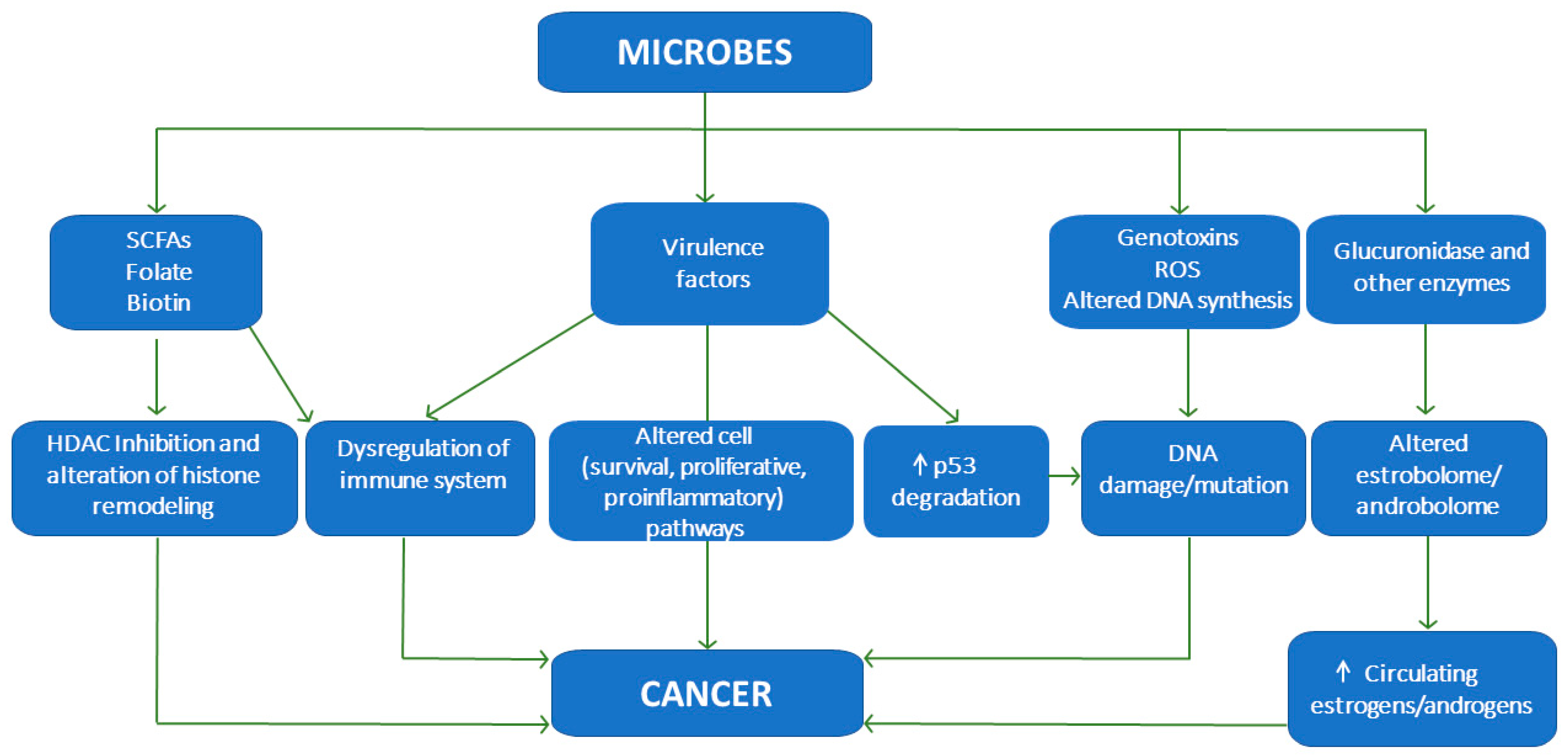
3. Detrimental Effects Exerted by the Gut Microbiota and Their Relationship with Cancer
3.1. Degradation of p53
3.2. Genomic Instability and DNA Damage
3.3. Metabolism of Endogenous and Exogenous Compounds
3.4. Alteration of Cell Proliferation and Survival Pathways (β-Catenin, MAPK and AKT)
3.5. Activation of Proinflammatory Pathways
3.6. Dysregulation of the Immune System
3.7. Epigenetic Mechanisms
4. Effects of Microbiota on Clinical Outcomes and Chemotherapy Resistance
4.1. Importance of Gut Microbiota in Cancer Therapies
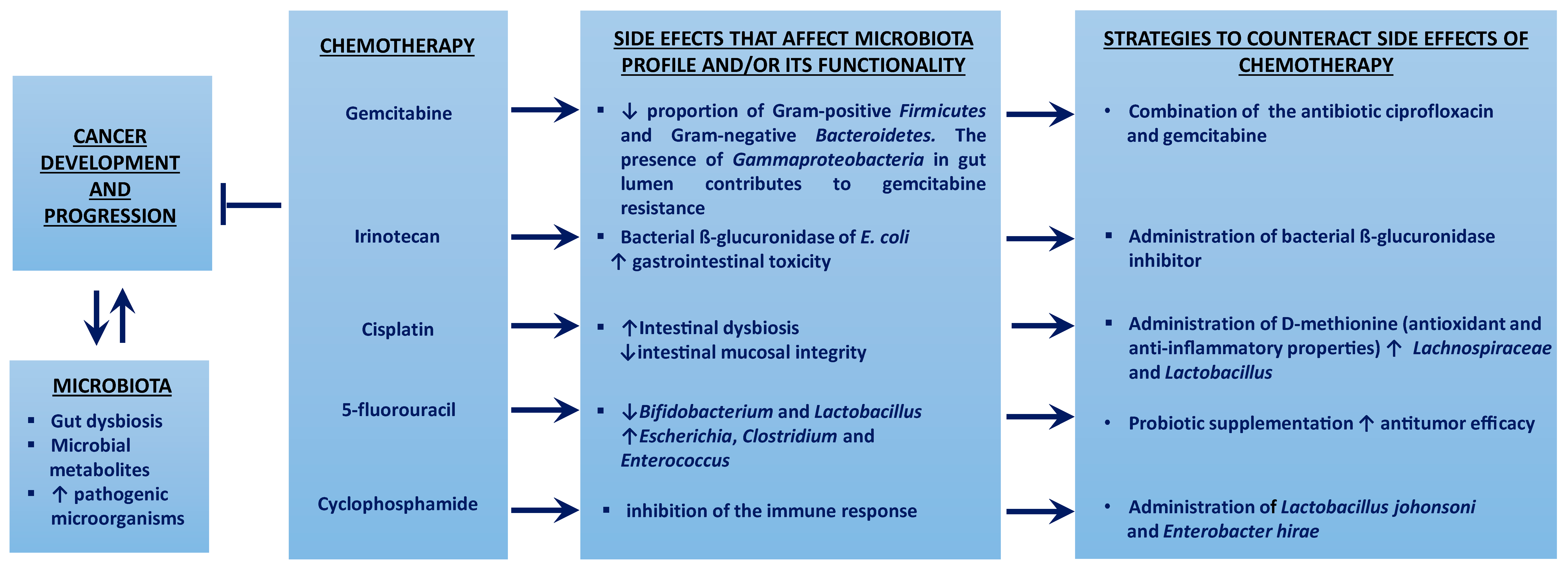
4.2. Intestinal Microbiota and Chemotherapy
4.2.1. Gemcitabine
4.2.2. Cyclophosphamide
4.2.3. Irinotecan
4.2.4. Cisplatin
4.2.5. 5-fluorouracil
4.3. Gut Microbiota and Immunotherapy
4.3.1. Anti-CTL-4
4.3.2. Anti-PD-L1
4.3.3. Anti-PD1
5. Clinical Studies Dealing with Gut Microbiota and Breast Cancer
5.1. Completed Clinical Trials
5.2. Ongoing Clinical Trials
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Álvarez-Mercado, A.; Navarro-Oliveros, M.; Robles-Sánchez, C.; Plaza-Díaz, J.; Sáez-Lara, M.; Muñoz-Quezada, S.; Fontana, L.; Abadía-Molina, F. Microbial Population Changes and Their Relationship with Human Health and Disease. Microorganisms 2019, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut microbiota and cancer: From pathogenesis to therapy. Cancers 2019, 11, 38. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.B.F. International Agency for Research on Cancer 2020. Glob. Cancer Obs. Cancer Today. 2020, 419, 1–2. [Google Scholar]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.J.; Alexander, J.L.; Merrifield, C.A.; Cunningham, D.; Jobin, C.; Brown, R.; Alverdy, J.; O’Keefe, S.J.; Gaskins, H.R.; Teare, J.; et al. International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut 2019, 68, 1624–1632. [Google Scholar] [CrossRef] [Green Version]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [Green Version]
- Paulos, C.M.; Wrzesinski, C.; Kaiser, A.; Hinrichs, C.S.; Chieppa, M.; Cassard, L.; Palmer, D.C.; Boni, A.; Muranski, P.; Yu, Z.; et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Investig. 2007, 117, 2197–2204. [Google Scholar] [CrossRef] [Green Version]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Buti, L.; Spooner, E.; Van Der Veen, A.G.; Rappuoli, R.; Covacci, A.; Ploegh, H.L. Helicobacter pylori cytotoxin-associated gene A (CagA) subverts the apoptosis-stimulating protein of p53 (ASPP2) tumor suppressor pathway of the host. Proc. Natl. Acad. Sci. USA 2011, 108, 9238–9243. [Google Scholar] [CrossRef]
- Bergounioux, J.; Elisee, R.; Prunier, A.L.; Donnadieu, F.; Sperandio, B.; Sansonetti, P.; Arbibe, L. Calpain activation by the Shigella flexneri effector VirA regulates key steps in the formation and life of the bacterium’s epithelial niche. Cell Host Microbe 2012, 11, 240–252. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reida, G. The microbiota of breast tissue and its association with breast cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. [Google Scholar] [CrossRef] [Green Version]
- Gori, S.; Inno, A.; Belluomini, L.; Bocus, P.; Bisoffi, Z.; Russo, A.; Arcaro, G. Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit. Rev. Oncol./Hematol. 2019, 143, 139–147. [Google Scholar] [CrossRef]
- Goodwin, A.C.; Destefano Shields, C.E.; Wu, S.; Huso, D.L.; Wu, X.Q.; Murray-Stewart, T.R.; Hacker-Prietz, A.; Rabizadeh, S.; Woster, P.M.; Sears, C.L.; et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15354–15359. [Google Scholar] [CrossRef] [Green Version]
- Peterson, C.T.; Rodionov, D.A.; Peterson, S.N.; Osterman, A.L. B vitamins and their role in immune regulation and cancer. Nutrients 2020, 12, 3380. [Google Scholar] [CrossRef]
- Komorowski, A.S.; Pezo, R.C. Untapped “-omics”: The microbial metagenome, estrobolome, and their influence on the development of breast cancer and response to treatment. Breast Cancer Res. Treat. 2020, 179, 287–300. [Google Scholar] [CrossRef]
- Kunc, M.; Gabrych, A.; Witkowski, J.M. Microbiome impact on metabolism and function of sex, thyroid, growth and parathyroid hormones. Acta Biochim. Pol. 2016, 63, 189–201. [Google Scholar] [CrossRef]
- Rea, D.; Coppola, G.; Palma, G.; Barbieri, A.; Luciano, A.; Del Prete, P.; Rossetti, S.; Berretta, M.; Facchini, G.; Perdonà, S.; et al. Microbiota effects on cancer: From risks to therapies. Oncotarget 2018, 9, 17915–17927. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Tan, Q.; Fu, Q.; Zhou, Y.; Hu, Y.; Tang, S.; Zhou, Y.; Zhang, J.; Qiu, J.; Lv, Q. Gastrointestinal microbiome and breast cancer: Correlations, mechanisms and potential clinical implications. Breast Cancer 2017, 24, 220–228. [Google Scholar] [CrossRef]
- Dabek, M.; McCrae, S.I.; Stevens, V.J.; Duncan, S.H.; Louis, P. Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 2008, 66, 487–495. [Google Scholar] [CrossRef] [Green Version]
- DeLuca, J.A.A.; Allred, K.F.; Menon, R.; Riordan, R.; Weeks, B.R.; Jayaraman, A.; Allred, C.D. Bisphenol-A alters microbiota metabolites derived from aromatic amino acids and worsens disease activity during colitis. Exp. Biol. Med. 2018, 243, 864–875. [Google Scholar] [CrossRef]
- Murata-Kamiya, N.; Kurashima, Y.; Teishikata, Y.; Yamahashi, Y.; Saito, Y.; Higashi, H.; Aburatani, H.; Akiyama, T.; Peek, R.M.; Azuma, T.; et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the β-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene 2007, 26, 4617–4626. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by codulating E-Cadherin/β-Catenin cignaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Rhee, K.J.; Zhang, M.; Franco, A.; Sears, C.L. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and γ-secretase-dependent E-cadherin cleavage. J. Cell Sci. 2007, 120, 1944–1952. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Wu, S.; Zhang, Y.G.; Xia, Y.; Liu, X.; Zheng, Y.; Chen, H.; Schaefer, K.L.; Zhou, Z.; Bissonnette, M.; et al. Enteric bacterial protein AvrA promotes colonic tumorigenesis and activates colonic beta-catenin signaling pathway. Oncogenesis 2014, 3, e105. [Google Scholar] [CrossRef] [Green Version]
- Bronte-Tinkew, D.M.; Terebiznik, M.; Franco, A.; Ang, M.; Ahn, D.; Mimuro, H.; Sasakawa, C.; Ropeleski, M.J.; Peek, R.M.; Jones, N.L. Helicobacter pylori cytotoxin-associated gene a activates the signal transducer and activator of transcription 3 pathway in vitro and in vivo. Cancer Res. 2009, 69, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Kuijl, C.; Savage, N.D.L.; Marsman, M.; Tuin, A.W.; Janssen, L.; Egan, D.A.; Ketema, M.; Van Den Nieuwendijk, R.; Van Den Eeden, S.J.F.; Geluk, A.; et al. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature 2007, 450, 725–730. [Google Scholar] [CrossRef]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Microbiome, inflammation, and cancer. Cancer J. 2014, 20, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Pagano, J.S.; Blaser, M.; Buendia, M.A.; Damania, B.; Khalili, K.; Raab-Traub, N.; Roizman, B. Infectious agents and cancer: Criteria for a causal relation. Semin. Cancer Biol. 2004, 14, 453–471. [Google Scholar] [CrossRef]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef]
- Dapito, D.H.; Mencin, A.; Gwak, G.Y.; Pradere, J.P.; Jang, M.K.; Mederacke, I.; Caviglia, J.M.; Khiabanian, H.; Adeyemi, A.; Bataller, R.; et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef] [Green Version]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef] [Green Version]
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin. Epigenetics 2012, 4, 4. [Google Scholar] [CrossRef]
- Paul, B.; Barnes, S.; Demark-Wahnefried, W.; Morrow, C.; Salvador, C.; Skibola, C.; Tollefsbol, T.O. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin. Epigenetics 2015, 7, 112. [Google Scholar] [CrossRef] [Green Version]
- Dashwood, R.H.; Ho, E. Dietary histone deacetylase inhibitors: From cells to mice to man. Semin. Cancer Biol. 2007, 17, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; Deroos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Fu, L.; Li, Y.; Wang, W.; Gong, M.; Zhang, J.; Dong, X.; Huang, J.; Wang, Q.; Mackay, C.R.; et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 2021, 33, 988–1000.e7. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Picardo, S.L.; Coburn, B.; Hansen, A.R. The microbiome and cancer for clinicians. Crit. Rev. Oncol./Hematol. 2019, 141, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [Green Version]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef] [Green Version]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.A.; Mani, S.; et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef] [Green Version]
- Joyce, K.; Saxena, S.; Williams, A.; Damurjian, C.; Auricchio, N.; Aluotto, S.; Tynan, H.; Demain, A.L. Antimicrobial spectrum of the antitumor agent, cisplatin. J. Antibiot. 2010, 63, 530–532. [Google Scholar] [CrossRef]
- Gui, Q.F.; Lu, H.F.; Zhang, C.X.; Xu, Z.R.; Yang, Y.H. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet. Mol. Res. 2015, 14, 5642–5651. [Google Scholar] [CrossRef]
- Perales-Puchalt, A.; Perez-Sanz, J.; Payne, K.K.; Svoronos, N.; Allegrezza, M.J.; Chaurio, R.A.; Anadon, C.; Calmette, J.; Biswas, S.; Mine, J.A.; et al. Frontline Science: Microbiota reconstitution restores intestinal integrity after cisplatin therapy. J. Leukoc. Biol. 2018, 103, 799–805. [Google Scholar] [CrossRef]
- Wu, C.H.; Ko, J.L.; Liao, J.M.; Huang, S.S.; Lin, M.Y.; Lee, L.H.; Chang, L.Y.; Ou, C.C. D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation. Ther. Adv. Med. Oncol. 2019, 11, 1758835918821021. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, S.; Li, H.; Yang, F.; Mushtaq, N.; Ullah, S.; Shi, Y.; An, C.; Xu, J. The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomed. Pharmacother. 2018, 108, 184–193. [Google Scholar] [CrossRef]
- Ferrara, R.; Caramella, C.; Besse, B. Hyperprogression-immunotherapy-related phenomenon vs Intrinsic Natural History of Cancer-In Reply. JAMA Oncol. 2019, 5, 743–744. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2019, 30, 2012. [Google Scholar] [CrossRef] [Green Version]
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia 2017, 19, 848–855. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Laborda-Illanes, A.; Sanchez-Alcoholado, L.; Dominguez-Recio, M.E.; Jimenez-Rodriguez, B.; Lavado, R.; Comino-Méndez, I.; Alba, E.; Queipo-Ortuño, M.I. Breast and gut microbiota action mechanisms in breast cancer pathogenesis and treatment. Cancers 2020, 12, 2465. [Google Scholar] [CrossRef]
- Darbre, P.D.; Fernandez, M.F. Environmental oestrogens and breast cancer: Long-term low-dose effects of mixtures of various chemical combinations. J. Epidemiol. Community Health 2013, 67, 203–205. [Google Scholar] [CrossRef]
- Fontana, L. Microbiota and cancer therapy. Ann. Nutr. Metab. 2020, 76 (Suppl. 4), 1–232. [Google Scholar]
- Fernández, M. Endocrine Disruptors and Gut Microbiome Interactions. Ann. Nutr. Metab. 2020, 69, 1–32. [Google Scholar] [CrossRef]
- Fernández, M.F.; Reina-Pérez, I.; Astorga, J.M.; Rodríguez-Carrillo, A.; Plaza-Díaz, J.; Fontana, L. Breast cancer and its relationship with the microbiota. Int. J. Environ. Res. Public Health 2018, 15, 1747. [Google Scholar] [CrossRef]
- Scarpellini, E.; Ianiro, G.; Attili, F.; Bassanelli, C.; De Santis, A.; Gasbarrini, A. The human gut microbiota and virome: Potential therapeutic implications. Dig. Liver Dis. 2015, 47, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Frugé, A.D.; Van der Pol, W.; Rogers, L.Q.; Morrow, C.D.; Tsuruta, Y.; Demark-Wahnefried, W. Fecal Akkermansia muciniphila is associated with body composition and microbiota diversity in overweight and obese women with breast cancer participating in a presurgical weight loss trial. J. Acad. Nutr. Diet. 2020, 120, 650–659. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.J.; Kim, H.N.; Bang, J.I.; Lim, W.; Moon, B.I.; Paik, N.S.; Kim, B.S.; Kim, H.L. Physiologic intestinal 18F-FDG uptake is associated with alteration of gut microbiota and proinflammatory cytokine levels in breast cancer. Sci. Rep. 2019, 9, 18273. [Google Scholar] [CrossRef] [Green Version]
- Klann, E.; Williamson, J.M.; Tagliamonte, M.S.; Ukhanova, M.; Asirvatham, J.R.; Chim, H.; Yaghjyan, L.; Mai, V. Microbiota composition in bilateral healthy breast tissue and breast tumors. Cancer Causes Control 2020, 31, 1027–1038. [Google Scholar] [CrossRef]
- Tzeng, A.; Sangwan, N.; Jia, M.; Liu, C.C.; Keslar, K.S.; Downs-Kelly, E.; Fairchild, R.L.; Al-Hilli, Z.; Grobmyer, S.R.; Eng, C. Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 2021, 13, 60. [Google Scholar] [CrossRef]
- Wang, H.; Altemus, J.; Niazi, F.; Green, H.; Calhoun, B.C.; Sturgis, C.; Grobmyer, S.R.; Eng, C. Breast tissue, oral and urinary microbiomes in breast cancer. Oncotarget 2017, 8, 88122–88138. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.J.; Ingle, J.N.; Tang, X.; Chia, N.; Jeraldo, P.R.; Walther-Antonio, M.R.; Kandimalla, K.K.; Johnson, S.; Yao, J.Z.; Harrington, S.C.; et al. A comprehensive analysis of breast cancer microbiota and host gene expression. PLoS ONE 2017, 12, e0188873. [Google Scholar] [CrossRef]
- Thyagarajan, S.; Zhang, Y.; Thapa, S.; Allen, M.S.; Phillips, N.; Chaudhary, P.; Kashyap, M.V.; Vishwanatha, J.K. Comparative analysis of racial differences in breast tumor microbiome. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Goedert, J.J.; Hua, X.; Bielecka, A.; Okayasu, I.; Milne, G.L.; Jones, G.S.; Fujiwara, M.; Sinha, R.; Wan, Y.; Xu, X.; et al. Postmenopausal breast cancer and oestrogen associations with the IgA-coated and IgA-noncoated faecal microbiota. Br. J. Cancer 2018, 118, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.; Pierre, J.F.; Makowski, L.; Tolley, E.; Lyn-Cook, B.; Lu, L.; Vidal, G.; Starlard-Davenport, A. Distinct microbial communities that differ by race, stage, or breast-tumor subtype in breast tissues of non-Hispanic Black and non-Hispanic White women. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Hieken, T.J.; Chen, J.; Hoskin, T.L.; Walther-Antonio, M.; Johnson, S.; Ramaker, S.; Xiao, J.; Radisky, D.C.; Knutson, K.L.; Kalari, K.R.; et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci. Rep. 2016, 6, 30751. [Google Scholar] [CrossRef]
- Zhu, J.; Liao, M.; Yao, Z.; Liang, W.; Li, Q.; Liu, J.; Yang, H.; Ji, Y.; Wei, W.; Tan, A.; et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome 2018, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Chen, B.; Yang, J.; Wang, J.; Zhu, D.; Meng, Q.; Zhang, L. Study of microbiomes in aseptically collected samples of human breast tissue using needle biopsy and the potential role of in situ tissue microbiomes for promoting malignancy. Front. Oncol. 2018, 8, 318. [Google Scholar] [CrossRef] [Green Version]
- Costantini, L.; Magno, S.; Albanese, D.; Donati, C.; Molinari, R.; Filippone, A.; Masetti, R.; Merendino, N. Characterization of human breast tissue microbiota from core needle biopsies through the analysis of multi hypervariable 16S-rRNA gene regions. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chiba, A.; Bawaneh, A.; Velazquez, C.; Clear, K.Y.J.; Wilson, A.S.; Howard-McNatt, M.; Levine, E.A.; Levi-Polyachenko, N.; Yates-Alston, S.A.; Diggle, S.P.; et al. Neoadjuvant chemotherapy shifts breast tumor microbiota populations to regulate drug responsiveness and the development of metastasis. Mol. Cancer Res. 2020, 18, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Horigome, A.; Okubo, R.; Hamazaki, K.; Kinoshita, T.; Katsumata, N.; Uezono, Y.; Xiao, J.Z.; Matsuoka, Y.J. Association between blood omega-3 polyunsaturated fatty acids and the gut microbiota among breast cancer survivors. Benef. Microbes 2019, 10, 751–758. [Google Scholar] [CrossRef]
- Wu, A.H.; Tseng, C.; Vigen, C.; Yu, Y.; Cozen, W.; Garcia, A.A.; Spicer, D. Gut microbiome associations with breast cancer risk factors and tumor characteristics: A pilot study. Breast Cancer Res. Treat. 2020, 182, 451–463. [Google Scholar] [CrossRef]
- Jones, G.S.; Feigelson, H.S.; Falk, R.T.; Hua, X.; Ravel, J.; Yu, G.; Flores, R.; Gail, M.H.; Shi, J.; Xu, X.; et al. Mammographic breast density and its association with urinary estrogens and the fecal microbiota in postmenopausal women. PLoS ONE 2019, 14, e0216114. [Google Scholar] [CrossRef]
- Ma, J.; Sun, L.; Liu, Y.; Ren, H.; Shen, Y.; Bi, F.; Zhang, T.; Wang, X. Alter between gut bacteria and blood metabolites and the anti-tumor effects of Faecalibacterium prausnitzii in breast cancer. BMC Microbiol. 2020, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.H.; Michel, C.; Bard, J.M.; Dravet, F.; Nazih, H.; Bobin-Dubigeon, C. Intestinal proportion of Blautia sp. is associated with clinical stage and histoprognostic grade in patients with early-stage breast cancer. Nutr. Cancer 2017, 69, 267–275. [Google Scholar] [CrossRef]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, M.D.; Peck, K.N.; DeMichele, A.M.; Alwine, J.C.; Robertson, E.S. Distinct microbial signatures associated with different breast cancer types. Front. Microbiol. 2018, 9, 951. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Gou, Y.K.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [Green Version]
- Sampson, J.N.; Falk, R.T.; Schairer, C.; Moore, S.C.; Fuhrman, B.J.; Dallal, C.M.; Bauer, D.C.; Dorgan, J.F.; Shu, X.O.; Zheng, W.; et al. Association of estrogen metabolism with breast cancer risk in different cohorts of postmenopausal women. Cancer Res. 2017, 77, 918–925. [Google Scholar] [CrossRef] [Green Version]
- Gierach, G.L.; Burke, A.; Anderson, W.F. Epidemiology of triple negative breast cancers. Breast Dis. 2010, 32, 5–24. [Google Scholar] [CrossRef] [Green Version]
- NCT01461070. Available online: https://clinicaltrials.gov/show/NCT01461070 (accessed on 20 December 2022).
- Ciernikova, S.; Mego, M.; Chovanec, M. Exploring the potential role of the gut microbiome in chemotherapy-induced neurocognitive disorders and cardiovascular toxicity. Cancers 2021, 13, 782. [Google Scholar] [CrossRef]
- NCT03290651. Available online: https://clinicaltrials.gov/show/NCT03290651 (accessed on 20 December 2022).
- NCT04138979. Available online: https://clinicaltrials.gov/show/NCT04138979 (accessed on 20 December 2022).
- NCT02370277. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02370277 (accessed on 20 December 2022).
- NCT03586297. Available online: https://clinicaltrials.gov/show/NCT03586297 (accessed on 20 December 2022).
- Plaza-DÍaz, J.; Álvarez-Mercado, A.I.; Ruiz-Marín, C.M.; Reina-Pérez, I.; Pérez-Alonso, A.J.; Sánchez-Andujar, M.B.; Torné, P.; Gallart-Aragón, T.; Sánchez-Barrón, M.T.; Reyes Lartategui, S.; et al. Association of breast and gut microbiota dysbiosis and the risk of breast cancer: A case-control clinical study. BMC Cancer 2019, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- NCT04303325. Available online: https://clinicaltrials.gov/show/NCT04303325 (accessed on 20 December 2022).
- NCT04784182. Available online: https://clinicaltrials.gov/show/NCT04784182 (accessed on 20 December 2022).
- NCT04139993. Available online: https://clinicaltrials.gov/show/NCT04139993 (accessed on 20 December 2022).
- NTC05113485. Available online: https://clinicaltrials.gov/ct2/show/NCT05113485 (accessed on 20 December 2022).
- NCT02266082. Available online: https://clinicaltrials.gov/show/NCT02266082 (accessed on 20 December 2022).
- NCT04088708. Available online: https://clinicaltrials.gov/show/NCT04088708 (accessed on 20 December 2022).
- Solis-Urra, P.; Plaza-Diaz, J.; Álvarez-Mercado, A.I.; Fernando, R.-R.; Cristi-Montero, C.; Zavala-Crichton, J.P.; Olivares-Arancibia, J.; Sanchez-Martinez, J.; Abadía-Molina, F. The mediation effect of self–report physical activity patterns in the relationship between educational level and cognitive impairment in elderly: A cross-sectional analysis of chilean health national survey 2016–2017. Int. J. Environ. Res. Public Health 2020, 17, 2619. [Google Scholar] [CrossRef] [Green Version]
- Schuch, F.B.; Vancampfort, D. Physical activity, exercise and mental disorders: It is time to move on. Trends Psychiatry Psychother. 2021, 43, 177–184. [Google Scholar] [CrossRef]
| Study | Sampling Materials and Site | Microbiota Detection and OTU Picking Method | Sample Size | Main Findings |
|---|---|---|---|---|
| Nejman et al., 2020 [10] | Breast tumor samples from cancer patients. Breast samples from healthy subjects. | 16S rRNA sequencing that amplifies five short regions along the 16S rRNA gene: the 5R 16S rRNA sequencing method. Greengenes database and Ribosomal Database Project classifier. | 256 normal breast samples from healthy subjects. 355 breast cancer samples. | ↑ Bacterial load and richness in breast tumor samples than those found in normal breast samples from healthy subjects. The microbiome of breast cancer is richer and more diverse than that of other tumor types. |
| Goedert et al., 2018 [71] | Fecal and urine samples. | 16S rRNA gene amplicon sequencing: SILVA was used to assign sequences to OTU and HPLC /MS used to assign 16S rRNA gene sequences to OTUs. | 48 postmenopausal breast cancer women (75% stage 0–1, 88% estrogen-receptor positive). 48 contemporaneous women, postmenopausal, normal-mammogram. | Women with breast cancer had non-significantly elevated estrogen levels. Estrogens in healthy control (but not cases) subjects were directly correlated with their IgA-negative microbiota α-diversity. Prostaglandin E metabolite levels were not associated with tumor status, estrogen levels, or α-diversity. breast cancer patients. ↓ α-diversity and altered composition of both their IgA-positive and IgA-negative fecal microbiota in breast cancer. ↑ Microbial IgA-positive imputed Immune System Diseases metabolic pathway genes. Cases women: ↑ Levels of Clostridiaceae, Faecalibacterium, and Ruminococcaceae. ↓ Levels of Dorea and Lachnospiraceae. |
| Frugé et al., 2020 [64] | Serum and fecal samples. | 16S-V4 rRNA gene amplicon sequencing. RDP classifier. | 32 female breast cancer patients randomized to weight-loss or attention-control arms from the time of diagnosis to tumorectomy. | In the early stage of breast cancer, body composition is associated with Akkermansia muciniphila, microbiota diversity, and interleukin-6 level. Different composition and functions of the gut microbial community between postmenopausal breast cancer patients and healthy controls. Akkermansia muciniphila is related to relevant health outcome parameters and to favorable dietary changes. |
| Zhu et al., 2018 [74] | Fecal samples. | Shotgun metagenomic analysis. | Premenopausal women: 18 breast cancer patients. 25 healthy controls. Postmenopausal women: 44 breast cancer patients. 46 healthy controls. | ↑ Microbial diversity in breast cancer patients than in controls. No differences in relative abundance in gut microbiota between premenopausal breast cancer patients and premenopausal controls. In postmenopausal breast cancer patients: ↑ Escherichia coli, Klebsiella sp_1_1_55, Prevotellaamnii, Enterococcus gallinarum, Actinomycessp. HPA0247, Shewanella putrefaciens, and Erwinia amylovora, and ↓ Eubacterium eligens and Lactobacillus vaginalis. |
| Klann et al., 2020 [66] | Breast tumors from cancer patients. Breast samples from healthy subjects. | 16S rRNA V1–V2 hypervariable regions. RDP classifier and verified against the Greengenes database. | Bilateral normal breast tissue samples (n = 36) collected from 10 women who received routine reduction mammoplasty. Archived breast tumor samples (n = 10) obtained from a biorepository. | Breast cancer samples differed in microbiota composition across individual women. The most abundant phyla in both tumor and normal tissues were Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria. Differences in the relative abundance of various bacterial taxa between groups. ↑ α-diversity in normal compared to tumor samples. |
| Meng, et al., 2018 [75] | Breast tissue samples. | V1-V2 16S rRNA Sequencing. | 22 Chinese patients with benign tumor and 72 malignant breast cancer patients. | Levels of Propionicimonas, Micrococcaceae, Caulobacteraceae, Rhodobacteraceae, Nocardioidaceae, and Methylobacteriaceae, in breast cancer tissues. Bacteroidaceae and Agrococcus associated with malignancy. |
| Costantini, L. et al., 2018 [76] | Breast tissue samples. | V3 16S-rRNA gene amplicons Sequencing. | 16 Mediterranean patients with breast cancer (12 samples were collected from core needle biopsies (CNB) and seven from surgical excision biopsies (SEB); three patients were processed with both procedures). Fresh tumor breast tissue and paired breast healthy tissue. | Ralstonia was the most prominent genus in tumor breast tissue. No differences between healthy adjacent breast tissue and breast cancer tissue. |
| Chiba, A. et al., 2019 [77] | Snap-frozen breast tumor tissue. | V4 16S rRNA amplicon sequencing (Illumina Miseq). Pipelinee: Mothur (v.1.39.5) Microarray for confirmation. | An amount of 15 women with breast cancer who were treated with neoadjuvant chemotherapy, 18 women with no prior therapy at the time of surgery, and nine women who had tumor recurrence. | Presence of Pseudomonas spp. in breast cancer tissue after neoadjuvant chemotherapy. Presence of Prevotella in the tumor tissue from non-treated patients. Presence of Brevundimonas and Staphylococcus in the primary breast tumors in patients developing distant metastases. |
| Horigome, A. et al., 2019 [78] | Capillary blood and fecal samples. | V3-V4 region of the bacterial 16S rRNA gene sequencing. (Illumina Miseq). Pipeline: QIIME2 Gas chromatography for Fatty acid composition. | 124 participants (46% history. of chemotherapy). (123 women and one man). | Actinobacteria and Bacteroidetes were associated with PUFAs in patients previously treated with chemotherapy. Bifidobacterium was associated to PUFAs in participants with no history of chemotherapy |
| Wu et al., 2020 [79] | Breast tissue and fecal samples (collected prior to chemotherapy). | 16S rRNA gene amplification and sequencing of the V3 and V4 hypervariable regions. | 37 breast cancer patients. | No differences in α-diversity or phyla differences by estrogen/progesterone receptor status, tumor grade, stage, parity, and body mass index. HER2+ women showed: ↓ α-diversity, Firmicutes abundance ↑ Abundance of Bacteroidetes. Early menarche associated with: ↓ OTU. ↓ Abundance of Firmicutes. ↑ High total body fat. |
| Jones et al., 2019 [80] | Urine and fecal samples. | 16SrRNAgeneV3-V4hypervariable region. OTUs were assigned by Ribosomal Data Project Naïve Bayesian classifier. | 54 postmenopausal women. (50–74 years old) with normal mammogram. | No association between breast density and fecal microbiota. Total urinary estrogens were strongly and inversely associated with breast density. Fecal microbiota α-diversity and richness did not differ between women with high versus low mammographic density. |
| Yoon et al., 2019 [65] | Fecal samples. | 16S rRNA gene V3-V4 region. Greengenes database. | 121 female participants between the ages of 32 and 78 who underwent a positron emission tomography PET/CT scan. | The physiologic intestinal uptake was positively correlated with the relative abundance of the genus Citrobacter, while negatively correlated with the unclassified Ruminococcaceae. |
| Ma et al., 2020 [81] | Fecal and blood samples. | 16S rDNA amplicon sequencing Mothur method and the SSUrRNA database of SILVA. | 25 breast cancer patients. 25 patients with benign breast disease. | In breast cancer group: ↓ Relative abundance of Firmicutes and Bacteroidetes. ↑ Relative abundance of verrucomicrobla, Proteobacteria and Actinobacteria↓ Faecalibacterium, which was negatively correlated with various phosphorylcholines. |
| Tzeng et al., 2021 [67] | Breast tissue samples. | Bacterial 16S rRNA gene V3–V4 and V7–V9 regions. Amplicon sequence variants (ASVs) were then classified against SILVA. | 221 patients with breast cancer and 87 patients without breast cancer. | Anaerococcus, Caulobacter, and Streptococcus, predominant in benign tissue networks, were absent from cancer-associated tissue. Propionibacterium and Staphylococcus were depleted in tumors and showed negative associations with oncogenic immune features. Streptococcus and Propionibacterium correlated positively with T-cell activation-related genes. Pseudomonas constituted a wide proportion of the breast microbiome in tumor vs. other tissues, and Proteus was the second most abundant genus in tumor tissue but absent from non-tumor tissues. |
| Thyagarajan et al., 2020 [70] | Breast cancer and matched normal tissue adjacent to tumor samples. | 16S rRNA gene-based sequencing. SILVA 16S rRNA database. | Six White non-Hispanic (WNH) of which two were tumor and two normal adjacent tissue. Seven Black non-Hispanic (BNH), triple-negative breast cancer (TNBC) Seven WNH, TNBC. Three BNH and triple-positive breast cancer (TPBC). | Microbial diversity was significantly lower in BNH TNBC tumor tissue as compared to matched normal tissue adjacent to the tumor zone. WNH cohort had an inverse pattern for the Shannon index, when TNBC tumor tissue was compared to the matched d normal tissue adjacent to the tumor. Unweighted PCoA revealed distinct clustering of tumor and d normal tissue adjacent to tumor microbiota in both BNH and WNH cohorts. |
| Smith et al., 2019 [72] | Breast tissue. | 16S rRNA gene sequencing. Greengenes as the reference database. | An amount of 83 breast tissue samples, of which pathologically adjacent normal breast tissues (normal pair) were obtained from 11 breast cancer patients. 64 breast tissue samples from women with stages I-IV breast cancer and eight from healthy women who underwent breast reduction mammoplasty. Approximately 24% of the study participants were NHB, 75% NHW, and 64% were premenopausal. | Proteobacteria was most abundant in normal tissue adjacent to tumor and breast tumors from NHB and NHW women with fewer Firmicutes, Bacteroidetes, and Actinobacteria. ↑ Abundance of genus Ralstonia in NHB women compared to NHW tumors. Enrichment of family Streptococcaceae in TNBC. ↑ Abundance of genus Bosea (phylum Proteobacteria) associated with the tumor stage. |
| Luu et al., 2017 [82] | Feces from women with early-stage breast cancer. | qRT-PCR. | 31 women with breast cancer [ER/PgR+ (90%), HER2+ (15%)]. | In the fecal samples, Firmicutes and Bacteroidetes were the most abundant phyla. ↑Richness of Bacteroidetes, Clostridium coccoides cluster, C. leptum cluster, F. prausnitzii, and Blautia spp. In clinical stage groups II/III compared with clinical stages 0/I Blautia spp. was associated with more severe histoprognostic grades. ↓ Total bacteria and three groups: Firmicutes, Faecalibacterium prausnitzii and Blautia spp. in overweight and obese women. |
| Wang et al., 2017 [68] | Urine and bilateral breast tissue from each control patient, and tumor and ipsilateral adjacent normal breast tissue for cases. | Illumina 16S V3-V4 rRNA amplification. OTUs were assigned using Greengenes database, specific method not disclosed. | An amount of 50 patients and 20 healthy controls. | No significant difference in overall diversity in microbiota content (number of observed OTUs) was detected in breast tissue from cancer and control women. ↓ Relative richness of Methylobacterium was found in women with breast cancer. Differences in the urinary microbiota of women with breast cancer: ↑ Abundance of Corynebacterium, Staphylococcus, Actinomyces, and Propionibacteriaceae gram-positive bacteria. ↓ Abundance of genus Lactobacillus. |
| Thompson et al., 2017 [69] | Breast tumor tissues and normal adjacent tissues from The Cancer Genome Atlas. | 16S-V3-V5 rRNA amplified, metagenome Seq package. Greengenes database. | An amount of 668 tumor tissues (HER2+, ER+ and TNCBC) and 72 normal adjacent tissues. | The most abundant phyla in breast tissues were Proteobacteria, Actinobacteria, and Firmicutes. In tumor samples, the most predominant phyla were Proteobacteria and Actinobacteria in normal tissue. Mycobacterium fortuitum and Mycobacterium phlei were two of the prevalent species observed differentially abundant in the tumor samples. ↑Prevalence of Escherichia coli in the breast tissues. |
| Banerjee et al., 2018 [83] | Breast cancer tissues (cases), breast control tissues from healthy individuals (reduction surgeries). | PathoChips array. | Breast cancer [ER+ (n = 50), HER2+ (n = 34), triple positive (n = 24), TNBC (n = 40)], and normal breast tissue (n = 20). | Unique viral, bacterial, fungal and parasitic signatures were found for each of the breast cancer types. The triple-negative and positive samples showed distinct microbial signature patterns than the ER and HER2 positive breast cancer samples. The most prevalent bacterial signatures were Proteobacteria followed by Firmicutes. The Mobiluncus family was detected in all four types. |
| Title | NCT Number and Location | Study Type | Objective | Type of Sample and Microbiota Detection Method | Time Frame | Intervention | Sample Size and Participants´Characteristics | Status of the Recruitment |
|---|---|---|---|---|---|---|---|---|
| Effects of Chemotherapy on Intestinal Bacteria in Patients With Newly Diagnosed Breast Cancer [91]. | NCT02370277 United States of America. | Observational Case-Control. | To establish changes in gut microbiota related to chemotherapy treatment. | Stool samples, measurement of the number of taxonomic groups. | Baseline to four months after final adjuvant (or neoadjuvant) chemotherapy course. | N/A. | n = 36 ≥18 years Gender: Female Current patients of breast cancer. | Completed |
| Intestine Bacteria and Breast Cancer Risk [87]. | NCT01461070 United States of America. | Observational Case-Control. | To establish association between fecal microbiome and systemic estrogens levels. | Stool samples, 16S rRNA metagenomics sequencing. | Cross-sectional | N/A | n = 175 50–69 years Gender: Female Current patients of breast cancer and healthy women. | Completed |
| Persistent Post Surgical Pain in Women With breast cancer [98]. | NCT02266082 United States of America. | Observational Cohort. | To establish changes in gut microbiome potentially associated with pain after surgery and mental disorders. | Stool samples, 16S rRNA, metagenomics sequencing. | Baseline, three months and six months post-surgery. | N/A. | n = 5 40–75 years Gender: Female. | Completed |
| Effect of Esketamine on Postoperative Depression Gut Microbiota Bispectral Index Data of Depression Patients Undergoing Breast Cancer Operation [94]. | NCT04303325 China. | Interventional Randomized. | To detemine the effect of esketamine on gut microbiome potentialy associated with depresion mental disorders. | Stool samples, method not indicated. | Baseline to 72 h post-surgery. | Esketamine or saline solution. | n = 36 18–65 years Gender: Female Current patients of breast cancer. | Not yet recruiting. |
| Anti-anxiety Biotics for Breast Cancer Survivors [95]. | NCT04784182 United States of America. | Interventional Randomized. | To evaluate the effect of probiotics in anxiety symptoms in breast cancer survivors. | Stool samples, 16S rRNA metagenomics sequencing. | Four weeks. | Daily dietary supplementation with at least five billion CFU per day of total bacteria including Lactobacillus helveticus and Bifidobacterium longum and prebiotic containing 4 g of fructooligosaccharides for four weeks. | n = 48 50–75 years Gender: Female breast cancer survivors. | Not yet recruiting |
| Intestinal Microbiota of Breast Cancer Patients Undergoing Chemotherapy [90]. | NCT04138979 China. | Observational Case-Control. | To determine transcriptional changes in gut microbiota during and after chemotherapy. | Stool samples, 16S rRNA metagenomics sequencing. | Baseline, 1, 7, 14, 22, 29, 36, 44, 51, 58, 66, 73, and 80 days. | Cyclophosphamide. | n = 80 18–65 years Gender: Female Current patients of breast cancer. | Recruiting |
| Breast Cancer and Its Relationship With the Microbiota [93]. | NCT03885648 Spain. | Observational Case-Control. | To detemine the contribution of bacteria, archaea, viruses and fungi together with exposure to environmental contaminants to the risk of breast cancer. | Stool and mamary samples, shotgun metagenomics sequencing. | Post surgery | N/A. | n = 200 25–70 years Gender: Female Current patients of breast cancer. | Recruiting |
| A Pilot Trial of Preoperative Oral Microbiota-based Investigational New Drugs [96]. | NCT04139993 United States. | Interventional Non Randomized. | Evaluation of RBX7455 effect on the restoration of microbiota. | Stool samples, bacterial taxonomy identification. | An amount of ≤2 days prior to surgery, as well as eight weeks and six months after treatment. | RBX7455, a novel microbiome restoration therapy™. | n = 30 ≥18 years Gender: Female Current patients of breast cancer. | Recruiting |
| Gut and Intratumoral Microbiome Effect on the Neoadjuvant Chemotherapy- induced Immunosurveillance in Triple Negative Breast Cancer [92]. | NCT03586297 United States of America. | Observational Cohort. | To determine correlations between responses in triple-negative breast cancer patients to standard chemotherapy and the variations in microbiota profile. | Stool and tumoral samples, 16S rRNA and shotgun metagenomics sequencing. | Completion of chemotherapy, (approximately 18 weeks). | N/A. | n = 49 ≥18 years Gender: Female Current patients of breast cancer. | Recruiting |
| Probiotics and Breast Health Study [89]. | NCT03290651 Canada. | Interventional Randomized. | To test the hypothesis that probiotics can reach the breast tissue and contribute to displace the harmful bacteria. | Mamary samples, method not indicated. | An amount of 90 days post collection. | Dietary supplement: One capsule containing 2.5 billion CFU of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 or placebo for 90 days. | n = 40 Children, adult an older adult Gender: Female Women at high risk of developing breast cancer who have never had breast cancer. | Recruiting |
| Gut Microbe Composition, Exercise, and Breast [99]. | NCT04088708 United States of America. | Interventional Randomized. | To determine the effects of exercise on the gut microbiome in breast cancer survivors and how these changes may relate to psychosocial symptoms such as fatigue. | Stool sample, method not indicated. | Baseline, 5, 10, 15 weeks. | Aerobic exercise training. | n = 126 18–70 years Gender: Female breast cancer survivors. | Recruiting |
| A Fiber-diverse, Anti-inflammatory Diet and Aerobic Exercise Reduce Risk of Breast Cancer Recurrence [97]. | NCT05113485 United States of America. | Interventional Randomized Intervention | To conduct two parallel, three-month behavior change interventions, contrasting the six-count highly-microbiota-accessible foods approach with the traditional diabetes prevention program calorie restriction approach To design a ramped-up randomized factorial trial. | Stool sample, method not indicated. | Baseline and six-month follow-up assessments of: low grade systemic inflammation, body composition including visceral fat estimation, cardiorespiratory fitness, inflammatory and cardiometabolic biomarkers. | Highly-Microbiota-Accessible Foods approach with the traditional or Diabetes Prevention Program calorie restriction | n = 30 50–75 years Gender: Female breast cancer survivors. interested in losing excess body fat. | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Mercado, A.I.; del Valle Cano, A.; Fernández, M.F.; Fontana, L. Gut Microbiota and Breast Cancer: The Dual Role of Microbes. Cancers 2023, 15, 443. https://doi.org/10.3390/cancers15020443
Álvarez-Mercado AI, del Valle Cano A, Fernández MF, Fontana L. Gut Microbiota and Breast Cancer: The Dual Role of Microbes. Cancers. 2023; 15(2):443. https://doi.org/10.3390/cancers15020443
Chicago/Turabian StyleÁlvarez-Mercado, Ana Isabel, Ana del Valle Cano, Mariana F. Fernández, and Luis Fontana. 2023. "Gut Microbiota and Breast Cancer: The Dual Role of Microbes" Cancers 15, no. 2: 443. https://doi.org/10.3390/cancers15020443
APA StyleÁlvarez-Mercado, A. I., del Valle Cano, A., Fernández, M. F., & Fontana, L. (2023). Gut Microbiota and Breast Cancer: The Dual Role of Microbes. Cancers, 15(2), 443. https://doi.org/10.3390/cancers15020443







