Role of Oxidative Stress in Metabolic Reprogramming of Brain Cancer
Abstract
Simple Summary
Abstract
1. Introduction
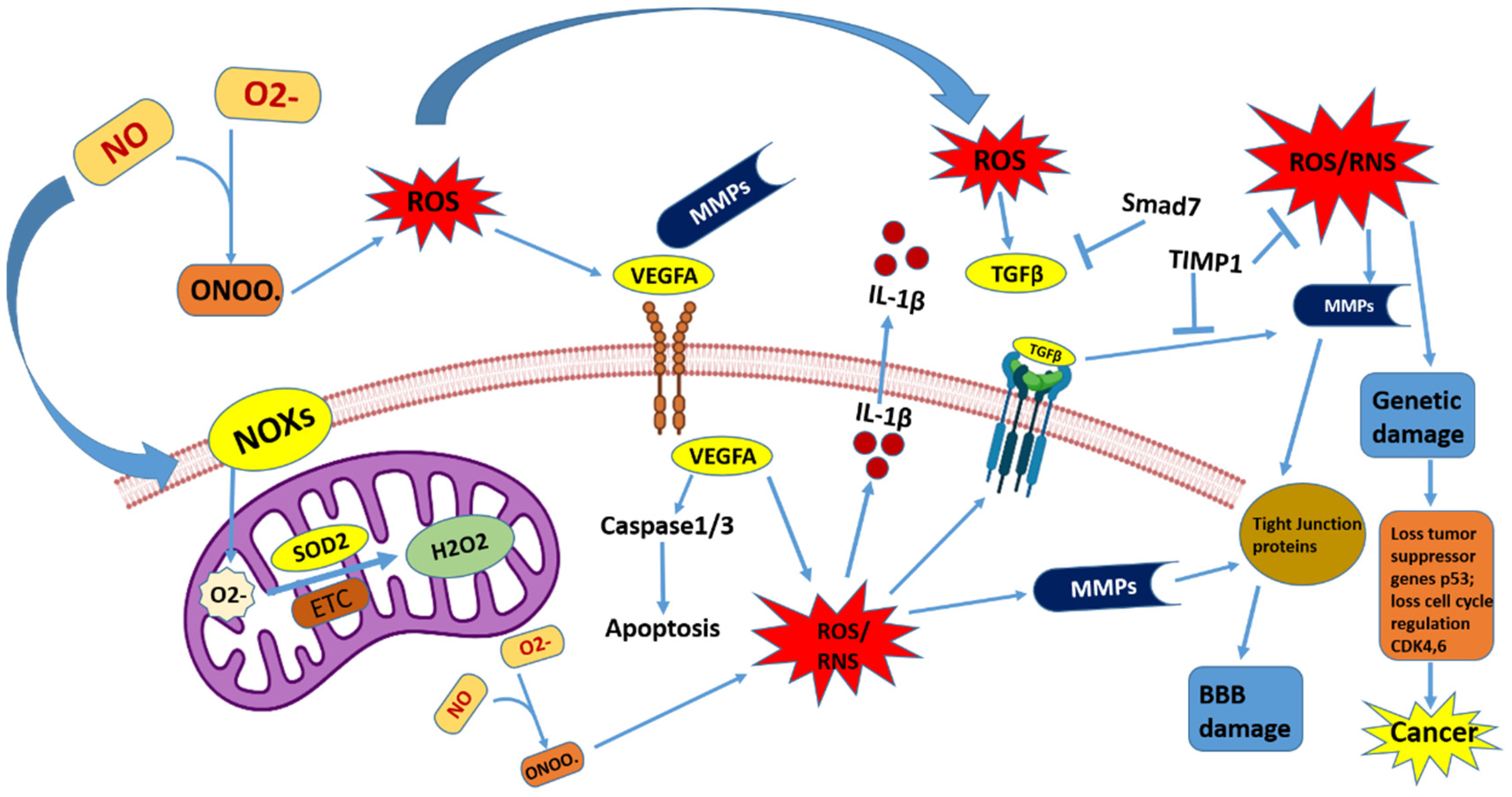
2. Function of ROS and RNS in the Brain
2.1. Reactive Oxygen Species (ROS)
2.2. Reactive Nitrogen Species (RNS)
3. Mitochondrial ROS in the Metabolism of Brain Cancer
4. Metabolic Reprogramming in Brain Cancer and Microenvironmental Stress
4.1. Aerobic Glycolysis and Lipogenesis in Medulloblastoma
4.2. Metabolism in Glioblastomas (GBMs)
4.3. Inflammation-Regulated Microenvironment in Brain Cancer
4.4. Role of Microenvionment in GBM
5. Targeting ROS and Glioma Stem-like Cells for Cancer Cell Death
5.1. Upregulation of ROS
5.2. Targeting Cancer Cell Proliferation by Downregulating ROS
5.3. Targeting Stem-like Gliomal Cells
6. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Finch, A.; Solomou, G.; Wykes, V.; Pohl, U.; Bardella, C.; Watts, C. Advances in research of adult gliomas. Int. J. Mol. Sci. 2021, 22, 924. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Muragaki, Y.; Chernov, M.F. Pathology and Genetics of Gliomas. Prog. Neurol. Surg. 2018, 31, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A. Overview of Primary Brain Tumors: Pathologic Classification, Epidemiology, Molecular Biology, and Prognostic Markers. Hematol. Oncol. Clin. N. Am. 2012, 26, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Medikonda, R.; Dunn, G.; Rahman, M.; Fecci, P.; Lim, M. A review of glioblastoma immunotherapy. J. Neurooncol. 2021, 151, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Preusser, M. The inflammatory microenvironment in brain metastases: Potential treatment target? Chin. Clin. Oncol. 2015, 4, 21. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Guzman, M.L.; Rivella, S. Iron and reactive oxygen species: Friends or foes of cancer cells? Antioxid. Redox Signal. 2014, 20, 1917–1924. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Saeed, S.A.; Shad, K.F.; Saleem, T.; Javed, F.; Khan, M.U. Some new prospects in the understanding of the molecular basis of the pathogenesis of stroke. Exp. Brain Res. 2007, 182, 1–10. [Google Scholar] [CrossRef]
- Ng, S.C.W.; Furman, R.; Axelsen, P.H.; Shchepinov, M.S. Free Radical Chain Reactions and Polyunsaturated Fatty Acids in Brain Lipids. ACS Omega 2022, 7, 25337–25345. [Google Scholar] [CrossRef]
- Lelièvre, P.; Sancey, L.; Coll, J.L.; Deniaud, A.; Busser, B. Iron dysregulation in human cancer: Altered metabolism, biomarkers for diagnosis, prognosis, monitoring and rationale for therapy. Cancers 2020, 12, 3524. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Andreux, P.; Poitry-Yamate, C.; Auwerx, J.; Hanahan, D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 19507–19512. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Letelier, M.E.; Lepe, A.M.; Faúndez, M.; Salazar, J.; Marín, R.; Aracena, P.; Speisky, H. Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity. Chem. Biol. Interact. 2005, 151, 71–82. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS generation and antioxidant defense systems in normal and malignant cells. Oxidative Med. Cell. Longev. 2020, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, V.R.; Orr, M.E.; Rodriguez, K.A.; Buffenstein, R. Mechanisms of oxidative stress resistance in the brain: Lessons learned from hypoxia tolerant extremophilic vertebrates. Arch. Biochem. Biophys. 2015, 576, 8–16. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. Ros homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Abdrakhmanov, A.; Yapryntseva, M.A.; Kaminskyy, V.O.; Zhivotovsky, B.; Gogvadze, V. Receptor-mediated mitophagy rescues cancer cells under hypoxic conditions. Cancers 2021, 13, 4027. [Google Scholar] [CrossRef]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2019, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.H.; Chan, S.H.H. Differential impacts of brain stem oxidative stress and nitrosative stress on sympathetic vasomotor tone. Pharmacol. Ther. 2019, 201, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, L.; Ying, Z.; Pan, C.; Huang, S.; Li, L.; Dai, M.; Yan, B.; Li, M.; Jiang, H.; et al. A Small Molecule That Protects the Integrity of the Electron Transfer Chain Blocks the Mitochondrial Apoptotic Pathway. Mol. Cell 2016, 63, 229–239. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Hirooka, Y.; Konno, S.; Sunagawa, K. Sympathoinhibition induced by centrally administered atorvastatin is associated with alteration of nad(p)h and mn superoxide dismutase activity in rostral ventrolateral medulla of stroke-prone spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 2010, 55, 184–190. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.M. ROS and brain diseases: The good, the bad, and the ugly. Oxidative Med. Cell. Longev. 2013, 2013, 963520. [Google Scholar] [CrossRef]
- Adusumilli, V.S.; Walker, T.L.; Overall, R.W.; Klatt, G.M.; Zeidan, S.A.; Zocher, S.; Kirova, D.G.; Ntitsias, K.; Fischer, T.J.; Sykes, A.M.; et al. ROS Dynamics Delineate Functional States of Hippocampal Neural Stem Cells and Link to Their Activity-Dependent Exit from Quiescence. Cell Stem Cell 2021, 28, 300–314.e6. [Google Scholar] [CrossRef]
- Tsuji, O.; Miura, K.; Okada, Y.; Fujiyoshi, K.; Mukaino, M.; Nagoshi, N.; Kitamura, K.; Kumagai, G.; Nishino, M.; Tomisato, S.; et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc. Natl. Acad. Sci. USA 2010, 107, 12704–12709. [Google Scholar] [CrossRef]
- Panthi, S.; Manandhar, S.; Gautam, K. Hydrogen sulfide, nitric oxide, and neurodegenerative disorders. Transl. Neurodegener. 2018, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Expósito, M.J.; Martínez-Martos, J.M. The Delicate Equilibrium between Oxidants and Antioxidants in Brain Glioma. Curr. Neuropharmacol. 2018, 17, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Dal-Pizzol, F.; Klamt, F.; Vianna, M.M.R.; Schröder, N.; Quevedo, J.; Benfato, M.S.; Moreira, J.C.F.; Walz, R. Lipid peroxidation in hippocampus early and late after status epilepticus induced by pilocarpine or kainic acid in Wistar rats. Neurosci. Lett. 2000, 17, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Bellissimo, M.I.; Amado, D.; Abdalla, D.S.P.; Ferreira, E.C.; Cavalheiro, E.A.; Da Graça Naffah-Mazzacoratti, M. Superoxide dismutase, glutathione peroxidase activities and the hydroperoxide concentration are modified in the hippocampus of epileptic rats. Epilepsy Res. 2001, 46, 121–128. [Google Scholar] [CrossRef]
- Lichtor, T. Clinical Management and Evolving Novel Therapeutic Strategies for Patients with Brain Tumors; Intechopen (Neuro-Oncology): London, UK, 2013. [Google Scholar] [CrossRef]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef]
- Calvani, M.; Pasha, A.; Favre, C. Nutraceutical boom in cancer: Inside the labyrinth of reactive oxygen species. Int. J. Mol. Sci. 2020, 21, 1936. [Google Scholar] [CrossRef]
- Orlicka-Płocka, M.; Fedoruk-Wyszomirska, A.; Gurda-Woźna, D.; Pawelczak, P.; Krawczyk, P.; Giel-Pietraszuk, M.; Framski, G.; Ostrowski, T.; Wyszko, E. Implications of oxidative stress in glioblastoma multiforme following treatment with purine derivatives. Antioxidants 2021, 10, 950. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef]
- Wilhelm, J.; Vytášek, R.; Uhlík, J.; Vajner, L. Oxidative stress in the developing rat brain due to production of reactive oxygen and nitrogen species. Oxidative Med. Cell. Longev. 2016, 2016, 5057610. [Google Scholar] [CrossRef]
- Poon, Y.Y.; Tsai, C.Y.; Cheng, C.D.; Chang, A.Y.W.; Chan, S.H.H. Endogenous nitric oxide derived from nos I or II in thoracic spinal cord exerts opposing tonic modulation on sympathetic vasomotor tone via disparate mechanisms in anesthetized rats. Am. J. Physiol.-Heart Circ. Physiol. 2016, 311, H555–H562. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Valdez, L.B.; Zaobornyj, T.; Boveris, A. Mitochondrial metabolic states and membrane potential modulate mtNOS activity. Biochim. Biophys. Acta-Bioenerg. 2006, 1757, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Dynnik, V.V.; Grishina, E.V.; Fedotcheva, N.I. The mitochondrial NO-synthase/guanylate cyclase/protein kinase G signaling system underpins the dual effects of nitric oxide on mitochondrial respiration and opening of the permeability transition pore. FEBS J. 2020, 287, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Riobó, N.A.; Melani, M.; Sanjuán, N.; Fiszman, M.L.; Gravielle, M.C.; Cecilia Carreras, M.; Cadenas, E.; Poderoso, J.J. The modulation of mitochondrial nitric-oxide synthase activity in rat brain development. J. Biol. Chem. 2002, 277, 42447–42455. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and the ugly. Am. J. Physiol.-Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Forman, H.J.; Torres, M. Redox signaling in macrophages. Mol. Asp. Med. 2001, 22, 189–216. [Google Scholar] [CrossRef]
- Mostofa, A.G.M.; Punganuru, S.R.; Madala, H.R.; Al-Obaide, M.; Srivenugopal, K.S. The process and regulatory components of inflammation in brain oncogenesis. Biomolecules 2017, 7, 34. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef]
- Idelchik, M.d.P.S.; Begley, U.; Begley, T.J.; Melendez, J.A. Mitochondrial ROS control of cancer. Semin. Cancer Biol. 2017, 47, 57–66. [Google Scholar] [CrossRef]
- Lu, J.; Sharma, L.K.; Bai, Y. Implications of mitochondrial DNA mutations and mitochondrial dysfunction in tumorigenesis. Cell Res. 2009, 19, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Geng, Z.; Li, H.; Zhang, Y.; Li, J.; Chang, H. Over-expression of TFB2M facilitates cell growth and metastasis via activating ROS-Akt-NF-κB signalling in hepatocellular carcinoma. Liver Int. 2020, 40, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Vithayathil, S.; Kumar, S.; Sung, P.L.; Dobrolecki, L.E.; Putluri, V.; Bhat, V.B.; Bhowmik, S.K.; Gupta, V.; Arora, K.; et al. Fatty Acid Oxidation-Driven Src Links Mitochondrial Energy Reprogramming and Oncogenic Properties in Triple-Negative Breast Cancer. Cell Rep. 2016, 14, 2154–2165. [Google Scholar] [CrossRef] [PubMed]
- Missiroli, S.; Perrone, M.; Genovese, I.; Pinton, P.; Giorgi, C. Cancer metabolism and mitochondria: Finding novel mechanisms to fight tumours. EBioMedicine 2020, 59, 102943. [Google Scholar] [CrossRef]
- Kovac, S.; Angelova, P.R.; Holmström, K.M.; Zhang, Y.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta-Gen. Subj. 2015, 1850, 794–801. [Google Scholar] [CrossRef]
- Lluis, J.M.; Buricchi, F.; Chiarugi, P.; Morales, A.; Fernandez-Checa, J.C. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: Activation of nuclear factor-KB via c-SRC- and oxidant-dependent cell death. Cancer Res. 2007, 67, 7368–7377. [Google Scholar] [CrossRef]
- Kung-Chun Chiu, D.; Pui-Wah Tse, A.; Law, C.T.; Ming-Jing Xu, I.; Lee, D.; Chen, M.; Kit-Ho Lai, R.; Wai-Hin Yuen, V.; Wing-Sum Cheu, J.; Wai-Hung Ho, D.; et al. Hypoxia regulates the mitochondrial activity of hepatocellular carcinoma cells through HIF/HEY1/PINK1 pathway. Cell Death Dis. 2019, 10, 934. [Google Scholar] [CrossRef]
- Venneti, S.; Mischel, P.S. Metabolic Reprogramming in Brain Cancer: A Coordinated Effort. Brain Pathol. 2015, 25, 753–754. [Google Scholar] [CrossRef][Green Version]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Vacanti, N.M.; Divakaruni, A.S.; Green, C.R.; Parker, S.J.; Henry, R.R.; Ciaraldi, T.P.; Murphy, A.N.; Metallo, C.M. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol. Cell 2014, 56, 425–435. [Google Scholar] [CrossRef]
- Reuss, A.M.; Groos, D.; Buchfelder, M.; Savaskan, N. The acidic brain—Glycolytic switch in the microenvironment of malignant glioma. Int. J. Mol. Sci. 2021, 22, 5518. [Google Scholar] [CrossRef]
- Bhatia, B.; Hsieh, M.; Kenney, A.M.; Nahlé, Z. Mitogenic Sonic hedgehog signaling drives E2F1-dependent lipogenesis in progenitor cells and medulloblastoma. Oncogene 2011, 30, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Tech, K.; Gershon, T.R. Energy metabolism in neurodevelopment and medulloblastoma. Transl. Pediatr. 2015, 4, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.B. Wnt meets Warburg: Another piece in the puzzle? EMBO J. 2014, 33, 1420–1422. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.W.; Dalton, J.; Kocak, M.; Nicholson, S.L.; Fraga, C.; Neale, G.; Kenney, A.M.; Brat, D.J.; Perry, A.; Yong, W.H.; et al. Medulloblastoma: Clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011, 121, 381–396. [Google Scholar] [CrossRef]
- Wolf, A.; Agnihotri, S.; Micallef, J.; Mukherjee, J.; Sabha, N.; Cairns, R.; Hawkins, C.; Guha, A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med. 2011, 208, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Libby, C.J.; Gc, S.; Benavides, G.A.; Fisher, J.L.; Williford, S.E.; Zhang, S.; Tran, A.N.; Gordon, E.R.; Jones, A.B.; Tuy, K.; et al. A role for GLUT3 in glioblastoma cell invasion that is not recapitulated by GLUT1. Cell Adhes. Migr. 2021, 15, 101–115. [Google Scholar] [CrossRef]
- Iranmanesh, Y.; Jiang, B.; Favour, O.C.; Dou, Z.; Wu, J.; Li, J.; Sun, C. Mitochondria’s Role in the Maintenance of Cancer Stem Cells in Glioblastoma. Front. Oncol. 2021, 11, 582694. [Google Scholar] [CrossRef]
- Virtuoso, A.; Giovannoni, R.; De Luca, C.; Gargano, F.; Cerasuolo, M.; Maggio, N.; Lavitrano, M.; Papa, M. The glioblastoma microenvironment: Morphology, metabolism, and molecular signature of glial dynamics to discover metabolic rewiring sequence. Int. J. Mol. Sci. 2021, 22, 3301. [Google Scholar] [CrossRef]
- Benny, S.; Mishra, R.; Manojkumar, M.K.; Aneesh, T.P. From Warburg effect to Reverse Warburg effect; the new horizons of anti-cancer therapy. Med. Hypotheses 2020, 144, 110216. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.A.; Asuthkar, S.; Guda, M.R.; Tsung, A.J.; Velpula, K.K. Cancer stem cell molecular reprogramming of the Warburg effect in glioblastomas: A new target gleaned from an old concept. CNS Oncol. 2016, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Chinopoulos, C.; Seyfried, T.N. Mitochondrial Substrate-Level Phosphorylation as Energy Source for Glioblastoma: Review and Hypothesis. ASN Neuro 2018, 10, 1759091418818261. [Google Scholar] [CrossRef]
- Lorger, M. Tumor microenvironment in the brain. Cancers 2012, 4, 218–243. [Google Scholar] [CrossRef]
- Nagashima, G.; Suzuki, R.; Asai, J.I.; Fujimoto, T. Immunohistochemical analysis of reactive astrocytes around glioblastoma: An immunohistochemical study of postmortem glioblastoma cases. Clin. Neurol. Neurosurg. 2002, 104, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, A.; Kwiatkowska, A.; Slomnicki, L.; Dembinski, M.; Master, A.; Sliwa, M.; Franciszkiewicz, K.; Chouaib, S.; Kaminska, B. Microglia-derived TGF-β as an important regulator of glioblastoma invasion-An inhibition of TGF-β-dependent effects by shRNA against human TGF-β type II receptor. Oncogene 2008, 27, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Buonfiglioli, A.; Hambardzumyan, D. Macrophages and microglia: The cerberus of glioblastoma. Acta Neuropathol. Commun. 2021, 9, 54. [Google Scholar] [CrossRef]
- Thaci, B.; Ahmed, A.U.; Ulasov, I.V.; Wainwright, D.A.; Nigam, P.; Auffinger, B.; Tobias, A.L.; Han, Y.; Zhang, L.; Moon, K.S.; et al. Depletion of myeloid-derived suppressor cells during interleukin-12 immunogene therapy does not confer a survival advantage in experimental malignant glioma. Cancer Gene Ther. 2014, 21, 38–44. [Google Scholar] [CrossRef]
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; Dechant, C.; Harrison Farber, S.; Elsamadicy, A.A.; Cui, X.; Koyama, S.; Jackson, C.; et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin. Cancer Res. 2018, 24, 4175–4186. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 2020. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Clavreul, A.; Guette, C.; Faguer, R.; Tétaud, C.; Boissard, A.; Lemaire, L.; Rousseau, A.; Avril, T.; Henry, C.; Coqueret, O.; et al. Glioblastoma-associated stromal cells (GASCs) from histologically normal surgical margins have a myofibroblast phenotype and angiogenic properties. J. Pathol. 2014, 233, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Civita, P.; Leite, D.M.; Pilkington, G.J. Pre-clinical drug testing in 2d and 3d human in vitro models of glioblastoma incorporating non-neoplastic astrocytes: Tunneling nano tubules and mitochondrial transfer modulates cell behavior and therapeutic respons. Int. J. Mol. Sci. 2019, 20, 6017. [Google Scholar] [CrossRef]
- Cha, J.; Kim, P. Biomimetic strategies for the glioblastoma microenvironment. Front. Mater. 2017, 4, 45. [Google Scholar] [CrossRef]
- Mannan, A.; Germon, Z.P.; Chamberlain, J.; Sillar, J.R.; Nixon, B.; Dun, M.D. Reactive oxygen species in acute lymphoblastic leukaemia: Reducing radicals to refine responses. Antioxidants 2021, 10, 1616. [Google Scholar] [CrossRef]
- Mansat-De Mas, V.; Bezombes, C.; Quillet-Mary, A.; Bettaïeb, A.; D’Orgeix, A.D.T.; Laurent, G.; Jaffrézou, J.P. Implication of radical oxygen species in ceramide generation, c-Jun N- terminal kinase activation and apoptosis induced by daunorubicin. Mol. Pharmacol. 1999, 56, 867–874. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative stress in cancer cell metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Marchetti, M.; Resnick, L.; Gamliel, E.; Kesaraju, S.; Weissbach, H.; Binninger, D. Sulindac enhances the killing of cancer cells exposed to oxidative stress. PLoS ONE 2009, 4, e5804. [Google Scholar] [CrossRef]
- Cheng, G.; Lanza-Jacoby, S. Metformin decreases growth of pancreatic cancer cells by decreasing reactive oxygen species: Role of NOX4. Biochem. Biophys. Res. Commun. 2015, 465, 41–46. [Google Scholar] [CrossRef]
- Mochizuki, T.; Furuta, S.; Mitsushita, J.; Shang, W.H.; Ito, M.; Yokoo, Y.; Yamaura, M.; Ishizone, S.; Nakayama, J.; Konagai, A.; et al. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene 2006, 25, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Teoh-Fitzgerald, M.L.; Fitzgerald, M.P.; Zhong, W.; Askeland, R.W.; Domann, F.E. Epigenetic reprogramming governs EcSOD expression during human mammary epithelial cell differentiation, tumorigenesis and metastasis. Oncogene 2014, 33, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Safa, A.R.; Saadatzadeh, M.R.; Cohen-Gadol, A.A.; Pollok, K.E.; Bijangi-Vishehsaraei, K. Emerging targets for glioblastoma stem cell therapy. J. Biomed. Res. 2016, 30, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Sareddy, G.R.; Kesanakurti, D.; Kirti, P.B.; Babu, P.P. Nonsteroidal anti-inflammatory drugs diclofenac and celecoxib attenuates Wnt/β-Catenin/Tcf signaling pathway in human glioblastoma cells. Neurochem. Res. 2013, 38, 2313–2322. [Google Scholar] [CrossRef]
- Kahlert, U.D.; Mooney, S.M.; Natsumeda, M.; Steiger, H.J.; Maciaczyk, J. Targeting cancer stem-like cells in glioblastoma and colorectal cancer through metabolic pathways. Int. J. Cancer 2017, 140, 10–22. [Google Scholar] [CrossRef]
- Colquhoun, A. Cell biology-metabolic crosstalk in glioma. Int. J. Biochem. Cell Biol. 2017, 89, 171–181. [Google Scholar] [CrossRef]
- Kinnaird, A.; Zhao, S.; Wellen, K.E.; Michelakis, E.D. Metabolic control of epigenetics in cancer. Nat. Rev. Cancer 2016, 16, 694–707. [Google Scholar] [CrossRef]
- Hochberg, F.H.; Wolfson, L.; Linggood, R.; Baker, W.H.; Kornblith, P. Quality and Duration of Survival in Glioblastoma Multiforme: Combined Surgical, Radiation, and Lomustine Therapy. JAMA J. Am. Med. Assoc. 1979, 241, 1016–1018. [Google Scholar] [CrossRef]
- Walker, M.D.; Alexander, E.; Hunt, W.E.; MacCarty, C.S.; Mahaley, M.S.; Mealey, J.; Norrell, H.A.; Owens, G.; Ransohoff, J.; Wilson, C.B.; et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J. Neurosurg. 1978, 49, 333–343. [Google Scholar] [CrossRef]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jääskeläinen, J.; Ram, Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro. Oncol. 2003, 49, 333–343. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.H.; Shen, Y.L.; Keegan, P.; Pazdur, R. FDA Drug Approval Summary: Bevacizumab (Avastin®) as Treatment of Recurrent Glioblastoma Multiforme. Oncologist 2009, 14, 1131–1138. [Google Scholar] [CrossRef] [PubMed]

| FDA-Approved Drug | Mechanism | Year of Approval | Reference |
|---|---|---|---|
| Lomustine | Non-selective alkylating agent induces the crosslinking of DNA/RNA in dividing cells, thereby initiating cell apoptosis | 1976 | [99] |
| Carmustine | Binds to and alters the activity of glutathione reductase. | 1977 | [100] |
| Carmustine wafer implants | Binds to and alters the activity of glutathione reductase. | 1996 & 2003 | [101] |
| Temozolomide (TMZ) | A non-specific alkylating agent activates mismatch repair in DNA through methylation at the O6 position of guanine. | 2005 | [102] |
| Bevacizumab (BVZ) | A targeted healing antibody that attaches to and hinders the activity of the VEGF protein within tumor cells. | 2009 | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, K.; Asthana, S.; Kumar, D. Role of Oxidative Stress in Metabolic Reprogramming of Brain Cancer. Cancers 2023, 15, 4920. https://doi.org/10.3390/cancers15204920
Agrawal K, Asthana S, Kumar D. Role of Oxidative Stress in Metabolic Reprogramming of Brain Cancer. Cancers. 2023; 15(20):4920. https://doi.org/10.3390/cancers15204920
Chicago/Turabian StyleAgrawal, Kirti, Shailendra Asthana, and Dhruv Kumar. 2023. "Role of Oxidative Stress in Metabolic Reprogramming of Brain Cancer" Cancers 15, no. 20: 4920. https://doi.org/10.3390/cancers15204920
APA StyleAgrawal, K., Asthana, S., & Kumar, D. (2023). Role of Oxidative Stress in Metabolic Reprogramming of Brain Cancer. Cancers, 15(20), 4920. https://doi.org/10.3390/cancers15204920






