Surgical Strategies for Recurrent Hepatocellular Carcinoma after Resection: A Review of Current Evidence
Abstract
:Simple Summary
Abstract
1. Introduction
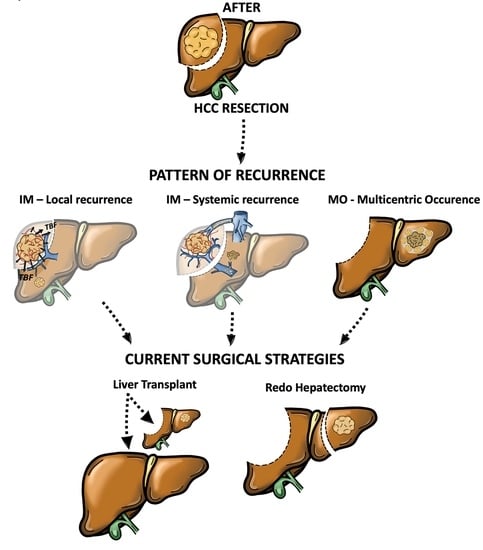
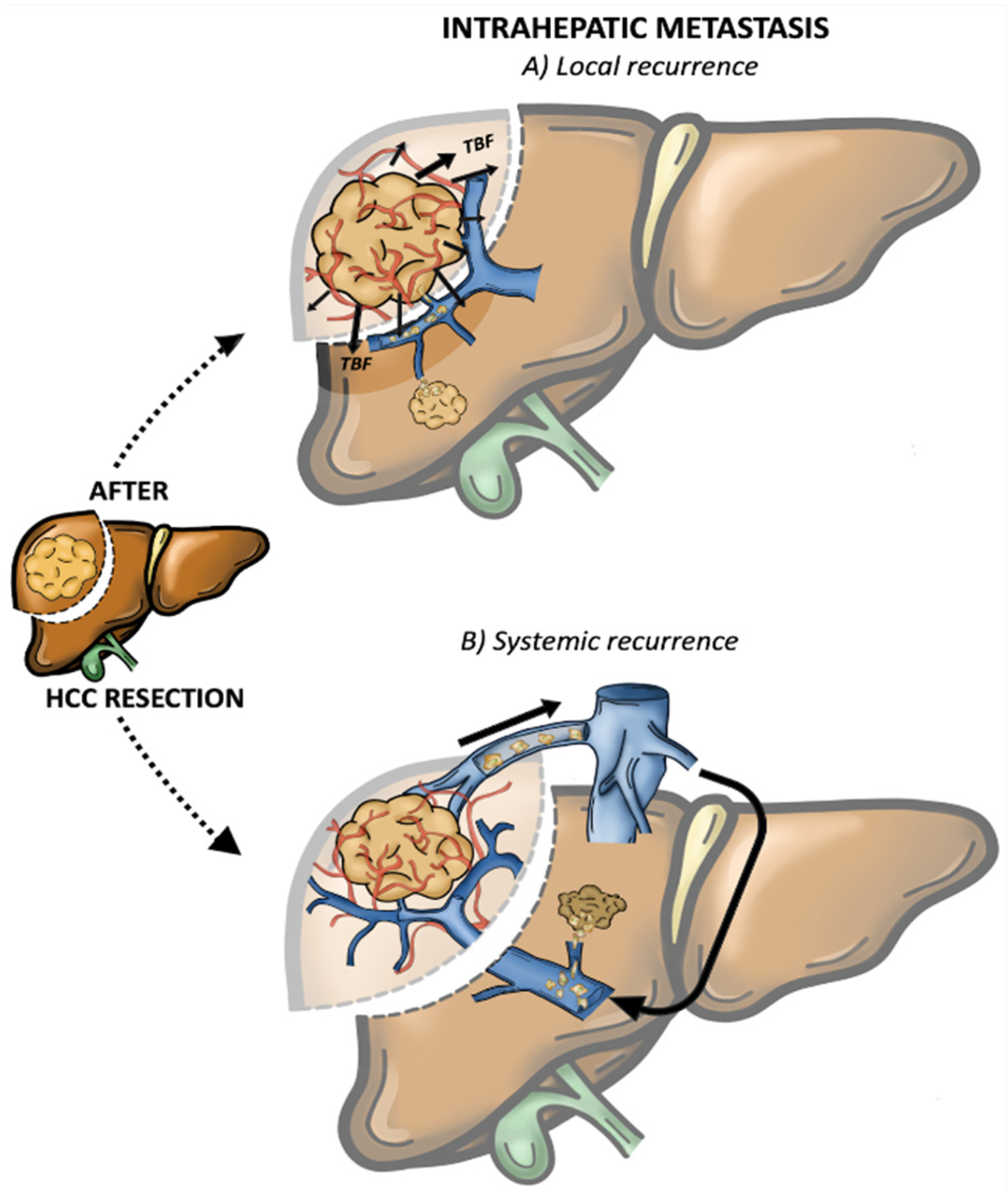
2. Pattern of Recurrence and Prognostic Significance
3. Adjuvant Postoperative Treatments
4. Surgical Treatments for rHCC
4.1. Redo Hepatectomy
4.2. Salvage Liver Transplantation
4.3. RH vs. SLT: Which Is the Best Option?
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Burroughs, A.; Bruix, J. Hepatocellular Carcinoma. Lancet 2003, 362, 1907–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the Epidemic of Nonalcoholic Fatty Liver Disease Demonstrates an Exponential Increase in Burden of Disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Belghiti, J.; Kianmanesh, R. Surgical Treatment of Hepatocellular Carcinoma. HPB 2005, 7, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torzilli, G.; Belghiti, J.; Kokudo, N.; Takayama, T.; Capussotti, L.; Nuzzo, G.; Vauthey, J.-N.; Choti, M.A.; De Santibanes, E.; Donadon, M.; et al. A Snapshot of the Effective Indications and Results of Surgery for Hepatocellular Carcinoma in Tertiary Referral Centers: Is It Adherent to the EASL/AASLD Recommendations?: An Observational Study of the HCC East-West Study Group. Ann. Surg. 2013, 257, 929–937. [Google Scholar] [CrossRef]
- Erridge, S.; Pucher, P.H.; Markar, S.R.; Malietzis, G.; Athanasiou, T.; Darzi, A.; Sodergren, M.H.; Jiao, L.R. Meta-Analysis of Determinants of Survival Following Treatment of Recurrent Hepatocellular Carcinoma. Br. J. Surg. 2017, 104, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Nagasue, N.; Uchida, M.; Makino, Y.; Takemoto, Y.; Yamanoi, A.; Hayashi, T.; Chang, Y.-C.; Kohno, H.; Nakamura, T.; Yukaya, H. Incidence and Factors Associated with Intrahepatic Recurrence Following Resection of Hepatocellular Carcinoma. Gastroenterology 1993, 105, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Vauthey, J.N.; Klimstra, D.; Franceschi, D.; Tao, Y.; Fortner, J.; Blumgart, L.; Brennan, M. Factors Affecting Long-Term Outcome after Hepatic Resection for Hepatocellular Carcinoma. Am. J. Surg. 1995, 169, 28–34; discussion 34–35. [Google Scholar] [CrossRef]
- Imamura, H.; Matsuyama, Y.; Tanaka, E.; Ohkubo, T.; Hasegawa, K.; Miyagawa, S.; Sugawara, Y.; Minagawa, M.; Takayama, T.; Kawasaki, S.; et al. Risk Factors Contributing to Early and Late Phase Intrahepatic Recurrence of Hepatocellular Carcinoma after Hepatectomy. J. Hepatol. 2003, 38, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.-Y.; Fan, H.-K.; Ren, Z.-G.; Fan, J.; Gao, Q. Identifying Clonal Origin of Multifocal Hepatocellular Carcinoma and Its Clinical Implications. Clin. Transl. Gastroenterol. 2019, 10, e00006. [Google Scholar] [CrossRef] [PubMed]
- Portolani, N.; Coniglio, A.; Ghidoni, S.; Giovanelli, M.; Benetti, A.; Tiberio, G.A.M.; Giulini, S.M. Early and Late Recurrence after Liver Resection for Hepatocellular Carcinoma: Prognostic and Therapeutic Implications. Ann. Surg. 2006, 243, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-Y.; Liang, B.-Y.; Xiong, M.; Zhan, D.-Q.; Wei, S.; Wang, G.-P.; Chen, Y.-F.; Chen, X.-P. Long-Term Outcomes of Repeat Hepatic Resection in Patients with Recurrent Hepatocellular Carcinoma and Analysis of Recurrent Types and Their Prognosis: A Single-Center Experience in China. Ann. Surg. Oncol. 2012, 19, 2515–2525. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhang, X.-F.; Bagante, F.; Ratti, F.; Marques, H.P.; Silva, S.; Soubrane, O.; Lam, V.; Poultsides, G.A.; Popescu, I.; et al. Early Versus Late Recurrence of Hepatocellular Carcinoma After Surgical Resection Based on Post-Recurrence Survival: An International Multi-Institutional Analysis. J. Gastrointest. Surg. 2021, 25, 125–133. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akamatsu, N.; Cillo, U.; Cucchetti, A.; Donadon, M.; Pinna, A.D.; Torzilli, G.; Kokudo, N. Surgery and Hepatocellular Carcinoma. Liver Cancer 2016, 6, 44–50. [Google Scholar] [CrossRef]
- Wen, T.; Jin, C.; Facciorusso, A.; Donadon, M.; Han, H.-S.; Mao, Y.; Dai, C.; Cheng, S.; Zhang, B.; Peng, B.; et al. Multidisciplinary Management of Recurrent and Metastatic Hepatocellular Carcinoma after Resection: An International Expert Consensus. Hepatobiliary Surg. Nutr. 2018, 7, 353–371. [Google Scholar] [CrossRef]
- Famularo, S.; Donadon, M.; Cipriani, F.; Bernasconi, D.P.; LaBarba, G.; Dominioni, T.; Iaria, M.; Molfino, S.; Conci, S.; Ferrari, C.; et al. Curative versus Palliative Treatments for Recurrent Hepatocellular Carcinoma: A Multicentric Weighted Comparison. HPB 2021, 23, 889–898. [Google Scholar] [CrossRef]
- Majno, P.E.; Sarasin, F.P.; Mentha, G.; Hadengue, A. Primary Liver Resection and Salvage Transplantation or Primary Liver Transplantation in Patients with Single, Small Hepatocellular Carcinoma and Preserved Liver Function: An Outcome-Oriented Decision Analysis. Hepatology 2000, 31, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, G.P.; Esposito, G.; Olivieri, T.; Magistri, P.; Ballarin, R.; Di Sandro, S.; Di Benedetto, F. Salvage versus Primary Liver Transplantation for Hepatocellular Carcinoma: A Twenty-Year Experience Meta-Analysis. Cancers 2022, 14, 3465. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M. Recurrence of Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Famularo, S.; Di Sandro, S.; Giani, A.; Lauterio, A.; Sandini, M.; De Carlis, R.; Buscemi, V.; Uggeri, F.; Romano, F.; Gianotti, L.; et al. Recurrence Patterns After Anatomic or Parenchyma-Sparing Liver Resection for Hepatocarcinoma in a Western Population of Cirrhotic Patients. Ann. Surg. Oncol. 2018, 25, 3974–3981. [Google Scholar] [CrossRef]
- Komuta, M. Histological Heterogeneity of Primary Liver Cancers: Clinical Relevance, Diagnostic Pitfalls and the Pathologist’s Role. Cancers 2021, 13, 2871. [Google Scholar] [CrossRef] [PubMed]
- Sweed, D.; Sweed, E.; Moaz, I.; Mosbeh, A.; Fayed, Y.; Elhamed, S.M.A.; Sweed, E.; Macshut, M.; Abdelsattar, S.; Kilany, S.; et al. The Clinicopathological and Prognostic Factors of Hepatocellular Carcinoma: A 10-Year Tertiary Center Experience in Egypt. World J. Surg. Oncol. 2022, 20, 298. [Google Scholar] [CrossRef]
- Calderaro, J.; Couchy, G.; Imbeaud, S.; Amaddeo, G.; Letouzé, E.; Blanc, J.-F.; Laurent, C.; Hajji, Y.; Azoulay, D.; Bioulac-Sage, P.; et al. Histological Subtypes of Hepatocellular Carcinoma Are Related to Gene Mutations and Molecular Tumour Classification. J. Hepatol. 2017, 67, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Boyault, S.; Rickman, D.S.; de Reyniès, A.; Balabaud, C.; Rebouissou, S.; Jeannot, E.; Hérault, A.; Saric, J.; Belghiti, J.; Franco, D.; et al. Transcriptome Classification of HCC Is Related to Gene Alterations and to New Therapeutic Targets. Hepatology 2007, 45, 42–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampaki, M.; Papatheodoridis, G.V.; Cholongitas, E. Intrahepatic Recurrence of Hepatocellular Carcinoma after Resection: An Update. Clin. J. Gastroenterol. 2021, 14, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Bahra, M.; Schmelzle, M.; Öllinger, R.; Sucher, R.; Sauer, I.M.; Guel-Klein, S.; Struecker, B.; Eurich, D.; Klein, F.; et al. Predictive Factors for Extrahepatic Recurrence of Hepatocellular Carcinoma Following Liver Transplantation. Clin. Transplant. 2016, 30, 819–827. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-L.; Luo, Y.-Y.; Chen, M.; Zhou, Y.-P.; Lu, F.-R.; Deng, D.-F.; Wu, Y.-R. A Systematic Review and Meta-Analysis Comparing the Prognosis of Multicentric Occurrence and vs. Intrahepatic Metastasis in Patients with Recurrent Hepatocellular Carcinoma after Hepatectomy. HPB 2017, 19, 835–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carissimi, F.; Barbaglia, M.N.; Salmi, L.; Ciulli, C.; Roccamatisi, L.; Cordaro, G.; Mallela, V.R.; Minisini, R.; Leone, B.E.; Donadon, M.; et al. Finding the Seed of Recurrence: Hepatocellular Carcinoma Circulating Tumor Cells and Their Potential to Drive the Surgical Treatment. World J. Gastrointest. Surg. 2021, 13, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Marubashi, S.; Gotoh, K.; Akita, H.; Takahashi, H.; Sugimura, K.; Miyoshi, N.; Motoori, M.; Kishi, K.; Noura, S.; Fujiwara, Y.; et al. Analysis of Recurrence Patterns After Anatomical or Non-Anatomical Resection for Hepatocellular Carcinoma. Ann. Surg. Oncol. 2015, 22, 2243–2252. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kajino, K.; Kudo, M.; Sasaki, Y.; Arakawa, Y.; Hino, O. Determination of the Clonal Origin of Multiple Human Hepatocellular Carcinomas by Cloning and Polymerase Chain Reaction of the Integrated Hepatitis B Virus DNA. Hepatology 1999, 29, 1446–1452. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Juzi, J.T.; Sun, Y.; Zheng, H.; Cui, Y.; Li, H.; Hao, X. Clonality Analysis for Multicentric Origin and Intrahepatic Metastasis in Recurrent and Primary Hepatocellular Carcinoma. J. Gastrointest. Surg. 2008, 12, 1540–1547. [Google Scholar] [CrossRef]
- Sakon, M.; Nagano, H.; Nakamori, S.; Dono, K.; Umeshita, K.; Murakami, T.; Nakamura, H.; Monden, M. Intrahepatic Recurrences of Hepatocellular Carcinoma after Hepatectomy: Analysis Based on Tumor Hemodynamics. Arch. Surg. 2002, 137, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Sakon, M.; Nagano, H.; Shimizu, J.; Kondo, M.; Nakamori, S.; Dono, K.; Umeshita, K.; Nakamura, H.; Murakami, T.; Monden, M. Hepatic Resection of Hepatocellular Carcinomas Based on Tumor Hemodynamics. J. Surg. Oncol. 2000, 73, 179–181. [Google Scholar] [CrossRef]
- Famularo, S.; Piardi, T.; Molfino, S.; Di Martino, M.; Ferrari, C.; Ielpo, B.; Diago, M.V.; Giani, A.; Griseri, G.; Terés, L.B.; et al. Factors Affecting Local and Intra Hepatic Distant Recurrence After Surgery for Hcc: An Alternative Perspective on Microvascular Invasion and Satellitosis—A Western European Multicentre Study. J. Gastrointest. Surg. 2021, 25, 104–111. [Google Scholar] [CrossRef]
- Wang, H.-L.; Mo, D.-C.; Zhong, J.-H.; Ma, L.; Wu, F.-X.; Xiang, B.-D.; Li, L.-Q. Systematic Review of Treatment Strategy for Recurrent Hepatocellular Carcinoma: Salvage Liver Transplantation or Curative Locoregional Therapy. Medicine 2019, 98, e14498. [Google Scholar] [CrossRef]
- Tajima, T.; Yoshimitsu, K.; Irie, H.; Aibe, H.; Shinozaki, K.; Nishie, A.; Honda, H.; Shimada, M. Detecting Postsurgical Recurrent Hepatocellular Carcinoma with Multiphasic Helical Computed Tomography: Intrahepatic Metastasis or Multicentric Occurrence? J. Comput. Assist. Tomogr. 2005, 29, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, O.; Kojiro, M. Recurrence of Hepatocellular Carcinoma: Multicentric Occurrence or Intrahepatic Metastasis? A Viewpoint in Terms of Pathology. J. Hepato-Biliary-Pancreat. Surg. 2001, 8, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Cucchetti, A.; Piscaglia, F.; Caturelli, E.; Benvegnù, L.; Vivarelli, M.; Ercolani, G.; Cescon, M.; Ravaioli, M.; Grazi, G.L.; Bolondi, L.; et al. Comparison of Recurrence of Hepatocellular Carcinoma after Resection in Patients with Cirrhosis to Its Occurrence in a Surveilled Cirrhotic Population. Ann. Surg. Oncol. 2009, 16, 413–422. [Google Scholar] [CrossRef]
- Tabrizian, P.; Jibara, G.; Shrager, B.; Schwartz, M.; Roayaie, S. Recurrence of Hepatocellular Cancer after Resection: Patterns, Treatments, and Prognosis. Ann. Surg. 2015, 261, 947–955. [Google Scholar] [CrossRef]
- Ng, I.O.-L.; Guan, X.-Y.; Poon, R.T.-P.; Fan, S.-T.; Lee, J.M.-F. Determination of the Molecular Relationship between Multiple Tumour Nodules in Hepatocellular Carcinoma Differentiates Multicentric Origin from Intrahepatic Metastasis. J. Pathol. 2003, 199, 345–353. [Google Scholar] [CrossRef]
- Niu, Z.-S.; Niu, X.-J.; Wang, W.-H. Genetic Alterations in Hepatocellular Carcinoma: An Update. World J. Gastroenterol. 2016, 22, 9069–9095. [Google Scholar] [CrossRef]
- Furuta, M.; Ueno, M.; Fujimoto, A.; Hayami, S.; Yasukawa, S.; Kojima, F.; Arihiro, K.; Kawakami, Y.; Wardell, C.P.; Shiraishi, Y.; et al. Whole Genome Sequencing Discriminates Hepatocellular Carcinoma with Intrahepatic Metastasis from Multi-Centric Tumors. J. Hepatol. 2017, 66, 363–373. [Google Scholar] [CrossRef]
- Fujiki, M.; Aucejo, F.; Kim, R. Adjuvant Treatment of Hepatocellular Carcinoma after Orthotopic Liver Transplantation: Do We Really Need This? Clin. Transplant. 2013, 27, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.-Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.-H.; Tak, W.Y.; et al. Adjuvant Sorafenib for Hepatocellular Carcinoma after Resection or Ablation (STORM): A Phase 3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef]
- Han, B.; Ding, H.; Zhao, S.; Zhang, Y.; Wang, J.; Zhang, Y.; Gu, J. Potential Role of Adjuvant Lenvatinib in Improving Disease-Free Survival for Patients With High-Risk Hepatitis B Virus-Related Hepatocellular Carcinoma Following Liver Transplantation: A Retrospective, Case Control Study. Front. Oncol. 2020, 10, 562103. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, E.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Arihara, F.; Kagaya, T.; Yamashita, T.; Fushimi, K.; Kaneko, S. Enhancement of Tumor-Associated Antigen-Specific T Cell Responses by Radiofrequency Ablation of Hepatocellular Carcinoma. Hepatology 2013, 57, 1448–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in Combination with Ablation in Patients with Advanced Hepatocellular Carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, M. Adjuvant Immunotherapy after Curative Treatment for Hepatocellular Carcinoma. Lichenologist 2021, 10, 399–403. [Google Scholar] [CrossRef]
- Harding, J.J.; Nandakumar, S.; Armenia, J.; Khalil, D.N.; Albano, M.; Ly, M.; Shia, J.; Hechtman, J.F.; Kundra, R.; El Dika, I.; et al. Prospective Genotyping of Hepatocellular Carcinoma: Clinical Implications of Next-Generation Sequencing for Matching Patients to Targeted and Immune Therapies. Clin. Cancer Res. 2019, 25, 2116–2126. [Google Scholar] [CrossRef] [Green Version]
- Morita, M.; Nishida, N.; Sakai, K.; Aoki, T.; Chishina, H.; Takita, M.; Ida, H.; Hagiwara, S.; Minami, Y.; Ueshima, K.; et al. Immunological Microenvironment Predicts the Survival of the Patients with Hepatocellular Carcinoma Treated with Anti-PD-1 Antibody. Liver Cancer 2021, 10, 380–393. [Google Scholar] [CrossRef]
- Sun, J.J.; Wang, K.; Zhang, C.Z.; Guo, W.X.; Shi, J.; Cong, W.M.; Wu, M.C.; Lau, W.Y.; Cheng, S.Q. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who Have Hepatocellular Carcinoma with Microvascular Invasion. Ann. Surg. Oncol. 2016, 23, 1344–1351. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Yao, X.; Rong, W.; Wu, F.; Chen, B.; Liu, M.; Lin, S.; Liu, Y.; Wu, J. Postoperative Adjuvant Radiotherapy Is Associated with Improved Survival in Hepatocellular Carcinoma with Microvascular Invasion. Oncotarget 2017, 8, 79971–79981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.-H.; Zhang, X.-P.; Zhou, T.-F.; Wang, K.; Wang, H.; Chai, Z.-T.; Shi, J.; Guo, W.-X.; Cheng, S.-Q. Adjuvant Transarterial Chemoembolization Improves Survival Outcomes in Hepatocellular Carcinoma with Microvascular Invasion: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2019, 45, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Nagasue, N.; Yukaya, H.; Ogawa, Y.; Sasaki, Y.; Chang, Y.C.; Niimi, K. Second Hepatic Resection for Recurrent Hepatocellular Carcinoma. Br. J. Surg. 1986, 73, 434–438. [Google Scholar] [CrossRef]
- Chan, D.L.; Morris, D.L.; Chua, T.C. Clinical Efficacy and Predictors of Outcomes of Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma—A Systematic Review. Surg. Oncol. 2013, 22, e23–e30. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific Clinical Practice Guidelines on the Management of Hepatocellular Carcinoma: A 2017 Update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [Green Version]
- Vitale, A.; Farinati, F.; Noaro, G.; Burra, P.; Pawlik, T.M.; Bucci, L.; Giannini, E.G.; Faggiano, C.; Ciccarese, F.; Rapaccini, G.L.; et al. Restaging Patients With Hepatocellular Carcinoma Before Additional Treatment Decisions: A Multicenter Cohort Study. Hepatology 2018, 68, 1232–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Child, C.G.; Turcotte, J.G. Surgery and Portal Hypertension. Major Probl. Clin. Surg. 1964, 1, 1–85. [Google Scholar] [PubMed]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the Oesophagus for Bleeding Oesophageal Varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Donadon, M.; Costa, G.; Cimino, M.; Procopio, F.; Fabbro, D.D.; Palmisano, A.; Torzilli, G. Safe Hepatectomy Selection Criteria for Hepatocellular Carcinoma Patients: A Validation of 336 Consecutive Hepatectomies. The BILCHE Score. World J. Surg. 2015, 39, 237–243. [Google Scholar] [CrossRef]
- Makuuchi, M.; Kosuge, T.; Takayama, T.; Yamazaki, S.; Kakazu, T.; Miyagawa, S.; Kawasaki, S. Surgery for Small Liver Cancers. Semin. Surg. Oncol. 1993, 9, 298–304. [Google Scholar] [CrossRef]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC Guidelines) 2019 Update. Hepatol. Res. 2019, 49, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Abraldes, J.G.; Berzigotti, A.; García-Pagan, J.C. The Clinical Use of HVPG Measurements in Chronic Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 573–582. [Google Scholar] [CrossRef]
- Shubert, C.R.; Habermann, E.B.; Truty, M.J.; Thomsen, K.M.; Kendrick, M.L.; Nagorney, D.M. Defining Perioperative Risk after Hepatectomy Based on Diagnosis and Extent of Resection. J. Gastrointest. Surg. 2014, 18, 1917–1928. [Google Scholar] [CrossRef]
- Roayaie, S.; Bassi, D.; Tarchi, P.; Labow, D.; Schwartz, M. Second Hepatic Resection for Recurrent Hepatocellular Cancer: A Western Experience. J. Hepatol. 2011, 55, 346–350. [Google Scholar] [CrossRef]
- Notake, T.; Kobayashi, A.; Shinkawa, H.; Kawahara, T.; Shimizu, A.; Yokoyama, T.; Hasegawa, K.; Kokudo, N.; Matsuyama, Y.; Makuuchi, M.; et al. Nomogram Predicting Long-Term Survival after the Diagnosis of Intrahepatic Recurrence of Hepatocellular Carcinoma Following an Initial Liver Resection. Int. J. Clin. Oncol. 2017, 22, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-M.; Lee, P.-H.; Shau, W.-Y.; Ho, M.-C.; Wu, Y.-M.; Hu, R.-H. Survival in Patients with Recurrent Hepatocellular Carcinoma after Primary Hepatectomy: Comparative Effectiveness of Treatment Modalities. Surgery 2012, 151, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sui, C.; Li, B.; Yin, Z.; Tan, Y.; Yang, J.; Liu, Z. Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma: A Local Experience and a Systematic Review. World J. Surg. Oncol. 2010, 8, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagano, Y.; Shimada, H.; Ueda, M.; Matsuo, K.; Tanaka, K.; Endo, I.; Kunisaki, C.; Togo, S. Efficacy of Repeat Hepatic Resection for Recurrent Hepatocellular Carcinomas. ANZ J. Surg. 2009, 79, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-H.; Chen, M.-S.; Peng, Z.-W.; Zhang, Y.-J.; Zhang, Y.-Q.; Li, J.-Q.; Lau, W.Y. Percutaneous Radiofrequency Ablation versus Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma: A Retrospective Study. Ann. Surg. Oncol. 2008, 15, 3484–3493. [Google Scholar] [CrossRef]
- Shimada, K.; Sakamoto, Y.; Esaki, M.; Kosuge, T.; Morizane, C.; Ikeda, M.; Ueno, H.; Okusaka, T.; Arai, Y.; Takayasu, K. Analysis of Prognostic Factors Affecting Survival after Initial Recurrence and Treatment Efficacy for Recurrence in Patients Undergoing Potentially Curative Hepatectomy for Hepatocellular Carcinoma. Ann. Surg. Oncol. 2007, 14, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-C.; Tang, Z.-Y.; Ma, Z.-C.; Qin, L.-X.; Wang, L.; Ye, Q.-H.; Fan, J.; Wu, Z.-Q.; Zhou, X.-D. The Prognostic Factor for Outcome Following Second Resection for Intrahepatic Recurrence of Hepatocellular Carcinoma with a Hepatitis B Virus Infection Background. J. Cancer Res. Clin. Oncol. 2005, 131, 284–288. [Google Scholar] [CrossRef]
- Minagawa, M.; Makuuchi, M.; Takayama, T.; Kokudo, N. Selection Criteria for Repeat Hepatectomy in Patients with Recurrent Hepatocellular Carcinoma. Ann. Surg. 2003, 238, 703–710. [Google Scholar] [CrossRef]
- Matsuda, M.; Fujii, H.; Kono, H.; Matsumoto, Y. Surgical Treatment of Recurrent Hepatocellular Carcinoma Based on the Mode of Recurrence: Repeat Hepatic Resection or Ablation Are Good Choices for Patients with Recurrent Multicentric Cancer. J. Hepato-Biliary-Pancreat. Surg. 2001, 8, 353–359. [Google Scholar] [CrossRef]
- Nakajima, Y.; Ko, S.; Kanamura, T.; Nagao, M.; Kanehiro, H.; Hisanaga, M.; Aomatsu, Y.; Ikeda, N.; Nakano, H. Repeat Liver Resection for Hepatocellular Carcinoma. J. Am. Coll. Surg. 2001, 192, 339–344. [Google Scholar] [CrossRef]
- Sugimachi, K.; Maehara, S.; Tanaka, S.; Shimada, M.; Sugimachi, K. Repeat Hepatectomy Is the Most Useful Treatment for Recurrent Hepatocellular Carcinoma. J. Hepato-Biliary-Pancreat. Surg. 2001, 8, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Kawasaki, S.; Miyagawa, S.-I.; Miwa, S.; Noike, T.; Takagi, S.; Iijima, S.; Miyagawa, Y. Results of 404 Hepatic Resections Including 80 Repeat Hepatectomies for Hepatocellular Carcinoma. Hepatogastroenterology 2006, 53, 736–741. [Google Scholar]
- Itamoto, T.; Nakahara, H.; Amano, H.; Kohashi, T.; Ohdan, H.; Tashiro, H.; Asahara, T. Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma. Surgery 2007, 141, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Tralhão, J.G.; Dagher, I.; Lino, T.; Roudié, J.; Franco, D. Treatment of Tumour Recurrence after Resection of Hepatocellular Carcinoma. Analysis of 97 Consecutive Patients. Eur. J. Surg. Oncol. 2007, 33, 746–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, S.; Takemura, S.; Uenishi, T.; Yamamoto, T.; Ohba, K.; Ogawa, M.; Hai, S.; Ichikawa, T.; Kodai, S.; Shinkawa, H.; et al. Second Hepatic Resection for Recurrent Hepatocellular Carcinoma in Patients with Chronic Hepatitis C. World J. Surg. 2008, 32, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Sasaki, A.; Kai, S.; Endo, Y.; Iwaki, K.; Uchida, H.; Shibata, K.; Ohta, M.; Kitano, S. Prognosis of Patients with Intrahepatic Recurrence after Hepatic Resection for Hepatocellular Carcinoma: A Retrospective Study. Eur. J. Surg. Oncol. 2009, 35, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Cheng, S.-B.; Yeh, D.-C.; Wang, J.; P’eng, F.-K. Second and Third Hepatectomies for Recurrent Hepatocellular Carcinoma Are Justified. Br. J. Surg. 2009, 96, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, E.; Yamashita, Y.-I.; Takeishi, K.; Matsuyama, A.; Tsutsui, S.-I.; Matsuda, H.; Toshima, T.; Taketomi, A.; Shirabe, K.; Ishida, T.; et al. Poor Prognostic Factors after Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma in the Modern Era. Am. Surg. 2012, 78, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Faber, W.; Seehofer, D.; Neuhaus, P.; Stockmann, M.; Denecke, T.; Kalmuk, S.; Warnick, P.; Bahra, M. Repeated Liver Resection for Recurrent Hepatocellular Carcinoma. J. Gastroenterol. Hepatol. 2011, 26, 1189–1194. [Google Scholar] [CrossRef]
- Umeda, Y.; Matsuda, H.; Sadamori, H.; Matsukawa, H.; Yagi, T.; Fujiwara, T. A Prognostic Model and Treatment Strategy for Intrahepatic Recurrence of Hepatocellular Carcinoma after Curative Resection. World J. Surg. 2011, 35, 170–177. [Google Scholar] [CrossRef]
- Chok, K.S.H.; Chan, S.C.; Poon, R.T.P.; Fan, S.T.; Lo, C.M. Re-Resection for Metachronous Primary Hepatocellular Carcinoma: Is It Justified? ANZ J. Surg. 2012, 82, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Yoh, T.; Seo, S.; Taura, K.; Iguchi, K.; Ogiso, S.; Fukumitsu, K.; Ishii, T.; Kaido, T.; Uemoto, S. Surgery for Recurrent Hepatocellular Carcinoma: Achieving Long-Term Survival. Ann. Surg. 2021, 273, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Chan, S.L.; Galle, P.R.; Rimassa, L.; Sangro, B. Systemic Treatment of Hepatocellular Carcinoma: An EASL Position Paper. J. Hepatol. 2021, 75, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Han, H.-S.; Yoon, Y.-S.; Cho, J.Y.; Kim, J.H. Laparoscopic Liver Resection in Patients with a History of Upper Abdominal Surgery. World J. Surg. 2011, 35, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Shelat, V.G.; Serin, K.; Samim, M.; Besselink, M.G.; Al Saati, H.; Di Gioia, P.; Pearce, N.W.; Abu Hilal, M. Outcomes of Repeat Laparoscopic Liver Resection Compared to the Primary Resection. World J. Surg. 2014, 38, 3175–3180. [Google Scholar] [CrossRef]
- Machairas, N.; Papaconstantinou, D.; Stamopoulos, P.; Prodromidou, A.; Garoufalia, Z.; Spartalis, E.; Kostakis, I.D.; Sotiropoulos, G.C. The Emerging Role of Laparoscopic Liver Resection in the Treatment of Recurrent Hepatocellular Carcinoma: A Systematic Review. Anticancer Res. 2018, 38, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Ciria, R.; Cherqui, D.; Geller, D.A.; Briceno, J.; Wakabayashi, G. Comparative Short-Term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann. Surg. 2016, 263, 761–777. [Google Scholar] [CrossRef]
- Belli, G.; Cioffi, L.; Fantini, C.; D’Agostino, A.; Russo, G.; Limongelli, P.; Belli, A. Laparoscopic Redo Surgery for Recurrent Hepatocellular Carcinoma in Cirrhotic Patients: Feasibility, Safety, and Results. Surg. Endosc. 2009, 23, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.K.P.; Teo, J.-Y.; Chan, C.-Y.; Lee, S.-Y.; Cheow, P.-C.; Chung, A.Y.F. Laparoscopic Repeat Liver Resection for Recurrent Hepatocellular Carcinoma. ANZ J. Surg. 2017, 87, E143–E146. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Z.-G.; Huang, Z.-X.; Yang, K.-L.; Chen, J.-C.; Chen, J.-B.; Xu, L.; Chen, M.-S.; Zhang, Y.-J. Prospective, Single-Center Cohort Study Analyzing the Efficacy of Complete Laparoscopic Resection on Recurrent Hepatocellular Carcinoma. Chin. J. Cancer 2016, 35, 25. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Lau, A.C.-H.; Chang, S.K.Y. Single-Incision Laparoscopic Hepatic Resection in Patients with Previous Hepatic Resections: A Mini Case Series. Asian J. Endosc. Surg. 2014, 7, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.C.Y.; Poon, R.T.P.; Chok, K.S.H.; Cheung, T.T.; Chan, S.C.; Lo, C.M. Feasibility of Laparoscopic Re-Resection for Patients with Recurrent Hepatocellular Carcinoma. World J. Surg. 2014, 38, 1141–1146. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.; Wu, X.; Huang, Z.; Lin, Z.; Jiang, J.; Tan, W.; Zhang, L. Laparoscopic Liver Re-Resection Is Feasible for Patients with Posthepatectomy Hepatocellular Carcinoma Recurrence: A Propensity Score Matching Study. Surg. Endosc. 2017, 31, 4790–4798. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, G.; Procopio, F.; Cimino, M.; Del Fabbro, D.; Palmisano, A.; Donadon, M.; Montorsi, M. Anatomical Segmental and Subsegmental Resection of the Liver for Hepatocellular Carcinoma: A New Approach by Means of Ultrasound-Guided Vessel Compression. Ann. Surg. 2010, 251, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Procopio, F.; Mimmo, A.; Donadon, M.; Terrone, A.; Cimino, M.; Fabbro, D.D.; Torzilli, G. Oncologic Superiority of Anatomic Resection of Hepatocellular Carcinoma by Ultrasound-Guided Compression of the Portal Tributaries Compared with Nonanatomic Resection: An Analysis of Patients Matched for Tumor Characteristics and Liver Function. Surgery 2018, 164, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Shindoh, J.; Makuuchi, M.; Matsuyama, Y.; Mise, Y.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; Kokudo, N. Complete Removal of the Tumor-Bearing Portal Territory Decreases Local Tumor Recurrence and Improves Disease-Specific Survival of Patients with Hepatocellular Carcinoma. J. Hepatol. 2016, 64, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, G.; Montorsi, M.; Donadon, M.; Palmisano, A.; Del Fabbro, D.; Gambetti, A.; Olivari, N.; Makuuchi, M. “Radical but Conservative” Is the Main Goal for Ultrasonography-Guided Liver Resection: Prospective Validation of This Approach. J. Am. Coll. Surg. 2005, 201, 517. [Google Scholar] [CrossRef]
- Is R1 Vascular Hepatectomy for Hepatocellular Carcinoma Oncologically Adequate? Analysis of 327 Consecutive Patients. Surgery 2019, 165, 897–904. [CrossRef]
- Ohba, T.; Yano, T.; Yoshida, T.; Kawano, D.; Tsukamoto, S.; Shoji, F.; Taketomi, A.; Saitsu, H.; Takeo, S.; Maehara, Y. Results of a Surgical Resection of Pulmonary Metastasis from Hepatocellular Carcinoma: Prognostic Impact of the Preoperative Serum Alpha-Fetoprotein Level. Surg. Today 2012, 42, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.-H.; Xing, B.-C.; Zhang, W.-G.; Chan, A.W.-H.; Chong, C.C.N.; Serenari, M.; Peng, N.; Huang, T.; Lu, S.-D.; Liang, Z.-Y.; et al. Repeat Hepatic Resection versus Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma: Retrospective Multicentre Study. Br. J. Surg. 2021, 109, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular Carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sevilla, E.; Allard, M.-A.; Selten, J.; Golse, N.; Vibert, E.; Sa Cunha, A.; Cherqui, D.; Castaing, D.; Adam, R. Recurrence of Hepatocellular Carcinoma after Liver Transplantation: Is There a Place for Resection? Liver Transpl. 2017, 23, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Bodzin, A.S.; Lunsford, K.E.; Markovic, D.; Harlander-Locke, M.P.; Busuttil, R.W.; Agopian, V.G. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma After Liver Transplantation: Impact of Treatment Modality and Recurrence Characteristics. Ann. Surg. 2017, 266, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Toso, C.; Cader, S.; Mentha-Dugerdil, A.; Meeberg, G.; Majno, P.; Morard, I.; Giostra, E.; Berney, T.; Morel, P.; Mentha, G.; et al. Factors Predicting Survival after Post-Transplant Hepatocellular Carcinoma Recurrence. J. Hepato-Biliary-Pancreat. Sci. 2013, 20, 342–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belghiti, J.; Cortes, A.; Abdalla, E.K.; Régimbeau, J.-M.; Prakash, K.; Durand, F.; Sommacale, D.; Dondero, F.; Lesurtel, M.; Sauvanet, A.; et al. Resection prior to Liver Transplantation for Hepatocellular Carcinoma. Ann. Surg. 2003, 238, 885–892; discussion 892–893. [Google Scholar] [CrossRef] [PubMed]
- Tribillon, E.; Barbier, L.; Goumard, C.; Irtan, S.; Perdigao-Cotta, F.; Durand, F.; Paradis, V.; Belghiti, J.; Scatton, O.; Soubrane, O. When Should We Propose Liver Transplant After Resection of Hepatocellular Carcinoma? A Comparison of Salvage and De Principe Strategies. J. Gastrointest. Surg. 2016, 20, 66–76; discussion 76. [Google Scholar] [CrossRef]
- Adam, R.; Azoulay, D.; Castaing, D.; Eshkenazy, R.; Pascal, G.; Hashizume, K.; Samuel, D.; Bismuth, H. Liver Resection as a Bridge to Transplantation for Hepatocellular Carcinoma on Cirrhosis: A Reasonable Strategy? Ann. Surg. 2003, 238, 508–518; discussion 518–519. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Shinkawa, H.; Hasegawa, K.; Bhangui, P.; Salloum, C.; Gomez Gavara, C.; Lahat, E.; Omichi, K.; Arita, J.; Sakamoto, Y.; et al. Salvage Liver Transplantation or Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma: An Intent-to-Treat Analysis. Liver Transpl. 2017, 23, 1553–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.-M.; Jiang, W.-T.; Pan, C.; Deng, Y.-L.; Zheng, H.; Shen, Z.-Y. Milan Criteria, University of California, San Francisco, Criteria, and Model for End-Stage Liver Disease Score as Predictors of Salvage Liver Transplantation. Transplant. Proc. 2015, 47, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver Transplantation for Hepatocellular Carcinoma: Expansion of the Tumor Size Limits Does Not Adversely Impact Survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A Model to Predict Survival in Patients with End-Stage Liver Disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef] [PubMed]
- de Haas, R.J.; Lim, C.; Bhangui, P.; Salloum, C.; Compagnon, P.; Feray, C.; Calderaro, J.; Luciani, A.; Azoulay, D. Curative Salvage Liver Transplantation in Patients with Cirrhosis and Hepatocellular Carcinoma: An Intention-to-Treat Analysis. Hepatology 2018, 67, 204–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaido, T.; Uemoto, S. Does Living Donation Have Advantages over Deceased Donation in Liver Transplantation? J. Gastroenterol. Hepatol. 2010, 25, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-M.; Fan, S.T.; Liu, C.L.; Yong, B.H.; Wong, Y.; Lau, G.K.; Lai, C.L.; Ng, I.O.; Wong, J. Lessons Learned from One Hundred Right Lobe Living Donor Liver Transplants. Ann. Surg. 2004, 240, 151–158. [Google Scholar] [CrossRef]
- Lee, S.-G.; Song, G.-W.; Yoon, Y.-I. An Exceptional Series: 5000 Living Donor Liver Transplantations at Asan Medical Center, Seoul, Korea. Transplantation 2019, 103, 1739–1741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-M.; Shi, Y.-X.; Sun, L.-Y.; Zhu, Z.-J. Hepatocellular Carcinoma Recurrence in Living and Deceased Donor Liver Transplantation: A Systematic Review and Meta-Analysis. Chin. Med. J. 2019, 132, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Sapisochin, G.; Gorgen, A.; Vitale, A.; Halazun, K.J.; Iesari, S.; Schaefer, B.; Bhangui, P.; Mennini, G.; Wong, T.C.L.; et al. Evaluation of the Intention-to-Treat Benefit of Living Donation in Patients With Hepatocellular Carcinoma Awaiting a Liver Transplant. JAMA Surg. 2021, 156, e213112. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-M.; Fan, S.-T.; Liu, C.-L.; Chan, S.-C.; Wong, J. The Role and Limitation of Living Donor Liver Transplantation for Hepatocellular Carcinoma. Liver Transpl. 2004, 10, 440–447. [Google Scholar] [CrossRef]
- Mak, K.S.W.; Tan, K.C. Liver Transplantation for Hepatocellular Carcinoma: An Asian Perspective. Asian J. Surg. 2002, 25, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Fisher, R.A.; Kulik, L.M.; Freise, C.E.; Lok, A.S.F.; Shearon, T.H.; Brown, R.S., Jr.; Ghobrial, R.M.; Fair, J.H.; Olthoff, K.M.; Kam, I.; et al. Hepatocellular Carcinoma Recurrence and Death Following Living and Deceased Donor Liver Transplantation. Am. J. Transplant 2007, 7, 1601–1608. [Google Scholar] [CrossRef]
- Lai, Q.; Avolio, A.W.; Lerut, J.; Singh, G.; Chan, S.C.; Berloco, P.B.; Tisone, G.; Agnes, S.; Chok, K.S.; Sharr, W.; et al. Recurrence of Hepatocellular Cancer after Liver Transplantation: The Role of Primary Resection and Salvage Transplantation in East and West. J. Hepatol. 2012, 57, 974–979. [Google Scholar] [CrossRef]
- Sapisochin, G.; Goldaracena, N.; Astete, S.; Laurence, J.M.; Davidson, D.; Rafael, E.; Castells, L.; Sandroussi, C.; Bilbao, I.; Dopazo, C.; et al. Benefit of Treating Hepatocellular Carcinoma Recurrence after Liver Transplantation and Analysis of Prognostic Factors for Survival in a Large Euro-American Series. Ann. Surg. Oncol. 2015, 22, 2286–2294. [Google Scholar] [CrossRef]
- Poon, R.T.-P.; Fan, S.T.; Lo, C.M.; Liu, C.L.; Wong, J. Long-Term Survival and Pattern of Recurrence after Resection of Small Hepatocellular Carcinoma in Patients with Preserved Liver Function: Implications for a Strategy of Salvage Transplantation. Ann. Surg. 2002, 235, 373–382. [Google Scholar] [CrossRef]
- Cucchetti, A.; Cescon, M.; Bigonzi, E.; Piscaglia, F.; Golfieri, R.; Ercolani, G.; Cristina Morelli, M.; Ravaioli, M.; Daniele Pinna, A. Priority of Candidates with Hepatocellular Carcinoma Awaiting Liver Transplantation Can Be Reduced after Successful Bridge Therapy. Liver Transpl. 2011, 17, 1344–1354. [Google Scholar] [CrossRef]
- Vitale, A.; D’Amico, F.; Frigo, A.C.; Grigoletto, F.; Brolese, A.; Zanus, G.; Neri, D.; Carraro, A.; D’Amico, F.E.; Burra, P.; et al. Response to Therapy as a Criterion for Awarding Priority to Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation. Ann. Surg. Oncol. 2010, 17, 2290–2302. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.-I.; Yoshida, Y.; Kurihara, T.; Itoh, S.; Harimoto, N.; Ikegami, T.; Yoshizumi, T.; Uchiyama, H.; Shirabe, K.; Maehara, Y. Surgical Results for Recurrent Hepatocellular Carcinoma after Curative Hepatectomy: Repeat Hepatectomy versus Salvage Living Donor Liver Transplantation. Liver Transpl. 2015, 21, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.W.; Chok, K.S.H.; She, W.H.; Chan, A.C.Y.; Cheung, T.T.; Dai, W.C.; Fung, J.Y.Y.; Lo, C.M. Defining Optimal Surgical Treatment for Recurrent Hepatocellular Carcinoma: A Propensity Score Matched Analysis. Liver Transpl. 2018, 24, 1062–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.-Z.; Xiang, L.; Hu, Y.-K.; Yang, Y.; Zhu, H.-D.; Lu, C.-D. Options for the Treatment of Intrahepatic Recurrent Hepatocellular Carcinoma: Salvage Liver Transplantation or Rehepatectomy? Clin. Transplant. 2020, 34, e13831. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.-I.; Song, G.-W.; Lee, S.; Moon, D.; Hwang, S.; Kang, W.-H.; Cho, H.-D.; Ha, S.-M.; Kim, M.-J.; Kim, S.-H.; et al. Salvage Living Donor Liver Transplantation versus Repeat Liver Resection for Patients with Recurrent Hepatocellular Carcinoma and Child-Pugh Class A Liver Cirrhosis: A Propensity Score-Matched Comparison. Am. J. Transplant 2022, 22, 165–176. [Google Scholar] [CrossRef]
- Kostakis, I.D.; Machairas, N.; Prodromidou, A.; Stamopoulos, P.; Garoufalia, Z.; Fouzas, I.; Sotiropoulos, G.C. Comparison Between Salvage Liver Transplantation and Repeat Liver Resection for Recurrent Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Transplant. Proc. 2019, 51, 433–436. [Google Scholar] [CrossRef] [PubMed]

| First Author | N° of Cases | Extent of Resection | Survivals | |||||
|---|---|---|---|---|---|---|---|---|
| Minor Resection n (%) | Major Resection n (%) | Median DFS | Median OS | 1-Year Survival (%) | 3-Year Survival (%) | 5-Year Survival (%) | ||
| Matsuda ● (2001) [79] | 25 | NR | NR | NR | NR | 84 | 68 | 56 |
| Nakajima (2001) [80] | 12 | 11 (92) | 1 (8) | 9 | NR | 90 | 80 | 55 |
| Sugimachi (2001) [81] | 78 | NR | NR | NR | NR | NR | 83 | 48 |
| Minagawa (2003) [78] | 67 | 61 (91) | 6 (10) | 12 | NR | 93 | 70 | 56 |
| Sun (2005) [77] | 57 | NR | NR | NR | 26 | 70 | 61 | 31 |
| Kobayashi (2006) [82] | 60 | NR | NR | 18 | 56 | 97 | 74 | 53 |
| Itamoto (2007) [83] | 84 | 73 (87) | 11 (13) | 9 | 60 | 88 | 67 | 50 |
| Shimada α (2007) [76] | 13 | NR | NR | NR | 63 | 97 | 67 | 57 |
| Tralhão (2007) [84] | 16 | 13 (81) | 3 (19) | 27 | 34 | 89 | 46 | 31 |
| Kubo (2008) [85] | 51 | NR | NR | 15 | 55 | 94 | 77 | 52 |
| Liang (2008) [75] | 44 | 42 (98) | 1 (2) | NR | 30 | 79 | 45 | 28 |
| Kawano (2009) [86] | 13 | NR | NR | NR | 36 | 100 | 50 | 26 |
| Nagano (2009) [74] | 24 | NR | NR | NR | 62 | 92 | 73 | 51 |
| Wu (2009) [87] | 149 | 148 (99) | 1 (1) | 27 | Not reached | 91 | 78 | 56 |
| Tsujita (2010) [88] | 121 | NR | NR | NR | Not reached | 97 | 88 | 83 |
| Zhou (2010) [73] | 37 | 33 (89) | 4 (11) | NR | 50 | 95 | 70 | 44 |
| Faber (2011) [89] | 27 | 25 (89) | 3 (11) | 17 | 36 | 96 | 70 | 42 |
| Roayaie (2011) [70] | 35 | 29 (83) | 6 (17) | 32 | Not reached | 90 | 67 | 67 |
| Umeda (2011) [90] | 29 | NR | NR | NR | 66 | 93 | 67 | 56 |
| Chok (2012) [91] | 47 | 41 (87) | 6 (13) | 11 | 54 | 81 | 55 | 44 |
| Ho (2012) [72] | 54 | NR | NR | NR | Not reached | 96 | 84 | 72 |
| Huang (2012) [14] | 82 | NR | NR | 7 | 22 | 71 | 41 | 22 |
| Chan (2013) [60] | 1125 | 89 (81–99) | 11 (1–19) | 15 (7–32) | 52 (22–66) | 92 (70–100) | 69 (41–88) | 52 (22–83) |
| Tabrizian (2015) [44] | 44 | 44 (100) | 0 (0) | NR | 56 | NR | NR | 47 |
| Famularo (2021) [19] | 156 * | NR | NR | 57 | Not reached | 100 | 70.3 | 52.7 |
| Yoh (2021) [92] | 128° | 100 (90) | 11 (10) | NR | 71.7 | 91 | 66.9 | 55.1 |
| Median value ♦ (range) | 44 (12–156) | 89.5 (81–100) | 10.5 (0–19) | 16 (7–57) | 54 (22–71.7) | 92 (70–100) | 69 (41–88) | 52 (22–83) |
| First Author | N° of Cases | Treatment at Recurrence | Survivals | |||||
|---|---|---|---|---|---|---|---|---|
| RH | SLT | RFS/DFS (RH) | RFS/DFS (SLT) | OS (RH) | OS (SLT) | p Value (RFS/DFS, OS) | ||
| Yamashita (2015) [137] | 159 | 146 | 13 | 16% (5 y) | 81% (5 y) | 61% (5 y) | 75% (5 y) | 0.0002, 0.1714 |
| Lim (2017) [119] | 99 | 81 | 18 | 18% (5 y) | 72% (5 y) | 71% (5 y) | 71% (5 y) | <0.001, 0.99 |
| Ma (2018) α [138] | 144 | 108 | 36 | 32.8% (5 y) | 71.6% (5 y) | 48.3% (5 y) | 72.8% (5 y) | <0.001, 0.01 |
| Fang (2020) [139] | 124 | 78 | 46 | 16.0 (8.0–27.3) | 32.0 (12.8–45.0) | 23.0 (15.0–32.5) | 36.5 (20.3–45) | <0.01, <0.01 |
| Yoon (2021) α [140] | 84 | 42 | 42 | 27.9 (5 y) | 78% (5 y) | 62.2% (5 y) | 89.2 (5 y) | <0.001, <0.001 |
| Median value ♦ (range) | 124 (84–159) | 81 (42–146) | 36 (13–46) | 22.95 (16–32.8) | 75 (71.6–81) | 61.6 (48.3–71) | 73.9 (71–89.2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milana, F.; Polidoro, M.A.; Famularo, S.; Lleo, A.; Boldorini, R.; Donadon, M.; Torzilli, G. Surgical Strategies for Recurrent Hepatocellular Carcinoma after Resection: A Review of Current Evidence. Cancers 2023, 15, 508. https://doi.org/10.3390/cancers15020508
Milana F, Polidoro MA, Famularo S, Lleo A, Boldorini R, Donadon M, Torzilli G. Surgical Strategies for Recurrent Hepatocellular Carcinoma after Resection: A Review of Current Evidence. Cancers. 2023; 15(2):508. https://doi.org/10.3390/cancers15020508
Chicago/Turabian StyleMilana, Flavio, Michela Anna Polidoro, Simone Famularo, Ana Lleo, Renzo Boldorini, Matteo Donadon, and Guido Torzilli. 2023. "Surgical Strategies for Recurrent Hepatocellular Carcinoma after Resection: A Review of Current Evidence" Cancers 15, no. 2: 508. https://doi.org/10.3390/cancers15020508
APA StyleMilana, F., Polidoro, M. A., Famularo, S., Lleo, A., Boldorini, R., Donadon, M., & Torzilli, G. (2023). Surgical Strategies for Recurrent Hepatocellular Carcinoma after Resection: A Review of Current Evidence. Cancers, 15(2), 508. https://doi.org/10.3390/cancers15020508