Body Composition as a Comorbidity-Independent Predictor of Survival following Nephroureterectomy for Urothelial Cancer of the Upper Urinary Tract
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Acquisition
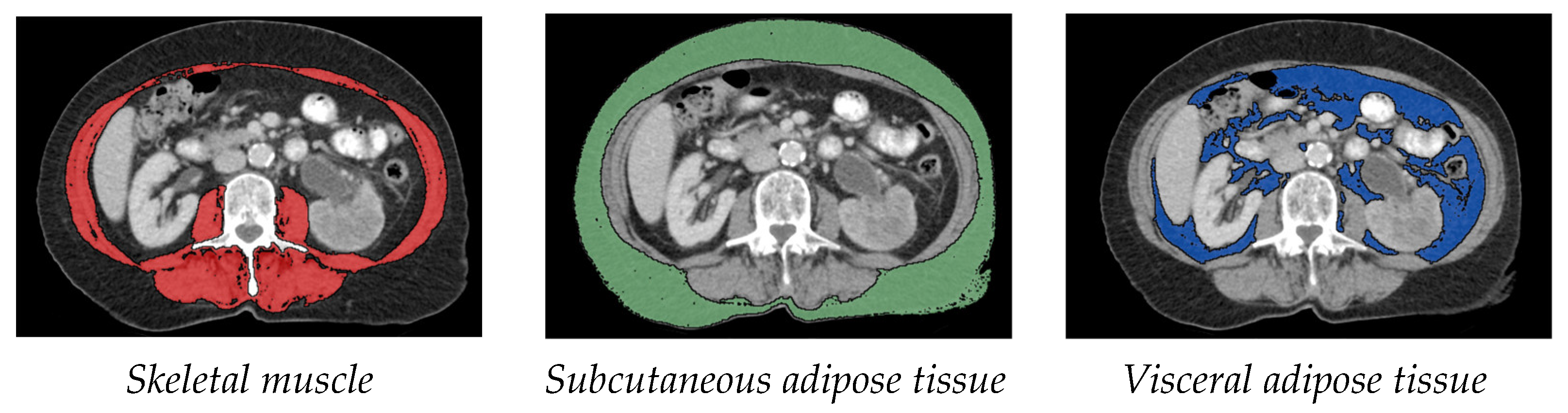
2.2. CT-Based Measurement of Body Composition
- Measurement of muscle tissue: As in various other sarcopenia studies, the cross-sectional skeletal muscle surface (cm2) was measured at the height of the third lumbar vertebra (L3) on two consecutive transversal computed tomography images. The range of –30 to +110 Hounsfield units was used for skeletal muscle [7,9].
- Measurement of subcutaneous and visceral fat tissue: The measurement of fat distribution was also done at the L3 level on two consecutive transversal CT images. Adipose tissue was identified as Hounsfield units –150 to –50. Visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) were normalized for height in metres squared, resulting in visceral adipose tissue index (VATI) and subcutaneous adipose tissue index (SATI) [10].
2.3. Sarcopenia
2.4. Comorbidity Survey
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raman, J.D.; Messer, J.; Sielatycki, J.A.; Hollenbeak, C.S. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int. 2011, 107, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Soria, F.; Shariat, S.F.; Lerner, S.P.; Fritsche, H.M.; Rink, M.; Kassouf, W.; Spiess, P.E.; Lotan, Y.; Ye, D.; Fernandez, M.I.; et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J. Urol. 2017, 35, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.D.; Wood, C.G. Upper Tract Urothelial Carcinoma CollaborationThe Upper Tract Urothelial Carcinoma C. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009, 115, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Botteri, E.; Iodice, S.; Boniol, M.; Lowenfels, A.B.; Maisonneuve, P.; Boyle, P. Tobacco smoking and cancer: A meta-analysis. Int. J. Cancer 2008, 122, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Nakanishi, Y.; Kataoka, M.; Tobisu, K.; Koga, F. Prognostic significance of sarcopenia in upper tract urothelial carcinoma patients treated with radical nephroureterectomy. Cancer Med. 2016, 5, 2213–2220. [Google Scholar] [CrossRef] [Green Version]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef]
- Mayr, R.; Gierth, M.; Zeman, F.; Reiffen, M.; Seeger, P.; Wezel, F.; Pycha, A.; Comploj, E.; Bonatti, M.; Ritter, M.; et al. Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 505–513. [Google Scholar] [CrossRef]
- Han, J.; Tang, M.; Lu, C.; Shen, L.; She, J.; Wu, G. Subcutaneous, but not visceral, adipose tissue as a marker for prognosis in gastric cancer patients with cachexia. Clin. Nutr. 2021, 40, 5156–5161. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Bamba, S.; Inatomi, O.; Takahashi, K.; Morita, Y.; Imai, T.; Ohno, M.; Kurihara, M.; Takebayashi, K.; Kojima, M.; Iida, H.; et al. Assessment of Body Composition From CT Images at the Level of the Third Lumbar Vertebra in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 27, 1435–1442. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Piccirillo, J.F.; Tierney, R.M.; Costas, I.; Grove, L.; Spitznagel, E.L., Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004, 291, 2441–2447. [Google Scholar] [CrossRef] [Green Version]
- Labeur, T.A.; van Vugt, J.L.A.; Ten Cate, D.W.G.; Takkenberg, R.B.; JNM, I.J.; Groot Koerkamp, B.; de Man, R.A.; van Delden, O.M.; Eskens, F.; Klumpen, H.J. Body Composition Is an Independent Predictor of Outcome in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Liver Cancer 2019, 8, 255–270. [Google Scholar] [CrossRef]
- Sun, M.Y.; Chang, C.L.; Lu, C.Y.; Wu, S.Y.; Zhang, J.Q. Sarcopenia as an Independent Risk Factor for Specific Cancers: A Propensity Score-Matched Asian Population-Based Cohort Study. Nutrients 2022, 14, 1910. [Google Scholar] [CrossRef]
- Psutka, S.P.; Carrasco, A.; Schmit, G.D.; Moynagh, M.R.; Boorjian, S.A.; Frank, I.; Stewart, S.B.; Thapa, P.; Tarrell, R.F.; Cheville, J.C.; et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: Impact on cancer-specific and all-cause mortality. Cancer 2014, 120, 2910–2918. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Surov, A.; Wienke, A. Prevalence of sarcopenia in patients with solid tumors: A meta-analysis based on 81,814 patients. JPEN J. Parenter Enter. Nutr. 2022, 46, 1761–1768. [Google Scholar] [CrossRef]
- Ishihara, H.; Kondo, T.; Omae, K.; Takagi, T.; Iizuka, J.; Kobayashi, H.; Hashimoto, Y.; Tanabe, K. Sarcopenia predicts survival outcomes among patients with urothelial carcinoma of the upper urinary tract undergoing radical nephroureterectomy: A retrospective multi-institution study. Int. J. Clin. Oncol. 2017, 22, 136–144. [Google Scholar] [CrossRef]
- Matsui, R.; Watanabe, J.; Banno, M.; Inaki, N.; Fukunaga, T. Association of visceral adipose tissue with postoperative outcome in upper gastrointestinal cancer: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2022, 116, 1540–1552. [Google Scholar] [CrossRef]
- Nakano, O.; Kawai, H.; Kobayashi, T.; Kohisa, J.; Ikarashi, S.; Hayashi, K.; Yokoyama, J.; Terai, S. Rapid decline in visceral adipose tissue over 1 month is associated with poor prognosis in patients with unresectable pancreatic cancer. Cancer Med. 2021, 10, 4291–4301. [Google Scholar] [CrossRef]
- Hariharan, N.; Ashcraft, K.A.; Svatek, R.S.; Livi, C.B.; Wilson, D.; Kaushik, D.; Leach, R.J.; Johnson-Pais, T.L. Adipose Tissue-Secreted Factors Alter Bladder Cancer Cell Migration. J. Obes. 2018, 2018, 9247864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, D.J.; Shabto, J.M.; Goyal, S.; Liu, Y.; Olsen, T.A.; Evans, S.T.; Magod, B.L.; Ravindranathan, D.; Brown, J.T.; Yantorni, L.; et al. Body Composition as an Independent Predictive and Prognostic Biomarker in Advanced Urothelial Carcinoma Patients Treated with Immune Checkpoint Inhibitors. Oncologist 2021, 26, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, Z.; Yang, L.; Wang, X.; Yi, Z.; Zhou, L.; Chen, Y.; Yang, L.; Zhuo, H.; Bao, Y.; et al. Body Composition Parameters May Be Prognostic Factors in Upper Urinary Tract Urothelial Carcinoma Treated by Radical Nephroureterectomy. Front. Oncol. 2021, 11, 679158. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Owari, T.; Iwamoto, T.; Morizawa, Y.; Hori, S.; Marugami, N.; Shimada, K.; Iida, K.; Ohnishi, K.; Gotoh, D.; et al. Clinical utility of bioelectrical impedance analysis in patients with locoregional muscle invasive or metastatic urothelial carcinoma: A subanalysis of changes in body composition during neoadjuvant systemic chemotherapy. Support Care Cancer 2018, 26, 1077–1086. [Google Scholar] [CrossRef]
- Beaudart, C.; Dawson, A.; Shaw, S.C.; Harvey, N.C.; Kanis, J.A.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.C.; Bruyere, O.; et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef]



| Entire Cohort N = 142 | Sarcopenic N = 53 | Non-Sarcopenic N = 89 | p-Value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 71 (64–77) | 76 (70–79.5) | 68 (61–74.5) | <0.001 * |
| Gender (male), n (%) | 87 (61.3) | 32 (60.4) | 55 (61.8) | 0.867 |
| BMI, median (IQR) | 25.75 (23.5–29.4) | 24.90 (22.2–27.4) | 26.70 (24.2–30.3) | 0.006 * |
| BMI-Category, n (%) | 0.019 * | |||
| <18.5 | 2 (1.4) | 1 (1.9) | 1 (1.1) | |
| 18.5–24.9 | 57 (40.1) | 27 (50.9) | 30 (30.7) | |
| 25–29.9 | 52 (36.6) | 18 (34.0) | 34 (38.2) | |
| >30 | 31 (21.8) | 7 (13.2) | 24 (27.0) | |
| Cystectomy in the course, n (%) | 11 (7.7) | 4 (7.5) | 7 (7.9) | 0.945 |
| ASA-Score, n (%) | 0.054 | |||
| 1 | 13 (9.2) | 4 (7.5) | 9 (10.1) | |
| 2 | 65 (45.8) | 19 (35.8) | 46 (51.7) | |
| 3 | 59 (41.5) | 28 (52.8) | 31 (34.8) | |
| 4 | 5 (3.5) | 2 (3.8) | 3 (3.4) | |
| 5 | - | - | - | |
| 6 | - | - | - | |
| ACE-27, n (%) | 0.016 * | |||
| None | 27 (19) | 5 (9.4) | 22 (24.7) | |
| Mild | 59 (41.5) | 23 (43.4) | 36 (40.4) | |
| Moderate | 45 (31.7) | 17 (32.1) | 28 (31.5) | |
| Severe | 11 (7.7) | 8 (15.1) | 3 (3.4) | |
| Diabetes mellitus, n (%) | 41 (28.9) | 11 (20.8) | 30 (33.7) | 0.099 |
| Creatinine preop. (mg/dL), median (IQR) | 1.15 (0.94–1.50) | 1.2 (0.98–1.54) | 1.15 (0.91–1.45) | 0.378 |
| pathol. T-stage, n (%) | 0.687 | |||
| pTa | 38 (26.8) | 13 (24.5) | 25 (28.1) | |
| pT1 | 19 (13.4) | 7 (13.7) | 12 (13.5) | |
| pT2 | 18 (12.7) | 8 (15.1) | 10 (11.2) | |
| pT3 | 59 (41.5) | 21 (39.6) | 38 (42.7) | |
| pT4 | 8 (5.6) | 4 (7.5) | 4 (4.5) | |
| pathol. T-stage-Category, n (%) | 0.711 | |||
| pTa, pT1 | 57 (40.1) | 20 (37.7) | 37 (41.6) | |
| pT2 | 18 (12.7) | 8 (15.1) | 10 (11.2) | |
| pT3 | 59 (41.5) | 21 (39.6) | 38 (42.7) | |
| pT4 | 8 (5.6) | 4 (7.5) | 4 (4.5) | |
| pathol. N-stage, n (%) | 0.929 | |||
| pN0 | 19 (13.4) | 4 (7.5) | 15 (16.9) | |
| pNx | 110 (77.5) | 40 (75.5) | 70 (78.7) | |
| pN+ | 13 (9.2) | 9 (17.0) | 4 (4.5) | |
| R-stage, n (%) | 0.429 | |||
| R0 | 127 (89.4) | 46 (86.8) | 81 (91.0) | |
| R1 | 15 (10,6) | 7 (13,2) | 8 (9,0) | |
| Concomitant CIS, n (%) | 14 (9.9) | 3 (5.7) | 11 (12.4) | 0.195 |
| LVI, n (%) | 38 (26.8) | 14 (26.4) | 24 (27.0) | 0.943 |
| Tumor necrosis, n (%) | 28 (19.7) | 14 (26.4) | 14 (15.7) | 0.122 |
| Multifocality, n (%) | 24 (16.9) | 8 (15.1) | 16 (18.0) | 0.657 |
| Location, n (%) | 0.652 | |||
| Renal pelvis | 99 (69.7) | 37 (69.8) | 62 (69.7) | |
| Upper third | 7 (4.9) | 4 (7.5) | 3 (3.4) | |
| Middle third | 16 (11.3) | 6 (11.3) | 10 (11.2) | |
| Distal third | 20 (14.1) | 6 (11.3) | 14 (15.7) | |
| Follow-up (month), median (IQR) | 37 (17–68) | 24 (12–57) | 42 (21–88) | 0.002 * |
| Overall mortality, n (%) | 66 (46.5) | 34 (64.2) | 32 (36.0) | 0.001 * |
| Cancer specific mortality, n (%) | 56 (39.4) | 30 (56.6) | 26 (29.2) | 0.001 * |
| SATI, median (IQR) | 60.98 (39.7–81.6) | 54.26 (39–81) | 62.95 (39.9–82) | 0.398 |
| VATI, median (IQR) | 68.00 (36.2–94) | 64.51 (36.1–68.6) | 68.44 (36.9–102.1) | 0.398 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age at NUE | 1.051 | 1.02–1.08 | <0.001 * | 1.01 | 0.98–1.05 | 0.438 |
| Gender (ref.: male) | 0.928 | 0.56–1.53 | 0.772 | - | - | - |
| Cystectomy in the course (ref.: absence) | 0.776 | 0.31–1.93 | 0.586 | - | - | - |
| BMI-Category (ref.: normal weight) | ||||||
| <18.5 (underweight) | 4.22 | 0.99–17.93 | 0.051 | - | - | - |
| 25–29.9 (Pre-Obesity) | 0.71 | 0.40–1.23 | 0.219 | - | - | - |
| >30 (Obesity) | 0.73 | 0.38–1.38 | 0.329 | - | - | - |
| ASA-Score (ref.: 1) | ||||||
| 2 | 1.29 | 0.45–3.69 | 0.635 | - | - | - |
| 3 | 1.81 | 0.64–5.13 | 0.262 | - | - | - |
| 4 | 2.90 | 0.64–13.09 | 0.165 | - | - | - |
| 5 | - | - | - | - | - | - |
| 6 | - | - | - | - | - | - |
| ACE-27 (ref.: none) | ||||||
| Mild | 1.77 | 0.80–3.91 | 0.160 | 1.00 | 0.39–2.58 | 0.994 |
| Moderate | 2.29 | 1.03–5.09 | 0.041 * | 2.12 | 0.85–5.29 | 0.108 |
| Severe | 3.54 | 1.28–9.81 | 0.015 * | 2.40 | 0.76–7.61 | 0.136 |
| Sarcopenia (ref.: absence) | 2.34 | 1.47–3.89 | <0.001 * | 1.77 | 1.02–3.07 | 0.042 * |
| Diabetes mellitus (ref.: absence) | 1.48 | 0.89–2.45 | 0.133 | - | - | - |
| Creatinine preop. (continuous) | 1.036 | 0.87–1.24 | 0.692 | - | - | - |
| pathol. T-stage (ref.: pTa) | ||||||
| pT1 | 0.76 | 0.20–2.86 | 0.685 | - | - | - |
| pT2 | 2.07 | 0.75–5.70 | 0.162 | - | - | - |
| pT3 | 4.60 | 2.15–9.85 | <0.001 * | - | - | - |
| pT4 | 13.22 | 4.89–35.67 | <0.001 * | - | - | - |
| pathol. T-stage-Category (ref.: pTa, pT1) | ||||||
| pT2 | 2.24 | 0.87–5.79 | 0.095 | 1.41 | 0.50–4.02 | 0.516 |
| pT3 | 5.00 | 2.56–9.76 | <0.001 * | 3.02 | 1.33–6.90 | 0.009 * |
| pT4 | 14.36 | 5.69–36.21 | <0.001 * | 6.28 | 1.90–20.72 | 0.003 * |
| R-stage (ref.: R0) | ||||||
| R1 | 4.65 | 2.48–8.71 | <0.001 * | 1.96 | 0.85–4.51 | 0.116 |
| Concomitant CIS (ref.: absence) | 1.06 | 0.48–2.31 | 0.892 | - | - | - |
| LVI (ref.: absence) | 3.91 | 2.39–6.40 | 0.001 * | 2.35 | 1.28–4.35 | 0.006 * |
| Tumor necrosis (ref. absence) | 1.98 | 1.16–3.37 | 0.013 * | 0.99 | 0.54–1.80 | 0.968 |
| Multifocality (ref. unifocal) | 1.31 | 0.72–2.41 | 0.382 | - | - | - |
| Location (ref.: renal pelvis) | ||||||
| Upper third | 0.95 | 0.34–2.66 | 0.927 | - | - | - |
| Middle third | 0.76 | 0.33–1.79 | 0.533 | - | - | - |
| Distal third | 1.28 | 0.68–2.42 | 0.451 | - | - | - |
| SATI (continuous) | 0.98 | 0.99–1.01 | 0.431 | - | - | - |
| VATI (continuous) | 0.99 | 0.99–1.00 | 0.098 | - | - | - |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age at NUE | 1.05 | 1.02–1.08 | 0.001 * | 1.00 | 0.97–1.04 | 0.822 |
| Gender (ref.: male) | 0.97 | 0.57–1.67 | 0.920 | - | - | - |
| Cystectomy in the course (ref.: absence) | 0.73 | 0.26–2.02 | 0.55 | - | - | - |
| BMI-Category (ref.: normal weight) | ||||||
| <18.5 (underweight) | 4.62 | 1.08–19.81 | 0.039 * | 17.96 | 3.35–96.39 | <0.001 * |
| 25–29.9 (Pre-Obesity) | 0.71 | 0.39–1.30 | 0.273 | 0.92 | 0.45–1.87 | 0.822 |
| >30 (Obesity) | 0.68 | 0.33–1.38 | 0.282 | 1.57 | 0.64–3.86 | 0.324 |
| ASA-Score (ref.: 1) | ||||||
| 2 | 1.72 | 0.52–5.68 | 0.372 | - | - | - |
| 3 | 1.80 | 0.54–5.99 | 0.336 | - | - | - |
| 4 | 2.41 | 0.40–14.53 | 0.336 | - | - | - |
| 5 | - | - | - | - | - | - |
| 6 | - | - | - | - | - | - |
| ACE-27 (ref.: none) | ||||||
| Mild | 1.84 | 0.79–4.28 | 0.155 | 0.79 | 0.27–2.33 | 0.668 |
| Moderate | 1.97 | 0.83–4.69 | 0.126 | 1.69 | 0.57–4.96 | 0.341 |
| Severe | 3.31 | 1.11–9.91 | 0.032 * | 2.01 | 0.57–7.12 | 0.278 |
| Sarcopenia (ref.: absence) | 2.56 | 1.50–4.34 | <0.001 * | 2.17 | 1.18–3.99 | 0.012 * |
| Diabetes mellitus (ref.: absence) | 1.00 | 0.55–1.81 | 1.0 | - | - | - |
| Creatinine preop. (continuous) | 1.06 | 0.83–1.25 | 0.88 | - | - | - |
| pathol. T-stage (ref.: pTa) | ||||||
| pT1 | 1.52 | 0.34–6.80 | 0.583 | - | - | - |
| pT2 | 4.08 | 1.19–13.94 | 0.025 * | - | - | - |
| pT3 | 7.67 | 2.72–21.65 | <0.001 * | - | - | - |
| pT4 | 24.75 | 7.37–83.13 | <0.001 * | - | - | - |
| pathol. T-stage-Category (ref.: pTa, pT1) | ||||||
| pT2 | 3.48 | 1.22–9.92 | 0.020 * | 2.55 | 0.70–9.29 | 0.156 |
| pT3 | 6.54 | 2.90–14.79 | <0.001 * | 4.04 | 1.30–12.56 | 0.016 * |
| pT4 | 21.11 | 7.56–59.00 | <0.001 * | 10.21 | 2.36–44.14 | 0.002 * |
| R-stage (ref.: R0) | ||||||
| R1 | 5.35 | 2.82–10.14 | <0.001 * | 2.52 | 0.98–6.46 | 0.055 |
| Concomitant CIS (ref.: absence) | 1.28 | 0.58–2.83 | 0.539 | |||
| LVI (ref.: absence) | 4.62 | 2.71–7.86 | <0.001 * | 2.91 | 1.43–5.90 | 0.003 * |
| Tumor necrosis (ref. absence) | 2.31 | 1.31–4.04 | 0.004 * | 1.56 | 0.72–2.57 | 0.347 |
| Multifocality (ref. unifocal) | 1.62 | 0.87–3.01 | 0.129 | - | - | - |
| Location (ref.: renal pelvis) | ||||||
| Upper third | 1.18 | 0.42–3.33 | 0.749 | - | - | - |
| Middle third | 0.61 | 0.22–1.72 | 0.351 | - | - | - |
| Distal third | 1.54 | 0.80–2.96 | 0.195 | - | - | - |
| SATI (continuous) | 1.00 | 0.99–1.01 | 0.459 | - | - | - |
| VATI (continuous) | 0.99 | 0.99–1.00 | 0.058 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pickl, C.; Engelmann, S.; Girtner, F.; Gužvić, M.; van Rhijn, B.W.G.; Hartmann, V.; Holbach, S.; Kälble, S.; Haas, M.; Rosenhammer, B.; et al. Body Composition as a Comorbidity-Independent Predictor of Survival following Nephroureterectomy for Urothelial Cancer of the Upper Urinary Tract. Cancers 2023, 15, 450. https://doi.org/10.3390/cancers15020450
Pickl C, Engelmann S, Girtner F, Gužvić M, van Rhijn BWG, Hartmann V, Holbach S, Kälble S, Haas M, Rosenhammer B, et al. Body Composition as a Comorbidity-Independent Predictor of Survival following Nephroureterectomy for Urothelial Cancer of the Upper Urinary Tract. Cancers. 2023; 15(2):450. https://doi.org/10.3390/cancers15020450
Chicago/Turabian StylePickl, Christoph, Simon Engelmann, Florian Girtner, Miodrag Gužvić, Bas W. G. van Rhijn, Valerie Hartmann, Sonja Holbach, Sebastian Kälble, Maximilian Haas, Bernd Rosenhammer, and et al. 2023. "Body Composition as a Comorbidity-Independent Predictor of Survival following Nephroureterectomy for Urothelial Cancer of the Upper Urinary Tract" Cancers 15, no. 2: 450. https://doi.org/10.3390/cancers15020450
APA StylePickl, C., Engelmann, S., Girtner, F., Gužvić, M., van Rhijn, B. W. G., Hartmann, V., Holbach, S., Kälble, S., Haas, M., Rosenhammer, B., Breyer, J., Burger, M., & Mayr, R. (2023). Body Composition as a Comorbidity-Independent Predictor of Survival following Nephroureterectomy for Urothelial Cancer of the Upper Urinary Tract. Cancers, 15(2), 450. https://doi.org/10.3390/cancers15020450







