Configuring Therapeutic Aspects of Immune Checkpoints in Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Significance of Immune Checkpoint Inhibitors
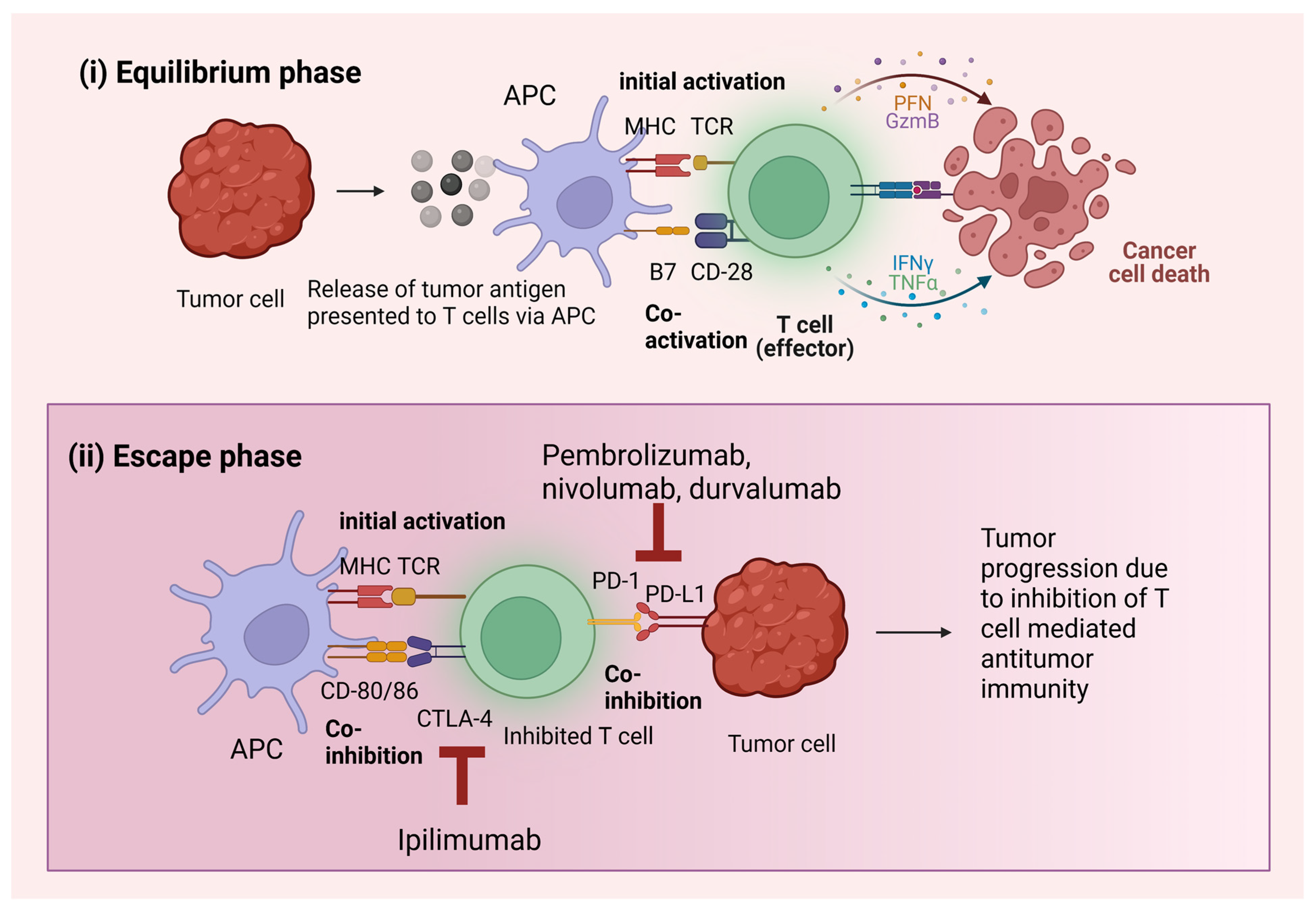
2.1. Immunoediting
2.2. Tumor Mutational Burden
3. Mechanism of Action of ICIs
3.1. PD-1 Pathway
3.2. CTLA-4 Pathway
3.3. Other Immune Checkpoints
3.3.1. Lymphocyte Activation Gene-3 (LAG-3)
3.3.2. V-Domain Ig Suppressor of T Cell Activation (VISTA)
3.3.3. Human Endogenous Retrovirus-H Long Terminal Repeat-Associating Protein 2 (HHLA2)
3.3.4. T Cell Immunoglobulin and Mucin-Containing Protein 3 (TIM-3)
3.3.5. T Cell Ig and Immunoreceptor Tyrosine-Based Inhibitory Motif Domain (TIGIT)
| Checkpoint Receptor | Checkpoint Ligand | Result of Interaction | Target ICI | References |
|---|---|---|---|---|
| PD-1 | PD-L1 | Repression of T cell-mediated tumor cell destruction by presenting tumor antigens via MHC | Anti-PD-1: pembrolizumab, nivolumab, cemiplimab Anti-PDL1: atezolizumab, avelumab, durvalumab | [93] |
| CTLA-4 | CD80, CD86 | Coinhibitory signals on T cells exert anti-tumor immune responses. | Ipilimumab | [94] |
| LAG-3 | MHC | Terminating CD-4 associated T cell activity. Induction of DC maturation | Relatlimab, LAG525, Eftilagimod | [95] |
| VISTA | Unknown binding partners | Dampening the activity of tumor-specific T cells and inducing cytokine secretion | HMBD-002, JNJ-61610588, CA-170 | [96] |
| HHLA2 | TIMGD2 | Co-stimulatory signal for T cell activation and cytokine release mediated by Akt dependent phosphorylation. | - | [97] |
| TIM-3 | Gal-9 | Apoptosis of T cells by inducing an influx of calcium into the cytoplasm | TSR-022 | [98] |
| TIGIT | CD155, CD112 | Inhibits the activation, proliferation, and signaling function of T cells Restricting the cytotoxic immune activity of NK cells | Etigilimab | [99] |
4. Role of ICIs in the Treatment of Lung Cancer
4.1. ICIs as Monotherapy
4.2. ICIs in Combination with Chemotherapy
4.3. ICIs in Combination with Radiotherapy
4.4. Combination of ICIs with Other Therapies
5. Clinical Trials of Various ICIs Targeting Immune Checkpoints
| Target Checkpoint | Trial No. | ICI Agent | Co-Administered with: | Phase of Trial | References |
|---|---|---|---|---|---|
| PD-1/PD-L1 | NCT01295827 (KEYNOTE-001) | Pembrolizumab | - | Phase 1 | [141] |
| NCT02259621 | Nivolumab | Carboplatin, Paclitaxel | Phase 2 | [148] | |
| NCT04944173 | Durvalumab | Stereotactic body radiotherapy | Phase 2 | [149] | |
| NCT04513925 (SKYSCRAPER-03) | Atezolizumab | Tiragolumab, Durvalumab | Phase 3 | [150] | |
| NCT02576574 (JAVELIN Lung 100) | Avelumab | Pemetrexed, Paclitaxel, Carboplatin, Gemcitabine | Phase 3 | [151] | |
| CTLA-4 | NCT02477826 (CheckMate 227) | Ipilimumab | Nivolumab, Pemetrexed, Paclitaxel, Carboplatin, Gemcitabine | Phase 3 | [152] |
| LAG-3 | NCT02465060 | Relatlimab | Nivolumab | Phase 2 | [153] |
| NCT02750514 | Relatlimab | Nivolumab | Phase 2 | [154] | |
| NCT03365791 | LAG525 400 mg | PDR001 | Phase 2 | [155] | |
| NCT02460224 | LAG525 0.3–10 mg/kg | PDR001 | Phase 1/2 | [156] | |
| NCT03625323 | Eftilagimod alpha 30 mg | Pembrolizumab | Phase 2 | [143] | |
| VISTA | NCT05082610 | HMBD-002 | Pembrolizumab | Phase 1 | [157] |
| NCT02671955 | JNJ-61610588 | - | Phase 1 | [158] | |
| NCT02812875 | CA-170 | - | Phase 1 | [159] | |
| TIM-3 | NCT03307785 | TSR-022 900 mg | Carboplatin + pemetrexed/nab-paclitaxel/paclitaxel | Phase 1 | [160] |
| NCT02817633 | TSR-022 | Nivolumab Docetaxel Cisplatin/carboplatin + pemetrexed | Phase 1 | [161] | |
| TIGIT | NCT05026606 | Etigilimab | Nivolumab | Phase 2 | |
| NCT03119428 | Etigilimab | Nivolumab | Phase 1 | [162] |
6. Limitations of ICI Therapy
6.1. Patients Harboring EGFR Mutation
6.2. Immune-Related Adverse Events (irAEs)
6.3. Coadministration of Steroids
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nath, A.; Sathishkumar, K.; Das, P.; Sudarshan, K.L.; Mathur, P. A clinicoepidemiological profile of lung cancers in India—Results from the National Cancer Registry Programme. Indian J. Med. Res. 2022, 155, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.H.; Kennedy, M.; Neal, R.D. Recognising lung cancer in primary care. Adv. Ther. 2019, 36, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Lung Cancer—Non-Small Cell: Statistics. Lancet 2022.
- De Ruysscher, D.; Wanders, R.; van Baardwijk, A.; Dingemans, A.-M.C.; Reymen, B.; Houben, R.; Bootsma, G.; Pitz, C.; van Eijsden, L.; Geraedts, W.; et al. Radical treatment of non–small-cell lung cancer patients with synchronous oligometastases: Long-term results of a prospective phase II trial (Nct01282450). J. Thorac. Oncol. 2012, 7, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Spiro, S.G.; Silvestri, G.A. One hundred years of lung cancer. Am. J. Respir. Crit. Care Med. 2005, 172, 523–529. [Google Scholar] [CrossRef]
- Waqar, S.N.; Morgensztern, D.J.P. Treatment advances in small cell lung cancer (SCLC). Pharmacol. Ther. 2017, 180, 16–23. [Google Scholar] [CrossRef]
- Khanna, P.; Blais, N.; Gaudreau, P.-O.; Corrales-Rodriguez, L. Immunotherapy comes of age in lung cancer. Clin. Lung Cancer 2017, 18, 13–22. [Google Scholar] [CrossRef]
- Shields, M.D.; Marin-Acevedo, J.A.; Pellini, B. Immunotherapy for advanced non–small cell lung cancer: A decade of progress. Clin. Oncol. Educ. Book 2021, 41, e105–e127. [Google Scholar] [CrossRef]
- Holt, G.E.; Podack, E.R.; Raez, L.E. Immunotherapy as a strategy for the treatment of non-small-cell lung cancer. Therapy 2011, 8, 43. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Kahnert, K.; Kauffmann-Guerrero, D.; Huber, R.M. SCLC–state of the art and what does the future have in store? Clin. Lung Cancer 2016, 17, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Saenger, Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist 2008, 13, 2–9. [Google Scholar] [CrossRef]
- Ishii, H.; Azuma, K.; Kawahara, A.; Yamada, K.; Imamura, Y.; Tokito, T.; Kinoshita, T.; Kage, M.; Hoshino, T. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J. Thorac. Oncol. 2015, 10, 426–430. [Google Scholar] [CrossRef]
- Long, L.; Zhao, C.; Ozarina, M.; Zhao, X.; Yang, J.; Chen, H. Targeting immune checkpoints in lung cancer: Current landscape and future prospects. Clin. Drug Investig. 2019, 39, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.S.; Baldwin, D.R. Recent advances in the management of lung cancer. Clin. Med. 2018, 18, s41–s46. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.; Jabbour, S.K.; Aisner, J. Current state of immunotherapy for non-small cell lung cancer. Transl. Lung Cancer Res. 2017, 6, 196–211. [Google Scholar] [CrossRef]
- Pluhar, G.E.; Pennell, C.A.; Olin, M.R. CD8⁺ T Cell-Independent Immune-Mediated Mechanisms of Anti-Tumor Activity. Crit. Rev. Immunol. 2015, 35, 153–172. [Google Scholar] [CrossRef]
- Kunimasa, K.; Goto, T. Immunosurveillance and immunoediting of lung cancer: Current perspectives and challenges. Int. J. Mol. Sci. 2020, 21, 597. [Google Scholar] [CrossRef]
- Desai, R.; Coxon, A.T.; Dunn, G.P. Therapeutic applications of the cancer immunoediting hypothesis. Semin. Cancer Biol. 2022, 78, 63–77. [Google Scholar] [CrossRef]
- Goto, T. Immunoediting and cancer priming. Cancer Immunol. Immunother. 2022, 5, 111–136. [Google Scholar]
- Barriga, V.; Kuol, N.; Nurgali, K.; Apostolopoulos, V. The Complex Interaction between the Tumor Micro-Environment and Immune Checkpoints in Breast Cancer. Cancers 2019, 11, 1205. [Google Scholar] [CrossRef]
- Ephraim, R.; Fraser, S.; Nurgali, K.; Apostolopoulos, V. Checkpoint Markers and Tumor Microenvironment: What Do We Know? Cancers 2022, 14, 3788. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Cui, J. Anti-angiogenesis: Opening a new window for immunotherapy. Life Sci. 2020, 258, 118163. [Google Scholar] [CrossRef]
- Nishii, K.; Ohashi, K.; Ninomiya, K.; Makimoto, G.; Watanabe, H.; Kano, H.; Hara, N.; Udono, H.; Kiura, K. Tumor immunoediting in a lung cancer mouse model harboring EGFR mutations. Cancer Res. 2019, 79, 582. [Google Scholar] [CrossRef]
- Chae, Y.K.; Chang, S.; Ko, T.; Anker, J.; Agte, S.; Iams, W.; Choi, W.M.; Lee, K.; Cruz, M. Epithelial-mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci. Rep. 2018, 8, 2918. [Google Scholar] [CrossRef]
- Terry, S.; Savagner, P.; Ortiz-Cuaran, S.; Mahjoubi, L.; Saintigny, P.; Thiery, J.P.; Chouaib, S. New insights into the role of EMT in tumor immune escape. Mol. Oncol. 2017, 11, 824–846. [Google Scholar] [CrossRef] [PubMed]
- Petty, A.J.; Yang, Y. Tumor-associated macrophages: Implications in cancer immunotherapy. Immunotherapy 2017, 9, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.; Fiander, M.; Tran, D.; Korytowsky, B.; Thomas, J.-M.; Calderon, F.; Zyczynski, T.M.; Brixner, D.; Stenehjem, D.D. Tumor mutational burden in lung cancer: A systematic literature review. Oncotarget 2019, 10, 6604. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Callahan, M.K.; Awad, M.M.; Calvo, E.; Ascierto, P.A.; Atmaca, A.; Rizvi, N.A.; Hirsch, F.R.; Selvaggi, G.; Szustakowski, J.D.; et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 2018, 33, 853–861.e854. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, N.; Adeni, A.; Hammerman, P.; Awad, M.; Gandhi, L.; Sholl, L. MA15.02 Non-Synonymous Mutation Burden in Lung Carcinoma is Associated with Durable Clinical Response to Immune Checkpoint Blockade. J. Thorac. Oncol. 2017, 12, S428–S429. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Ciuleanu, T.-E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Chamoto, K.; Al-Habsi, M.; Honjo, T. Role of PD-1 in immunity and diseases. Emerg. Concepts Target. Immune Checkp. Cancer Autoimmun. 2017, 410, 75–97. [Google Scholar]
- Sharma, P.; Allison, J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8+ T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancers 2020, 20, 218–232. [Google Scholar] [CrossRef]
- Sheppard, K.-A.; Fitz, L.J.; Lee, J.M.; Benander, C.; George, J.A.; Wooters, J.; Qiu, Y.; Jussif, J.M.; Carter, L.L.; Wood, C.R.; et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3ζ signalosome and downstream signaling to PKCθ. FEBS Lett. 2004, 574, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Honjo, T. PD-1 and PD-1 ligands: From discovery to clinical application. Int. Immunol. 2007, 19, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. PD-1/PD-L1 in disease. Immunotherapy 2018, 10, 149–160. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. The mechanisms tumor cells utilize to evade the host’s immune system. Maturitas 2017, 105, 8–15. [Google Scholar] [CrossRef]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Thommen, D.S.; Schumacher, T.N. T cell dysfunction in cancer. Cancer Cell 2018, 33, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I.; et al. T cell costimulatory receptor CD28 is a primary target for PD-1–mediated inhibition. Science 2017, 355, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Frontera, O.A.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Jure-Kunkel, M.; Masters, G.; Girit, E.; Dito, G.; Lee, F. Antitumor activity of anti-CTLA-4 monoclonal antibody (mAb) in combination with ixabepilone in preclinical tumor models. J. Clin. Oncol. 2008, 26, 3048. [Google Scholar] [CrossRef]
- Lee, K.-M.; Chuang, E.; Griffin, M.; Khattri, R.; Hong, D.K.; Zhang, W.; Straus, D.; Samelson, L.E.; Thompson, C.B.; Bluestone, J.A.; et al. Molecular basis of T cell inactivation by CTLA-4. Science 1998, 282, 2263–2266. [Google Scholar] [CrossRef] [PubMed]
- Kalbasi, A.; Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef]
- Sansom, D. CD28, CTLA-4 and their ligands: Who does what and to whom? Immunology 2000, 101, 169. [Google Scholar] [CrossRef] [PubMed]
- Brunner-Weinzierl, M.C.; Rudd, C.E. CTLA-4 and PD-1 control of T-cell motility and migration: Implications for tumor immunotherapy. Front. Immunol. 2018, 9, 2737. [Google Scholar] [CrossRef]
- Kolar, P.; Knieke, K.; Hegel, J.K.E.; Quandt, D.; Burmester, G.R.; Hoff, H.; Brunner-Weinzierl, M.C. CTLA-4 (CD152) controls homeostasis and suppressive capacity of regulatory T cells in mice. Arthritis Rheum. 2009, 60, 123–132. [Google Scholar] [CrossRef]
- Martin, M.; Schneider, H.; Azouz, A.; Rudd, C.E. Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function. J. Exp. Med. 2001, 194, 1675–1682. [Google Scholar] [CrossRef]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. The Journal of the American Society of Hematology. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Malas, S.; Harrasser, M.; Lacy, K.E.; Karagiannis, S.N. Antibody therapies for melanoma: New and emerging opportunities to activate immunity. Oncol. Rep. 2014, 32, 875–886. [Google Scholar] [CrossRef]
- Torphy, R.J.; Schulick, R.D.; Zhu, Y. Newly emerging immune checkpoints: Promises for future cancer therapy. Int. J. Mol. Sci. 2017, 18, 2642. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.J.; Dugger, K.J.; Vignali, D.A. Cutting edge: Molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J. Immunol. 2002, 169, 5392–5395. [Google Scholar] [CrossRef]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef]
- Durham, N.M.; Nirschl, C.J.; Jackson, C.M.; Elias, J.; Kochel, C.M.; Anders, R.A.; Drake, C.G. Lymphocyte Activation Gene 3 (LAG-3) modulates the ability of CD4 T-cells to be suppressed in vivo. PLoS ONE 2014, 9, e109080. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.R.; Peinado, P.; Fuentes-Antrás, J.; Pérez-Segura, P.; Pandiella, A.; Amir, E.; Ocaña, A. Prognostic value of lymphocyte-activation gene 3 (LAG3) in cancer: A meta-analysis. Front. Immunol. 2019, 9, 1040. [Google Scholar] [CrossRef]
- Woo, S.-R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-cell Function to Promote Tumoral Immune EscapeLAG-3 and PD-1 Synergistically Prevent Antitumor Immunity. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, H.; Rozeboom, L.; Rivard, C.J.; Ellison, K.; Dziadziuszko, R.; Suda, K.; Ren, S.; Wu, C.; Hou, L.J.; et al. LAG-3 protein expression in non–small cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J. Thorac. Oncol. 2017, 12, 814–823. [Google Scholar] [CrossRef]
- Hahn, A.W.; Gill, D.M.; Pal, S.K.; Agarwal, N. The future of immune checkpoint cancer therapy after PD-1 and CTLA-4. Immunotherapy 2017, 9, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, Q.; Keyaerts, M.; Devoogdt, N.; Breckpot, K. The next-generation immune checkpoint LAG-3 and its therapeutic potential in oncology: Third time’sa charm. Int. J. Mol. Sci. 2020, 22, 75. [Google Scholar] [CrossRef] [PubMed]
- Lines, J.L.; Pantazi, E.; Mak, J.; Sempere, L.F.; Wang, L.; O’Connell, S.; Ceeraz, S.; Suriawinata, A.A.; Yan, S.; Ernstoff, M.S.; et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014, 74, 1924–1932. [Google Scholar] [CrossRef]
- ElTanbouly, M.A.; Zhao, Y.; Nowak, E.; Li, J.; Schaafsma, E.; Le Mercier, I.; Ceeraz, S.; Lines, J.L.; Peng, C.; Carriere, C.; et al. VISTA is a checkpoint regulator for naïve T cell quiescence and peripheral tolerance. Science 2020, 367, eaay0524. [Google Scholar] [CrossRef]
- Flies, D.B.; Han, X.; Higuchi, T.; Zheng, L.; Sun, J.; Ye, J.J.; Chen, L. Coinhibitory receptor PD-1H preferentially suppresses CD4+ T cell–mediated immunity. J. Clin. Investig. 2014, 124, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ward, J.F.; Pettaway, C.A.; Shi, L.Z.; Subudhi, S.K.; Vence, L.M.; Zhao, H.; Chen, J.; Chen, H.; Efstathiou, E.; et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017, 23, 551–555. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, X.; Li, E.; Zhang, G.; Wang, X.; Tang, T.; Bai, X.; Liang, T. VISTA: An immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Villarroel-Espindola, F.; Yu, X.; Datar, I.; Mani, N.; Sanmamed, M.; Velcheti, V.; Syrigos, K.; Toki, M.; Zhao, H.; Chen, L.; et al. Spatially Resolved and Quantitative Analysis of VISTA/PD-1H as a Novel Immunotherapy Target in Human Non–Small Cell Lung CancerRole of VISTA/PD-1H in NSCLC. Clin. Cancer Res. 2018, 24, 1562–1573. [Google Scholar] [CrossRef]
- Zhao, R.; Chinai, J.M.; Buhl, S.; Scandiuzzi, L.; Ray, A.; Jeon, H.; Ohaegbulam, K.C.; Ghosh, K.; Zhao, A.; Scharff, M.D.; et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc. Natl. Acad. Sci. USA 2013, 110, 9879–9884. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yao, S.; Iliopoulou, B.P.; Han, X.; Augustine, M.M.; Xu, H.; Phennicie, R.T.; Flies, S.J.; Broadwater, M.; Ruff, W.; et al. B7-H5 costimulates human T cells via CD28H. Nat. Commun. 2013, 4, 2043. [Google Scholar] [CrossRef]
- Janakiram, M.; Chinai, J.M.; Fineberg, S.; Fiser, A.; Montagna, C.; Medavarapu, R.; Castano, E.; Jeon, H.; Ohaegbulam, K.C.; Zhao, R.; et al. Expression, Clinical Significance, and Receptor Identification of the Newest B7 Family Member HHLA2 ProteinHHLA Is the Newest Immune Checkpoint in Human Cancers. Clin. Cancer Res. 2015, 21, 2359–2366. [Google Scholar] [CrossRef]
- Sun, W.; Li, S.; Tang, G.; Sun, S.; Luo, Y.; Bai, R.; Han, L.; Jiang, X.; Gao, Y.; Huang, Z.; et al. HHLA2 deficiency inhibits non-small cell lung cancer progression and THP-1 macrophage M2 polarization. Cancer Med. 2021, 10, 5256–5269. [Google Scholar] [CrossRef]
- Cheng, H.; Janakiram, M.; Borczuk, A.; Lin, J.; Qiu, W.; Liu, H.; Chinai, J.M.; Halmos, B.; Perez-Soler, R.; Zang, X. HHLA2, a New Immune Checkpoint Member of the B7 Family, Is Widely Expressed in Human Lung Cancer and Associated with EGFR Mutational StatusHHLA2 in Lung Cancer. Clin. Cancer Res. 2017, 23, 825–832. [Google Scholar] [CrossRef]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, Y.; Li, G.; Huang, H.; Zhang, G.; Wang, F.; Sun, J.; Yang, Q.; Zhang, X.; Lu, B. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS ONE 2012, 7, e30676. [Google Scholar] [CrossRef]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Commun. 2005, 6, 1245–1252. [Google Scholar] [CrossRef]
- Rangachari, M.; Zhu, C.; Sakuishi, K.; Xiao, S.; Karman, J.; Chen, A.; Angin, M.; Wakeham, A.; Greenfield, E.A.; Sobel, R.A.; et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3–mediated cell death and exhaustion. Nat. Med. 2012, 18, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, J.; Sun, Z.; Benallaoua, M.; Guillaume, P.; Luescher, I.F.; Sander, C.; Kirkwood, J.M.; Kuchroo, V.; Zarour, H.M. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen–specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 2010, 207, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef]
- Levin, S.D.; Taft, D.W.; Brandt, C.S.; Bucher, C.; Howard, E.D.; Chadwick, E.M.; Johnston, J.; Hammond, A.; Bontadelli, K.; Ardourel, D.; et al. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur. J. Immunol. 2011, 41, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Bottino, C.; Castriconi, R.; Pende, D.; Rivera, P.; Nanni, M.; Carnemolla, B.; Cantoni, C.; Grassi, J.; Marcenaro, S.; Reymond, N.; et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J. Exp. Med. 2003, 198, 557–567. [Google Scholar] [CrossRef]
- Joller, N.; Hafler, J.P.; Brynedal, B.; Kassam, N.; Spoerl, S.; Levin, S.D.; Sharpe, A.H.; Kuchroo, V.K. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J. Immunol. 2011, 186, 1338–1342. [Google Scholar] [CrossRef] [PubMed]
- Stanietsky, N.; Simic, H.; Arapovic, J.; Toporik, A.; Levy, O.; Novik, A.; Levine, Z.; Beiman, M.; Dassa, L.; Achdout, H.; et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 17858–17863. [Google Scholar] [CrossRef]
- Yu, X.; Harden, K.C.; Gonzalez, L.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8+ T cell effector function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef]
- Taguchi, K.; Onoe, T.; Yoshida, T.; Yamashita, Y.; Tanaka, Y.; Ohdan, H. Tumor Endothelial Cell–Mediated Antigen-Specific T-cell Suppression via the PD-1/PD-L1 PathwayTECs Suppress Antitumor T-cell Responses. Mol. Cancer Res. 2020, 18, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Neeve, S.C.; Robinson, B.W.; Fear, V.S. The role and therapeutic implications of T cells in cancer of the lung. Clin. Transl. Immunol. 2019, 8, e1076. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mao, K.; Germain, R.N. Thinking differently about ILC s—Not just tissue resident and not just the same as CD 4+ T-cell effectors. Immunol. Rev. 2018, 286, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Franco, F.; Jaccard, A.; Romero, P.; Yu, Y.-R.; Ho, P.-C. Metabolic and epigenetic regulation of T-cell exhaustion. Nat. Metab. 2020, 2, 1001–1012. [Google Scholar] [CrossRef]
- Shaaban, M.; Othman, H.; Ibrahim, T.; Ali, M.; Abdelmoaty, M.; Abdel-Kawi, A.-R.; Mostafa, A.; El Nakeeb, A.; Emam, H.; Refaat, A. Immune Checkpoint Regulators: A New Era Toward Promising Cancer Therapy. Curr. Cancer Drug Targets 2020, 20, 429–460. [Google Scholar] [CrossRef]
- Ji, J.; Yin, Y.; Ju, H.; Xu, X.; Liu, W.; Fu, Q.; Hu, J.; Zhang, X.; Sun, B. Long non-coding RNA Lnc-Tim3 exacerbates CD8 T cell exhaustion via binding to Tim-3 and inducing nuclear translocation of Bat3 in HCC. Cell Death Dis. 2018, 9, 478. [Google Scholar] [CrossRef]
- Chauvin, J.-M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8, 957. [Google Scholar] [CrossRef]
- Facchinetti, F.; Di Maio, M.; Perrone, F.; Tiseo, M. First-line immunotherapy in non-small cell lung cancer patients with poor performance status: A systematic review and meta-analysis. Transl. Lung Cancer Res. 2021, 10, 2917. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.; Rizvi, N.A.; Chow, L.Q.; Borghaei, H.; Brahmer, J.; Ready, N.; Gerber, D.E.; Shepherd, F.A.; Antonia, S.; Goldman, J.W.; et al. Nivolumab monotherapy for first-line treatment of advanced non–small-cell lung cancer. J. Clin. Oncol. 2016, 34, 2980. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for first-line treatment of PD-L1–selected patients with NSCLC. J. Clin. Oncol. 2020, 383, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Hrinczenko, B.; Iannotti, N.; Goel, S.; Spigel, D.; Safran, H.; Taylor, M.H.; Bennouna, J.; Wong, D.J.; Kelly, K.; Verschraegen, C.; et al. Long-term avelumab in advanced non-small-cell lung cancer: Summaries and post hoc analyses from JAVELIN Solid Tumor. Future Med. 2022, 18, 1333–1342. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Pennell, N.A.; Fidler, M.J.; Halmos, B.; Bonomi, P.; Stevenson, J.; Schneider, B.; Sukari, A.; Ventimiglia, J.; Chen, W.; et al. Phase II study of maintenance pembrolizumab in patients with extensive-stage small cell lung cancer (SCLC). J. Thorac. Oncol. 2018, 13, 1393–1399. [Google Scholar] [CrossRef]
- Goldman, J.W.; Dowlati, A.; Antonia, S.J.; Nemunaitis, J.J.; Butler, M.O.; Segal, N.H.; Smith, P.A.; Weiss, J.; Zandberg, D.P.; Xiao, F.; et al. Safety and antitumor activity of durvalumab monotherapy in patients with pretreated extensive disease small-cell lung cancer (ED-SCLC). J. Clin. Oncol. 2018, 36, 8518. [Google Scholar] [CrossRef]
- Horn, L.; Reck, M.; Gettinger, S.N.; Spigel, D.R.; Antonia, S.J.; Rupnow, B.A.; Pieters, A.; Selvaggi, G.; Fairchild, J.P.; Peters, S. CheckMate 331: An open-label, randomized phase III trial of nivolumab versus chemotherapy in patients (pts) with relapsed small cell lung cancer (SCLC) after first-line platinum-based chemotherapy (PT-DC). J. Clin. Oncol. 2016, 34, TPS8578. [Google Scholar] [CrossRef]
- Girard, N.; Popat, S.; Shoshkova, S.; Fear, S.; Gracia, J.P. 1033P IMreal Cohort 2: Second interim analysis of efficacy and safety data in patients (pts) with locally advanced or metastatic non-small cell lung cancer (NSCLC) receiving atezolizumab (atezo) under real-world conditions. Ann. Oncol. 2022, 33, S1027–S1028. [Google Scholar] [CrossRef]
- Govindan, R.; Szczesna, A.; Jassem, J.; Ahn, M.-J.; Lee, K.H.; Schneider, C.-P.; Von Pawel, J.; Reck, M.; Gonzalez Mella, P.; Barlesi, F.; et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J. Clin. Oncol. 2017, 35, 3449–3459. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Juergens, R.; Hao, D.; Laurie, S.; Mates, M.; Tehfe, M.; Bradbury, P.; Kollmannsberger, C.; Ellis, P.; Hilton, J.; Brown-Walker, P. MA09.03 cisplatin/pemetrexed+ durvalumab+/-tremelimumab in Pts with advanced non-squamous NSCLC: A CCTG phase IB study-IND. 226. J. Thorac. Oncol. 2017, 12, S392–S393. [Google Scholar] [CrossRef]
- Kanda, S.; Goto, K.; Shiraishi, H.; Kubo, E.; Tanaka, A.; Utsumi, H.; Sunami, K.; Kitazono, S.; Mizugaki, H.; Horinouchi, H.; et al. Safety and efficacy of nivolumab and standard chemotherapy drug combination in patients with advanced non-small-cell lung cancer: A four arms phase Ib study. Ann. Oncol. 2016, 27, 2242–2250. [Google Scholar] [CrossRef]
- Nishio, M.; Saito, H.; Goto, K.; Watanabe, S.; Sueoka-Aragane, N.; Okuma, Y.; Kasahara, K.; Chikamori, K.; Nakagawa, Y.; Kawakami, T. IMpower132: Atezolizumab plus platinum-based chemotherapy vs. chemotherapy for advanced NSCLC in Japanese patients. Cancer Sci. 2021, 112, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Gogishvili, M.; Melkadze, T.; Makharadze, T.; Giorgadze, D.; Dvorkin, M.; Penkov, K.; Laktionov, K.; Nemsadze, G.; Nechaeva, M.; Rozhkova, I.; et al. LBA51 EMPOWER-Lung 3: Cemiplimab in combination with platinum doublet chemotherapy for first-line (1L) treatment of advanced non-small cell lung cancer (NSCLC). Ann. Oncol. 2021, 32, S1328. [Google Scholar] [CrossRef]
- Tfayli, A.; Al Assaad, M.; Fakhri, G.; Akel, R.; Atwi, H.; Ghanem, H.; El Karak, F.; Farhat, F.; Al Rabi, K.; Sfeir, P.; et al. Neoadjuvant chemotherapy and Avelumab in early stage resectable nonsmall cell lung cancer. Cancer Med. 2020, 9, 8406–8411. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keam, B.; Ock, C.-Y.; Song, S.; Kim, M.; Kim, S.H.; Kim, K.H.; Kim, J.-S.; Kim, T.M.; Kim, D.-W.; et al. A phase II study of pembrolizumab and paclitaxel in refractory extensive disease small cell lung cancer. J. Clin. Oncol. 2018, 36, 8575. [Google Scholar] [CrossRef]
- Weiss, G.J.; Waypa, J.; Blaydorn, L.; Coats, J.; McGahey, K.; Sangal, A.; Niu, J.; Lynch, C.A.; Farley, J.H.; Khemka, V. A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br. J. Cancer 2017, 117, 33–40. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Brahmer, J.R.; Juergens, R.A.; Borghaei, H.; Gettinger, S.; Chow, L.Q.; Gerber, D.E.; Laurie, S.A.; Goldman, J.W.; et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J. Clin. Oncol. 2016, 34, 2969. [Google Scholar] [CrossRef] [PubMed]
- Arriola, E.; Wheater, M.; Galea, I.; Cross, N.; Maishman, T.; Hamid, D.; Stanton, L.; Cave, J.; Geldart, T.; Mulatero, C.; et al. Outcome and biomarker analysis from a multicenter phase 2 study of ipilimumab in combination with carboplatin and etoposide as first-line therapy for extensive-stage SCLC. J. Thorac. Oncol. 2016, 11, 1511–1521. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.C.; Raben, D.; Formenti, S.C. The integration of radiotherapy with immunotherapy for the treatment of non–small cell lung cancer. Clin. Cancer Res. 2018, 24, 5792–5806. [Google Scholar] [CrossRef] [PubMed]
- Theelen, W.S.; Chen, D.; Verma, V.; Hobbs, B.P.; Peulen, H.M.; Aerts, J.G.; Bahce, I.; Niemeijer, A.L.N.; Chang, J.Y.; de Groot, P.M.; et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: A pooled analysis of two randomised trials. J. Immunother. Cancer 2021, 9, 467–475. [Google Scholar] [CrossRef]
- Altorki, N.K.; McGraw, T.E.; Borczuk, A.C.; Saxena, A.; Port, J.L.; Stiles, B.M.; Lee, B.E.; Sanfilippo, N.J.; Scheff, R.J.; Pua, B.B.; et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: A single-centre, randomised phase 2 trial. Lancet 2021, 22, 824–835. [Google Scholar] [CrossRef]
- Fiorica, F.; Belluomini, L.; Stefanelli, A.; Santini, A.; Urbini, B.; Giorgi, C.; Frassoldati, A. Immune checkpoint inhibitor nivolumab and radiotherapy in pretreated lung cancer patients. Am. J. Clin. Oncol. 2018, 41, 1101–1105. [Google Scholar] [CrossRef]
- Golden, E.; Chachoua, A.; Fenton-Kerimian, M.; Demaria, S.; Formenti, S.C. Abscopal responses in metastatic non-small cell lung cancer (NSCLC) patients treated on a phase 2 study of combined radiation therapy and ipilimumab: Evidence for the in situ vaccination hypothesis of radiation. Int. J. Radiat. Oncol. 2015, 93, S66–S67. [Google Scholar] [CrossRef]
- Lin, S.H.; Lin, Y.; Yao, L.; Kalhor, N.; Carter, B.W.; Altan, M.; Blumenschein, G.; Byers, L.A.; Fossella, F.; Gibbons, D.L.; et al. Phase II trial of concurrent atezolizumab with chemoradiation for unresectable NSCLC. J. Thorac. Oncol. 2020, 15, 248–257. [Google Scholar] [CrossRef]
- Moreno, V.; Gil-Martin, M.; Johnson, M.; Aljumaily, R.; Lopez-Criado, M.; Northfelt, D.; Crittenden, M.; Jabbour, S.; Rosen, L.; Calvo, E.; et al. MA04. 01 cemiplimab, a human monoclonal anti-PD-1, alone or in combination with radiotherapy: Phase 1 NSCLC expansion cohorts. J. Thorac. Oncol. 2018, 13, S366. [Google Scholar] [CrossRef]
- Wong, P.; Florescu, M.; Plourde, M.-E.; Panet-Raymond, V.; Pavic, M.; Owen, S.P.; Masson-Cote, L.; Menard, C.; Routy, B.; Tehfe, M.; et al. A phase II trial combining nivolumab and stereotactic brain radiosurgery for treatment of brain metastases in patients with NSCLC. J. Clin. Oncol. 2021, 39, 2303. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Higgins, K.A.; Chen, Z.; Zhang, C.; Pillai, R.N.; Steuer, C.E.; Saba, N.F.; Pakkala, S.; Shin, D.M.; Zhang, G.; et al. A randomized phase II study of tremelimumab and durvalumab with or without radiation for patients with relapsed small cell lung cancer (SCLC). J. Clin. Oncol. 2019, 37, 8515. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.; Goldberg, S.B.; Balmanoukian, A.; Chaft, J.E.; Sanborn, R.E.; Gupta, A.; Narwal, R.; Steele, K.; Gu, Y.; Karakunnel, J.; et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: A multicentre, phase 1b study. Lancet 2016, 17, 299–308. [Google Scholar] [CrossRef]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet 2016, 17, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Ready, N.; Farago, A.F.; de Braud, F.; Atmaca, A.; Hellmann, M.D.; Schneider, J.G.; Spigel, D.R.; Moreno, V.; Chau, I.; Hann, C.L.; et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J. Thorac. Oncol. 2019, 14, 237–244. [Google Scholar] [CrossRef]
- Wrangle, J.M.; Velcheti, V.; Patel, M.R.; Garrett-Mayer, E.; Hill, E.G.; Ravenel, J.G.; Miller, J.S.; Farhad, M.; Anderton, K.; Lindsey, K.; et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: A non-randomised, open-label, phase 1b trial. Lancet 2018, 19, 694–704. [Google Scholar] [CrossRef]
- Dajon, M.; Iribarren, K.; Cremer, I. Dual roles of TLR7 in the lung cancer microenvironment. Oncoimmunology 2015, 4, e991615. [Google Scholar] [CrossRef]
- Nesbit, E.G.; Leal, T.A.; Kruser, T. What is the role of radiotherapy for extensive-stage small cell lung cancer in the immunotherapy era? Transl. Lung Cancer Res. 2019, 8, S153. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zheng, F.; Ren, D.; Du, F.; Dong, Q.; Wang, Z.; Zhao, F.; Ahmad, R.; Zhao, J. Anlotinib: A novel multi-targeting tyrosine kinase inhibitor in clinical development. J. Hematol. Oncol. 2018, 11, 120. [Google Scholar] [CrossRef]
- Schettini, F.; De Santo, I.; Rea, C.G.; De Placido, P.; Formisano, L.; Giuliano, M.; Arpino, G.; De Laurentiis, M.; Puglisi, F.; De Placido, S.; et al. CDK 4/6 inhibitors as single agent in advanced solid tumors. Front. Immunol. 2018, 8, 608. [Google Scholar] [CrossRef]
- US National Institutes of Health. Study of Pembrolizumab (MK-3475) in Participants with Progressive Locally Advanced or Metastatic Carcinoma, Melanoma, or Non-Small Cell Lung Carcinoma (P07990/MK-3475-001/KEYNOTE-001); American Medical Association: Chicago, IL, USA, 2015.
- Sun, H.; Dai, J.; Zhao, L.; Zhu, J.; Wang, H.; Chen, P.; Lu, H.; Chen, Q.; Zhang, Z. Lymphocyte activation gene-3 is associated with programmed death-ligand 1 and programmed cell death protein 1 in small cell lung cancer. Ann. Oncol. 2021, 9, 1468. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Doger, B.; Majem, M.; Carcereny, E.; Krebs, M.; Peguero, J.A.; Roxburgh, P.; Forster, M.; Bajaj, P.; Clay, T.D. Initial Results from a Phase II Study (TACTI-002) in Metastatic Non-Small Cell Lung or Head and Neck Carcinoma Patients Receiving Eftilagimod Alpha (Soluble LAG-3 Protein) and Pembrolizumab; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2020. [Google Scholar]
- Liu, J.; Yuan, Y.; Chen, W.; Putra, J.; Suriawinata, A.A.; Schenk, A.D.; Miller, H.E.; Guleria, I.; Barth, R.J.; Huang, Y.H.; et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc. Natl. Acad. Sci. USA 2015, 112, 6682–6687. [Google Scholar] [CrossRef] [PubMed]
- ElTanbouly, M.A.; Schaafsma, E.; Smits, N.C.; Shah, P.; Cheng, C.; Burns, C.; Blazar, B.R.; Noelle, R.J.; Mabaera, R. VISTA re-programs macrophage biology through the combined regulation of tolerance and anti-inflammatory pathways. Front. Immunol. 2020, 2515. [Google Scholar] [CrossRef]
- Shaffer, A. Novel Immunotherapy Combos Target TIM-3 and PD-1/PD-L1 Networks. Oncol. Live 2022, 22. [Google Scholar]
- Chen, Y.; Huang, H.; Li, Y.; Xiao, W.; Liu, Y.; Chen, R.; Zhu, Y.; Zheng, X.; Wu, C.; Chen, L. TIGIT Blockade Exerts Synergistic Effects on Microwave Ablation Against Cancer. Front. Immunol. 2022, 13, 832230. [Google Scholar] [CrossRef]
- Ettinger, D.; Hann, C.; Kelly, R.; Hales, R.; Feller-Kopman, D.; Kang, H.; Marrone, K.; Anagnostou, V.; Naidoo, J.; Zahurak, B.M. Study Title: Neoadjuvant Nivolumab, or Nivolumab in Combination with Ipilimumab, in Resectable Non-Small-Cell Lung Cancer JHMI Protocol ID number: NA_00092076/J1414. Available online: https://www.clinicaltrials.gov/ProvidedDocs/21/NCT02259621/Prot_SAP_001.pdf (accessed on 28 November 2022).
- Mohamed, I.; Rao, S. P1. 05-01 Phase II Study of ctDNA Directed Consolidation Durvalumab After Induction and Concurrent Durvalumab with SABR for Stage I NSCLC. J. Thorac. Oncol. 2022, 17, S102–S103. [Google Scholar] [CrossRef]
- Dziadziuszko, R.; Ahn, M.; Kelly, K.; Popat, S.; Wakelee, H.; Baird, A.; Rooney, I.; Afshari, M.; Yao, E.; Zhang, Z.; et al. 1190TiP SKYSCRAPER-03: Phase III, open-label randomised study of atezolizumab+ tiragolumab vs. durvalumab in patients with locally advanced, unresectable, stage III non-small cell lung cancer (NSCLC) who have not progressed after platinum-based concurrent chemoradiation (cCRT). Ann. Oncol. 2021, 32, S947–S948. [Google Scholar]
- Verschraegen, C.F.; Jerusalem, G.; McClay, E.F.; Iannotti, N.; Redfern, C.H.; Bennouna, J.; Chen, F.L.; Kelly, K.; Mehnert, J.; Morris, J.C.; et al. Efficacy and safety of first-line avelumab in patients with advanced non-small cell lung cancer: Results from a phase Ib cohort of the JAVELIN Solid Tumor study. J. Immunother. Cancer 2020, 8, e001064. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Ciuleanu, T.E.; Pluzanski, A.; Lee, J.-S.; Schenker, M.; Bernabe Caro, R.; Lee, K.H.; Zurawski, B.; Audigier-Valette, C.; Provencio, M.; et al. Nivolumab+ ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: Three-year update from CheckMate 227 Part 1. J. Clin. Oncol. 2020, 38, 9500. [Google Scholar] [CrossRef]
- Kozłowski, M.; Borzyszkowska, D.; Cymbaluk-Płoska, A. The Role of TIM-3 and LAG-3 in the Microenvironment and Immunotherapy of Ovarian Cancer. Biomedicines 2022, 10, 2826. [Google Scholar] [CrossRef]
- Du, H.; Yi, Z.; Wang, L.; Li, Z.; Niu, B.; Ren, G. The co-expression characteristics of LAG3 and PD-1 on the T cells of patients with breast cancer reveal a new therapeutic strategy. Int. Immunopharmacol. 2020, 78, 106113. [Google Scholar] [CrossRef]
- Uboha, N.V.; Milhem, M.M.; Kovacs, C.; Amin, A.; Magley, A.; Purkayastha, D.D.; Piha-Paul, S.A. Phase II study of spartalizumab (PDR001) and LAG525 in advanced solid tumors and hematologic malignancies. J. Clin. Oncol. 2019, 37, 2553. [Google Scholar] [CrossRef]
- Hong, D.S.; Schoffski, P.; Calvo, A.; Sarantopoulos, J.; Ochoa De Olza, M.; Carvajal, R.D.; Prawira, A.; Kyi, C.; Esaki, T.; Akerley, W.L.; et al. Phase I/II study of LAG525±spartalizumab (PDR001) in patients (pts) with advanced malignancies. J. Clin. Oncol. 2018, 36, 3012. [Google Scholar] [CrossRef]
- Haber, T.; Symons, J.; Dharmadhikari, B.; Müller, T.; Thakkar, D.; Rodon, J.A.; Gruber, J.; Telli, M.; Mita, M.; Mita, A.; et al. 623 Trial in progress: A phase 1 first-in-human study of HMBD-002, an IgG4 monoclonal antibody targeting VISTA, as a monotherapy and combined with pembrolizumab in patients with advanced solid malignancies. J. Immunother. Cancer 2022, 9, A498. [Google Scholar]
- Scott, F.; Wichmann, C.; Burvenich, I.; McDonald, A.; Guo, N.; Rigopoulos, A.; Soikes, R.; Angelides, S.; von Roemeling, R.; Scott, A. 324 Preclinical evaluation of anti-VISTA antibody CI-8993 in a syngeneic huVISTA-KI model. J. Immunother. Cancer 2021, 9, A349. [Google Scholar] [CrossRef]
- Radhakrishnan, V.; Banavali, S.; Gupta, S.; Kumar, A.; Deshmukh, C.; Nag, S.; Beniwal, S.; Gopichand, M.; Naik, R.; Lakshmaiah, K.; et al. Excellent CBR and prolonged PFS in non-squamous NSCLC with oral CA-170, an inhibitor of VISTA and PD-L1. Ann. Oncol. 2019, 30, v494. [Google Scholar] [CrossRef]
- Gabrail, N.Y.; Bessudo, A.; Hamilton, E.P.; Sachdev, J.C.; Patel, M.R.; Rodon Ahnert, J.; Evilevitch, L.; Duncan, M.; Guo, W.; Lu, S.; et al. IOLite: Multipart, phase 1b, dose-finding study of the PD-1 inhibitor dostarlimab in combination with the PARP inhibitor niraparib±bevacizumab (bev), or with platinum-based chemotherapy±bev for advanced cancer. Eur. J. Cancer 2019, 37, 2560. [Google Scholar] [CrossRef]
- Falchook, G.S.; Ribas, A.; Davar, D.; Eroglu, Z.; Wang, J.S.; Luke, J.J.; Hamilton, E.P.; Di Pace, B.; Wang, T.; Ghosh, S.; et al. Phase 1 trial of TIM-3 inhibitor cobolimab monotherapy and in combination with PD-1 inhibitors nivolumab or dostarlimab (AMBER). Eur. J. Cancer 2022, 40, 2504. [Google Scholar] [CrossRef]
- Sharma, S.; Moore, K.; Mettu, N.; Garrido-Laguna, I.; Ulahannan, S.; Khemka, V. Initial results from a phase 1a/b study of etigilimab (OMP-313M32), an anti-T cell immunoreceptor with Ig and ITIM domains (TIGIT) antibody, in advanced solid tumors. J. Immunother. Cancer 2018, 6, 114. [Google Scholar]
- Hastings, K.; Yu, H.; Wei, W.; Sanchez-Vega, F.; DeVeaux, M.; Choi, J.; Rizvi, H.; Lisberg, A.; Truini, A.; Lydon, C.; et al. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann. Oncol. 2019, 30, 1311–1320. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Hellmann, M.D. Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 1165. [Google Scholar] [CrossRef]
- Michot, J.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.-P.; Du, X.-J.; Liu, J.-Q.; Huang, C.-L.; Chen, L.; Zhou, G.-Q.; Li, W.-F.; Mao, Y.-P.; Hsu, C.; et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. Br. Med. J. 2018, 363, k4226. [Google Scholar] [CrossRef]
- Onoi, K.; Chihara, Y.; Uchino, J.; Shimamoto, T.; Morimoto, Y.; Iwasaku, M.; Kaneko, Y.; Yamada, T.; Takayama, K.J. Immune checkpoint inhibitors for lung cancer treatment: A review. J. Clin. Med. 2020, 9, 1362. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Proc. Natl. Acad. Sci. USA 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.J.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714. [Google Scholar] [CrossRef]
- Stamatouli, A.M.; Quandt, Z.; Perdigoto, A.L.; Clark, P.L.; Kluger, H.; Weiss, S.A.; Gettinger, S.; Sznol, M.; Young, A.; Rushakoff, R.; et al. Collateral damage: Insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 2018, 67, 1471–1480. [Google Scholar] [CrossRef]
- Griss, J.; Bauer, W.; Wagner, C.; Simon, M.; Chen, M.; Grabmeier-Pfistershammer, K.; Maurer-Granofszky, M.; Roka, F.; Penz, T.; Bock, C.; et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat. Commun. 2019, 10, 4186. [Google Scholar] [CrossRef]
- König, D.; Läubli, H. Mechanisms of immune-related complications in cancer patients treated with immune checkpoint inhibitors. Pharmacology 2021, 106, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Meng, C.; Ivashkiv, L.B. Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proc. Natl. Acad. Sci. USA 2000, 97, 9573–9578. [Google Scholar] [CrossRef] [PubMed]
- Im, S.J.; Hashimoto, M.; Gerner, M.Y.; Lee, J.; Kissick, H.T.; Burger, M.C.; Shan, Q.; Hale, J.S.; Lee, J.; Nasti, T.H.; et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 2016, 537, 417–421. [Google Scholar] [CrossRef]
- Ricciuti, B.; Dahlberg, S.E.; Adeni, A.; Sholl, L.M.; Nishino, M.; Awad, M.M. Immune checkpoint inhibitor outcomes for patients with non–small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J. Clin. Oncol. 2019, 37, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Santini, F.C.; Rizvi, H.; Plodkowski, A.J.; Ni, A.; Lacouture, M.E.; Gambarin-Gelwan, M.; Wilkins, O.; Panora, E.; Halpenny, D.F.; Long, N.M. Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLCRetreatment with Immunotherapy after Serious irAE. Cancer Immunol. Res. 2018, 6, 1093–1099. [Google Scholar] [CrossRef]
- Coulie, P.G.; Van den Eynde, B.J.; Van Der Bruggen, P.; Boon, T. Tumour antigens recognized by T lymphocytes: At the core of cancer immunotherapy. Nat. Rev. Cancer 2014, 14, 135–146. [Google Scholar] [CrossRef]
- Ito, A.; Kitano, S.; Kim, Y.; Inoue, M.; Fuse, M.; Tada, K.; Yoshimura, K.J. Cancer neoantigens: A promising source of immunogens for cancer immunotherapy. J. Clin. Cell Immunol. 2015, 6, 2. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khadela, A.; Chavda, V.P.; Postwala, H.; Ephraim, R.; Apostolopoulos, V.; Shah, Y. Configuring Therapeutic Aspects of Immune Checkpoints in Lung Cancer. Cancers 2023, 15, 543. https://doi.org/10.3390/cancers15020543
Khadela A, Chavda VP, Postwala H, Ephraim R, Apostolopoulos V, Shah Y. Configuring Therapeutic Aspects of Immune Checkpoints in Lung Cancer. Cancers. 2023; 15(2):543. https://doi.org/10.3390/cancers15020543
Chicago/Turabian StyleKhadela, Avinash, Vivek P. Chavda, Humzah Postwala, Ramya Ephraim, Vasso Apostolopoulos, and Yesha Shah. 2023. "Configuring Therapeutic Aspects of Immune Checkpoints in Lung Cancer" Cancers 15, no. 2: 543. https://doi.org/10.3390/cancers15020543
APA StyleKhadela, A., Chavda, V. P., Postwala, H., Ephraim, R., Apostolopoulos, V., & Shah, Y. (2023). Configuring Therapeutic Aspects of Immune Checkpoints in Lung Cancer. Cancers, 15(2), 543. https://doi.org/10.3390/cancers15020543










