Current Advances and Future Strategies for BCL-2 Inhibitors: Potent Weapons against Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
| Compounds | Targets | Status | Applicable Cancer Types | Refs. |
|---|---|---|---|---|
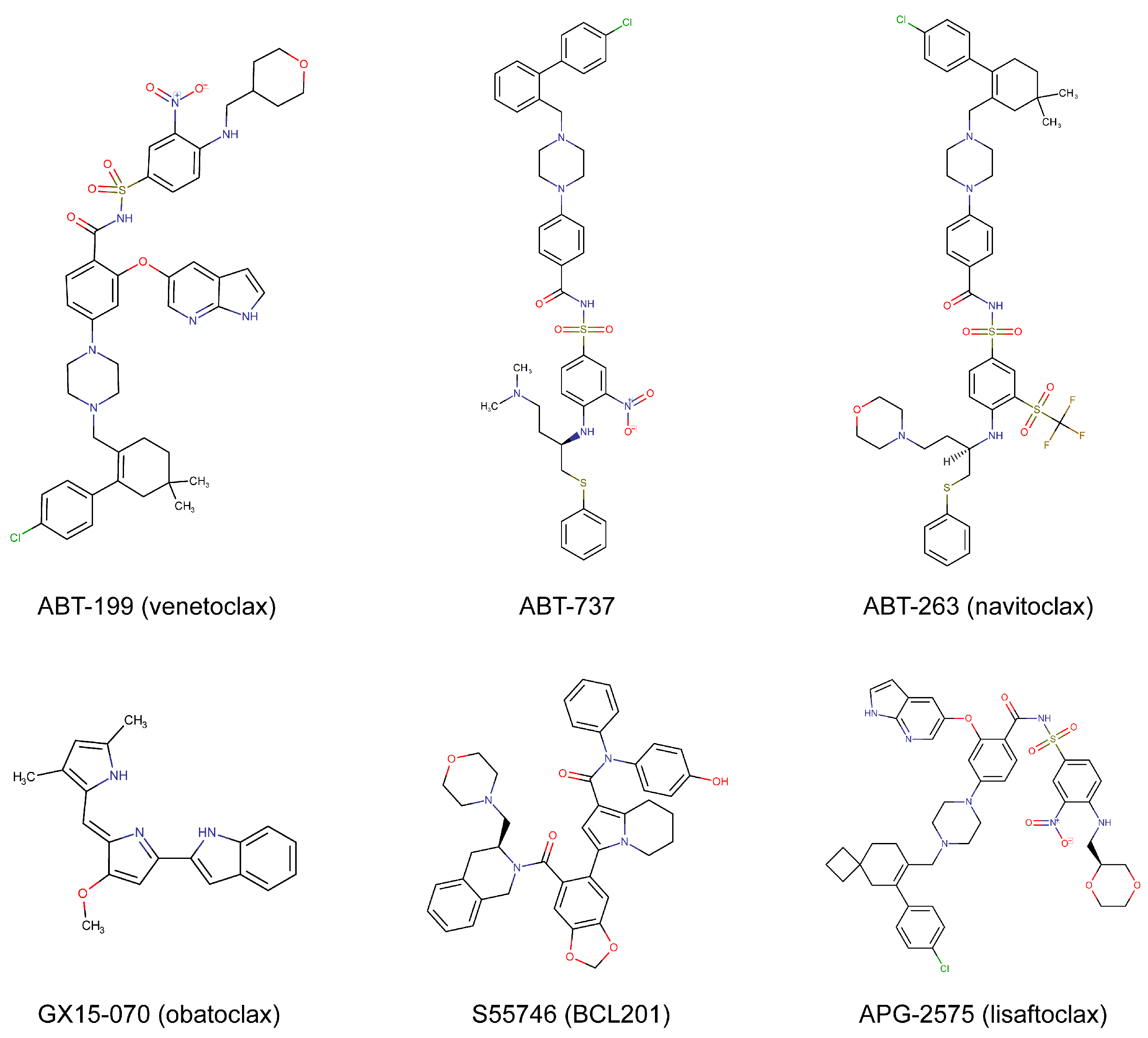
| ABT199 (venetoclax) | BCL-2 | FDA-approved | Hematologic cancers, solid tumors | [11,12,13,14] |
| S55746 (BCL201) | BCL-2 | Phase 1 | Hematologic cancers | [15,16] |
| APG-2575 (lisaftoclax) | BCL-2 | Phase 1/2 | Hematologic cancers | [17,18] |
| G3139 (oblimersen) | BCL-2 | Phase 1/2/3 | Hematologic cancers, solid tumors | [19,20,21] |
| AZD4320 | BCL-2 and BCL-XL | Preclinical | Hematologic cancers, malignant pleural mesothelioma | [22,23] |
| AZD0466 | BCL-2 and BCL-XL | Phase 1/2 | Hematologic cancers, solid tumors | [23,24] |
| APG-1252 (pelcitoclax) | BCL-2 and BCL-XL | Phase 1/2 | NSCLC, NPC, colorectal cancer, AML | [25,26,27,28] |
| BM-1197 | BCL-2 and BCL-XL | Preclinical | SCLC, adenoid cystic carcinoma, NHL | [29,30,31] |
| S44563 | BCL-2 and BCL-XL | Preclinical | SCLC, uveal melanoma | [32,33] |
| ABT-737 | BCL-2, BCL-XL, and BCL-W | Preclinical | Hematologic cancers, solid tumors | [3,4,5,6] |
| ABT-263 (navitoclax) | BCL-2, BCL-XL, and BCL-W | Phase 1/2 | Hematologic cancers, solid tumors | [7,8,9,10] |
| GX15-070 (obatoclax) | BCL-2, BCL-XL, BCL-W, and MCL-1 | Phase 1/2 | Hematologic cancers, solid tumors | [34,35,36] |
| AT-101 | BCL-2, BCL-XL, BCL-W, and MCL-1 | Phase 1/2 | Hematologic cancers, solid tumors | [37,38] |
2. Mechanisms of Drug Action
3. Substantial Progress in Hematologic Malignancies
3.1. Chronic Lymphocytic Leukemia
| Disease | Indications | Combined Agents | Treatment Outcomes | Phase | Clinical Trials | Ref. |
|---|---|---|---|---|---|---|
| CLL | R/R CLL or SLL | None | ORR: 79%; CR: 20%; 15-month PFS: 69% | 1 | NCT01328626 | [12] |
| R/R CLL with del 17p | None | ORR: 79%; 2-year PFS: 54% | 2 | NCT01889186 | [44] | |
| R/R CLL | Rituximab | ORR: 86%; CR: 51%; uMRD: 57% | 1 | NCT01682616 | [45] | |
| R/R CLL | Rituximab | 4-year PFS: 57%; 4-year OS: 85% | 3 | NCT02005471 (MURANO) | [47] | |
| Previously untreated CLL | Obinutuzumab | 2-year PFS: 88% | 3 | NCT02242942 (CLL14) | [49] | |
| R/R CLL | Ibrutinib | ORR: 89%; CR: 51%; uMRD: 53% | 2 | ISCRTN13751862 (CLARITY) | [51] | |
| Previously untreated CLL | Ibrutinib | uMRD: 75% | 2 | NCT02910583 (CAPTIVATE) | [52] | |
| R/R CLL | Obinutuzumab and ibrutinib | ORR: 92%; CR or CRi: 42% | 1 | NCT02427451 | [53] | |
| AML | R/R AML or unfit for intensive chemotherapy | None | ORR: 19%; CR: 13% | 2 | NCT01994837 | [55] |
| Previously untreated elderly AML patients ineligible for intensive chemotherapy | Azacytidine or decitabine | CR or CRi: 61% | 1 | NCT02203773 | [13] | |
| ND intensive chemotherapy ineligible and R/R AML | Decitabine | ORR: 74% | 2 | NCT03404193 | [56] | |
| Previously untreated elderly AML patients ineligible for intensive chemotherapy | Azacytidine | ORR: 37%; CR or CRi: 66%; median OS: 14.7 months | 1 | NCT02993523 (VIALE-A) | [57] | |
| Previously untreated elderly AML patients ineligible for intensive chemotherapy | Cytarabine | CR or CRi: 54%; median OS: 10.1 months; median DOR: 8.1 months | 1/2 | NCT02287233 | [58] | |
| ND-AML ineligible for intensive chemotherapy | Cytarabine | CR or CRi: 48%; median OS: 7.2 months | 3 | NCT03069352 | [59] | |
| ND-AML | FLAG- IDA | ORR: 97%; CRc: 90%; uMRD: 96%; 1-year OS: 94% | 1/2 | NCT03214562 | [60] | |
| R/R AML | ORR: 72%; CRc: 66%; uMRD: 69%; 1-year OS: 78% | |||||
| ND-AML | Decitabine and FLT3 inhibitor | CRc: 92%; uMRD: 56% | 2 | NCT03404193 | [61] | |
| R/R AML | CRc: 62%; uMRD: 63% | |||||
| MM | R/R MM | None | ORR: 21%; VGPR: 15% | 1 | NCT01794520 | [62] |
| R/R MM | Bortezomib and dexamethasone | ORR: 67%; VGPR: 42%; median DOR: 9.7 months | 1 | NCT01794507 | [63] | |
| R/R MM | Bortezomib and dexamethasone | Median PFS: 22%; TE-SAEs: 48% | 3 | NCT02755597 (BELLINI) | [64] | |
| R/R MM patients refractory to lenalidomide | Pomalidomide and dexamethasone | ORR: 53%; median DOR: 12.9 months; median PFS: 10.5 months | 2 | NCT03567616 | [65] | |
| R/R MM | Carfilzomib and dexamethasone | ORR: 80%; CR: 41%; median PFS: 22.8 months | 2 | NCT02899052 | [66] | |
| R/R MM with t(11;14) | Daratumumab and dexamethasone | ORR: 96%; 1.5-year PFS: 91% | 1 | NCT03314181 | [67] | |
| R/R MM | Daratumumab, dexamethasone, and bortezomib | ORR: 92%; 1.5-year PFS: 67% | ||||
| NHL | R/R MCL | None | ORR: 75%; median PFS: 14 months | 1 | NCT01328626 | [68] |
| R/R FL | ORR: 38%; median PFS: 11 months | |||||
| R/R DLBCL | ORR: 18%; median PFS: 1 month | |||||
| MCL | Ibrutinib | CR: 42%; uMRD: 67% | 2 | NCT02471391 (AIM) | [69] | |
| Relapsed MCL | Ibrutinib and obinutuzumab | CR: 67%; uMRD: 72%; 2-year PFS: 70% | 1/2 | NCT02558816 | [70] | |
| Untreated MCL | CR: 87%; uMRD: 100%; 1-year PFS: 93% | |||||
| B-cell NHL | R-/G-CHOP | ORR: 88% | 1 | NCT02055820 (CAVALLI) | [71] | |
| Previously untreated DLBCL | R-CHOP | CR: 69% | 2 | NCT02055820 (CAVALLI) | [72] | |
| R/R FL | Rituximab | CR: 17% | 2 | NCT02187861 (CONTRALTO) | [73] | |
| Rituximab and bendamustine | CR: 75% | |||||
| ALL | R/R ALL or LBL | Navitoclax | CR: 60% | 1 | NCT03181126 | [74] |
3.2. Acute Myeloid Leukemia
3.3. Multiple Myeloma
3.4. Other Hematologic Malignancies
4. Genetic Mutations Associated with Treatment Effectiveness
4.1. BCL-2 Mutations
4.2. Therapeutically Favorable Mutations
4.3. Therapeutically Adverse Mutations
5. Potential Applications in Solid Tumors
5.1. Breast Cancer
5.2. Lung Cancer
5.3. Pancreatic Cancer
5.4. Sarcoma
5.5. Other Solid Tumors
| Cancers | Cell Types | Synergistic Drugs | Drug Attributes | Ref. |
|---|---|---|---|---|
| Breast cancer | ER(+) breast cancer cell lines, patient-derived organoid, patient-derived xenograft | Palbociclib | CDK4/6 inhibitor | [129] |
| LM2-4, BT549, MDA-157 | Dasatinib | SRC inhibitor | [130] | |
| SKBR3, MDAMB468, T47D, CAMA-1 | AZD1775 | WEE1 inhibitor | [135] | |
| SUM149, BT474, xenografts | Neratinib | ERBB1/2/4 inhibitor | [136] | |
| MCF7, BT549, MDAMB231 | GSIXII | Gamma-secretase inhibitor | [134] | |
| Patient samples, TNBC cell lines, and xenografts | Metformin | AMPK activator | [132] | |
| Lung cancer | NCI-H146, H1963, xenografts | ABBV-075 | BET inhibitor | [143] |
| SCLC cell lines and xenografts | Dinaciclib | CDK9 inhibitor | [144] | |
| H69 and H82 | KAN0441571C | ROR1 inhibitor | [145] | |
| PC-9 and xenografts | Gefitinib | EGFR-tyrosine kinase inhibitor | [149] | |
| H157, H460, H1299, xenografts | Decitabine | DNA methyltransferase inhibitor | [148] | |
| DMS53, H460, xenografts | BKA-073 | BAK activator | [151] | |
| Pancreatic cancer | MIAPacCa-2, SW1990, xenografts | Gemcitabine | Chemotherapeutics | [153] |
| SUIT-2, MIAPaCa-2, BxPC-3 | Prexasertib | CHK1 inhibitor | [156] | |
| S2013 and MIAPaCa-2 | Analog 24 | CDK5 inhibitor | [157] | |
| Soft tissue sarcomas | STS cell lines and tumor-derived cells | Bortezomib | Proteasome inhibitor | [159] |
| RD, TE381.T, RH30, primary-derived RMS cells | JNJ | Histone deacetylase inhibitor | [160] | |
| Primary cells, ES cell lines, patient-derived and cell line-derived xenografts | Olaparib | PARP inhibitor | [162] | |
| Colorectal cancer | RKO cell line and xenografts | LZT-106 | CDK9 inhibitor | [168] |
| RKO | Birinapant/AT-406 | IAP antagonist | [166] | |
| HT-29 and HCT-116 | Perifosine | AKT inhibitor | [171] | |
| Hepatocellular carcinoma | HepG2, Hep3B, xenografts | Osimertinib | EGFR-tyrosine kinase inhibitor | [169] |
| HepG2 | Curcumin | Plant polyphenol | [172] | |
| HepG2 and SMMC-7721 | Norcantharidin | Herbal components | [173] |
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ashkenazi, A.; Fairbrother, W.J.; Leverson, J.D.; Souers, A.J. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov. 2017, 16, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Diepstraten, S.T.; Anderson, M.A.; Czabotar, P.E.; Lessene, G.; Strasser, A.; Kelly, G.L. The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs. Nat. Rev. Cancer 2021, 22, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Oltersdorf, T.; Elmore, S.W.; Shoemaker, A.R.; Armstrong, R.C.; Augeri, D.J.; Belli, B.A.; Bruncko, M.; Deckwerth, T.L.; Dinges, J.; Hajduk, P.J.; et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435, 677–681. [Google Scholar] [CrossRef]
- Del Gaizo Moore, V.; Brown, J.R.; Certo, M.; Love, T.M.; Novina, C.D.; Letai, A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J. Clin. Investig. 2007, 117, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Del Gaizo Moore, V.; Schlis, K.D.; Sallan, S.E.; Armstrong, S.A.; Letai, A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood 2008, 111, 2300–2309. [Google Scholar] [CrossRef]
- Hikita, H.; Takehara, T.; Shimizu, S.; Kodama, T.; Shigekawa, M.; Iwase, K.; Hosui, A.; Miyagi, T.; Tatsumi, T.; Ishida, H.; et al. The Bcl-xL inhibitor, ABT-737, efficiently induces apoptosis and suppresses growth of hepatoma cells in combination with sorafenib. Hepatology 2010, 52, 1310–1321. [Google Scholar] [CrossRef]
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P.; et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008, 68, 3421–3428. [Google Scholar] [CrossRef]
- Wilson, W.H.; O’Connor, O.A.; Czuczman, M.S.; LaCasce, A.S.; Gerecitano, J.F.; Leonard, J.P.; Tulpule, A.; Dunleavy, K.; Xiong, H.; Chiu, Y.L.; et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: A phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010, 11, 1149–1159. [Google Scholar] [CrossRef]
- Roberts, A.W.; Seymour, J.F.; Brown, J.R.; Wierda, W.G.; Kipps, T.J.; Khaw, S.L.; Carney, D.A.; He, S.Z.; Huang, D.C.; Xiong, H.; et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: Results of a phase I study of navitoclax in patients with relapsed or refractory disease. J. Clin. Oncol. 2012, 30, 488–496. [Google Scholar] [CrossRef]
- Nor Hisam, N.S.; Ugusman, A.; Rajab, N.F.; Ahmad, M.F.; Fenech, M.; Liew, S.L.; Mohamad Anuar, N.N. Combination Therapy of Navitoclax with Chemotherapeutic Agents in Solid Tumors and Blood Cancer: A Review of Current Evidence. Pharmaceutics 2021, 13, 1353. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Pratz, K.W.; Letai, A.; Jonas, B.A.; Wei, A.H.; Thirman, M.; Arellano, M.; Frattini, M.G.; Kantarjian, H.; Popovic, R.; et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: A non-randomised, open-label, phase 1b study. Lancet Oncol. 2018, 19, 216–228. [Google Scholar] [CrossRef]
- Lok, S.W.; Whittle, J.R.; Vaillant, F.; Teh, C.E.; Lo, L.L.; Policheni, A.N.; Bergin, A.R.T.; Desai, J.; Ftouni, S.; Gandolfo, L.C.; et al. A Phase Ib Dose-Escalation and Expansion Study of the BCL2 Inhibitor Venetoclax Combined with Tamoxifen in ER and BCL2-Positive Metastatic Breast Cancer. Cancer Discov. 2019, 9, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Casara, P.; Davidson, J.; Claperon, A.; Le Toumelin-Braizat, G.; Vogler, M.; Bruno, A.; Chanrion, M.; Lysiak-Auvity, G.; Le Diguarher, T.; Starck, J.B.; et al. S55746 is a novel orally active BCL-2 selective and potent inhibitor that impairs hematological tumor growth. Oncotarget 2018, 9, 20075–20088. [Google Scholar] [CrossRef]
- Moujalled, D.M.; Pomilio, G.; Ghiurau, C.; Ivey, A.; Salmon, J.; Rijal, S.; Macraild, S.; Zhang, L.; Teh, T.C.; Tiong, I.S.; et al. Combining BH3-mimetics to target both BCL-2 and MCL1 has potent activity in pre-clinical models of acute myeloid leukemia. Leukemia 2019, 33, 905–917. [Google Scholar] [CrossRef]
- Deng, J.; Paulus, A.; Fang, D.D.; Manna, A.; Wang, G.; Wang, H.; Zhu, S.; Chen, J.; Min, P.; Yin, Y.; et al. Lisaftoclax (APG-2575) Is a Novel BCL-2 Inhibitor with Robust Antitumor Activity in Preclinical Models of Hematologic Malignancy. Clin. Cancer Res. 2022, 28, 5455–5468. [Google Scholar] [CrossRef]
- Ailawadhi, S.; Chen, Z.; Huang, B.; Paulus, A.; Collins, M.C.; Fu, L.T.; Li, M.; Ahmad, M.; Men, L.; Wang, H.; et al. Novel BCL-2 Inhibitor Lisaftoclax in Relapsed or Refractory Chronic Lymphocytic Leukemia and Other Hematologic Malignancies: First-in-Human Open-Label Trial. Clin. Cancer Res. 2023, 29, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.; Chen, H.; Yang, D.; Figueira, M.; Bouker, K.B.; Ling, Y.; Lippman, M.; Frankel, S.R.; Hayes, D.F. A phase I trial of a Bcl-2 antisense (G3139) and weekly docetaxel in patients with advanced breast cancer and other solid tumors. Ann. Oncol. 2004, 15, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Moore, J.O.; Boyd, T.E.; Larratt, L.M.; Skotnicki, A.B.; Koziner, B.; Chanan-Khan, A.A.; Seymour, J.F.; Gribben, J.; Itri, L.M.; et al. 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. J. Clin. Oncol. 2009, 27, 5208–5212. [Google Scholar] [CrossRef]
- Walker, A.R.; Marcucci, G.; Yin, J.; Blum, W.; Stock, W.; Kohlschmidt, J.; Mrozek, K.; Carroll, A.J.; Eisfeld, A.K.; Wang, E.S.; et al. Phase 3 randomized trial of chemotherapy with or without oblimersen in older AML patients: CALGB 10201 (Alliance). Blood Adv. 2021, 5, 2775–2787. [Google Scholar] [CrossRef]
- Balachander, S.B.; Criscione, S.W.; Byth, K.F.; Cidado, J.; Adam, A.; Lewis, P.; Macintyre, T.; Wen, S.; Lawson, D.; Burke, K.; et al. AZD4320, A Dual Inhibitor of Bcl-2 and Bcl-x(L), Induces Tumor Regression in Hematologic Cancer Models without Dose-limiting Thrombocytopenia. Clin. Cancer Res. 2020, 26, 6535–6549. [Google Scholar] [CrossRef]
- Arulananda, S.; O’Brien, M.; Evangelista, M.; Jenkins, L.J.; Poh, A.R.; Walkiewicz, M.; Leong, T.; Mariadason, J.M.; Cebon, J.; Balachander, S.B.; et al. A novel BH3-mimetic, AZD0466, targeting BCL-XL and BCL-2 is effective in pre-clinical models of malignant pleural mesothelioma. Cell Death Discov. 2021, 7, 122. [Google Scholar] [CrossRef]
- Patterson, C.M.; Balachander, S.B.; Grant, I.; Pop-Damkov, P.; Kelly, B.; McCoull, W.; Parker, J.; Giannis, M.; Hill, K.J.; Gibbons, F.D.; et al. Design and optimisation of dendrimer-conjugated Bcl-2/x(L) inhibitor, AZD0466, with improved therapeutic index for cancer therapy. Commun. Biol. 2021, 4, 112. [Google Scholar] [CrossRef]
- Wang, J.; Yang, D.; Luo, Q.; Qiu, M.; Zhang, L.; Li, B.; Chen, H.; Yi, H.; Yan, X.; Li, S.; et al. APG-1252-12A induces mitochondria-dependent apoptosis through inhibiting the antiapoptotic proteins Bcl-2/Bcl-xl in HL-60 cells. Int. J. Oncol. 2017, 51, 563–572. [Google Scholar] [CrossRef]
- Luo, F.; Lu, F.T.; Qiu, M.Z.; Zhou, T.; Ma, W.J.; Luo, M.; Zeng, K.M.; Luo, Q.Y.; Pan, W.T.; Zhang, L.; et al. Gemcitabine and APG-1252, a novel small molecule inhibitor of BCL-2/BCL-XL, display a synergistic antitumor effect in nasopharyngeal carcinoma through the JAK-2/STAT3/MCL-1 signaling pathway. Cell Death Dis. 2021, 12, 772. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Vallega, K.A.; Yao, W.; Wang, D.; Zhai, Y.; He, X.; Sun, S.Y. Therapeutic potential of the novel Bcl-2/Bcl-X(L) dual inhibitor, APG1252, alone or in combination against non-small cell lung cancer. Mol. Carcinog. 2022, 61, 1031–1042. [Google Scholar] [CrossRef]
- Yao, W.; Bai, L.; Wang, S.; Zhai, Y.; Sun, S.Y. Mcl-1 levels critically impact the sensitivities of human colorectal cancer cells to APG-1252-M1, a novel Bcl-2/Bcl-X(L) dual inhibitor that induces Bax-dependent apoptosis. Neoplasia 2022, 29, 100798. [Google Scholar] [CrossRef]
- Bai, L.; Chen, J.; McEachern, D.; Liu, L.; Zhou, H.; Aguilar, A.; Wang, S. BM-1197: A novel and specific Bcl-2/Bcl-xL inhibitor inducing complete and long-lasting tumor regression in vivo. PLoS ONE 2014, 9, e99404. [Google Scholar] [CrossRef] [PubMed]
- Acasigua, G.A.; Warner, K.A.; Nor, F.; Helman, J.; Pearson, A.T.; Fossati, A.C.; Wang, S.; Nor, J.E. BH3-mimetic small molecule inhibits the growth and recurrence of adenoid cystic carcinoma. Oral Oncol. 2015, 51, 839–847. [Google Scholar] [CrossRef]
- Sun, Y.L.; Jiang, W.Q.; Luo, Q.Y.; Yang, D.J.; Cai, Y.C.; Huang, H.Q.; Sun, J. A novel Bcl-2 inhibitor, BM-1197, induces apoptosis in malignant lymphoma cells through the endogenous apoptotic pathway. BMC Cancer 2019, 20, 1. [Google Scholar] [CrossRef]
- Loriot, Y.; Mordant, P.; Dugue, D.; Geneste, O.; Gombos, A.; Opolon, P.; Guegan, J.; Perfettini, J.L.; Pierre, A.; Berthier, L.K.; et al. Radiosensitization by a novel Bcl-2 and Bcl-XL inhibitor S44563 in small-cell lung cancer. Cell Death Dis. 2014, 5, e1423. [Google Scholar] [CrossRef]
- Nemati, F.; de Montrion, C.; Lang, G.; Kraus-Berthier, L.; Carita, G.; Sastre-Garau, X.; Berniard, A.; Vallerand, D.; Geneste, O.; de Plater, L.; et al. Targeting Bcl-2/Bcl-XL induces antitumor activity in uveal melanoma patient-derived xenografts. PLoS ONE 2014, 9, e80836. [Google Scholar] [CrossRef]
- Trudel, S.; Li, Z.H.; Rauw, J.; Tiedemann, R.E.; Wen, X.Y.; Stewart, A.K. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood 2007, 109, 5430–5438. [Google Scholar] [CrossRef]
- Hwang, J.J.; Kuruvilla, J.; Mendelson, D.; Pishvaian, M.J.; Deeken, J.F.; Siu, L.L.; Berger, M.S.; Viallet, J.; Marshall, J.L. Phase I dose finding studies of obatoclax (GX15-070), a small molecule pan-BCL-2 family antagonist, in patients with advanced solid tumors or lymphoma. Clin. Cancer Res. 2010, 16, 4038–4045. [Google Scholar] [CrossRef]
- Rahmani, M.; Aust, M.M.; Attkisson, E.; Williams, D.C., Jr.; Ferreira-Gonzalez, A.; Grant, S. Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood 2012, 119, 6089–6098. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, K.; Zhang, L.; Feng, L.; Fu, G.; Jiang, S.; Bi, S.; Lin, C.; Zhou, Y.; Zhao, H.; et al. Synthetic lethality of combined AT-101 with idarubicin in acute myeloid leukemia via blockade of DNA repair and activation of intrinsic apoptotic pathway. Cancer Lett. 2019, 461, 31–43. [Google Scholar] [CrossRef]
- Renner, O.; Mayer, M.; Leischner, C.; Burkard, M.; Berger, A.; Lauer, U.M.; Venturelli, S.; Bischoff, S.C. Systematic Review of Gossypol/AT-101 in Cancer Clinical Trials. Pharmaceuticals 2022, 15, 144. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Roberts, A.W.; Wei, A.H.; Huang, D.C.S. BCL2 and MCL1 inhibitors for hematologic malignancies. Blood 2021, 138, 1120–1136. [Google Scholar] [CrossRef]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Coutre, S.; Seymour, J.F.; Munir, T.; Puvvada, S.D.; Wendtner, C.M.; Roberts, A.W.; Jurczak, W.; et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016, 17, 768–778. [Google Scholar] [CrossRef]
- Seymour, J.F.; Ma, S.; Brander, D.M.; Choi, M.Y.; Barrientos, J.; Davids, M.S.; Anderson, M.A.; Beaven, A.W.; Rosen, S.T.; Tam, C.S.; et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: A phase 1b study. Lancet Oncol. 2017, 18, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Kater, A.P.; Seymour, J.F.; Hillmen, P.; Eichhorst, B.; Langerak, A.W.; Owen, C.; Verdugo, M.; Wu, J.; Punnoose, E.A.; Jiang, Y.; et al. Fixed Duration of Venetoclax-Rituximab in Relapsed/Refractory Chronic Lymphocytic Leukemia Eradicates Minimal Residual Disease and Prolongs Survival: Post-Treatment Follow-Up of the MURANO Phase III Study. J. Clin. Oncol. 2019, 37, 269–277. [Google Scholar] [CrossRef]
- Kater, A.P.; Wu, J.Q.; Kipps, T.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Robak, T.; de la Serna, J.; et al. Venetoclax Plus Rituximab in Relapsed Chronic Lymphocytic Leukemia: 4-Year Results and Evaluation of Impact of Genomic Complexity and Gene Mutations From the MURANO Phase III Study. J. Clin. Oncol. 2020, 38, 4042–4054. [Google Scholar] [CrossRef]
- Ma, S.; Seymour, J.F.; Brander, D.M.; Kipps, T.J.; Choi, M.Y.; Anderson, M.A.; Humphrey, K.; Al Masud, A.; Pesko, J.; Nandam, R.; et al. Efficacy of venetoclax plus rituximab for relapsed CLL: 5-year follow-up of continuous or limited- duration therapy. Blood 2021, 138, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Zhang, C.; Tandon, M.; Sinha, A.; Fink, A.M.; Robrecht, S.; Samoylova, O.; Liberati, A.M.; Pinilla-Ibarz, J.; Opat, S.; et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): Follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1188–1200. [Google Scholar] [CrossRef]
- Hillmen, P.; Rawstron, A.C.; Brock, K.; Munoz-Vicente, S.; Yates, F.J.; Bishop, R.; Boucher, R.; MacDonald, D.; Fegan, C.; McCaig, A.; et al. Ibrutinib Plus Venetoclax in Relapsed/Refractory Chronic Lymphocytic Leukemia: The CLARITY Study. J. Clin. Oncol. 2019, 37, 2722–2729. [Google Scholar] [CrossRef]
- Wierda, W.G.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Opat, S.; Tedeschi, A.; Badoux, X.C.; Kuss, B.J.; Jackson, S.; Moreno, C.; et al. Ibrutinib Plus Venetoclax for First-Line Treatment of Chronic Lymphocytic Leukemia: Primary Analysis Results From the Minimal Residual Disease Cohort of the Randomized Phase II CAPTIVATE Study. J. Clin. Oncol. 2021, 39, 3853–3865. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.A.; Huang, Y.; Ruppert, A.S.; Awan, F.T.; Heerema, N.A.; Hoffman, C.; Lozanski, G.; Maddocks, K.J.; Moran, M.E.; Reid, M.A.; et al. Phase 1b study of obinutuzumab, ibrutinib, and venetoclax in relapsed and refractory chronic lymphocytic leukemia. Blood 2018, 132, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Tambaro, F.P.; Wierda, W.G. Tumour lysis syndrome in patients with chronic lymphocytic leukaemia treated with BCL-2 inhibitors: Risk factors, prophylaxis, and treatment recommendations. Lancet Haematol. 2020, 7, e168–e176. [Google Scholar] [CrossRef]
- Konopleva, M.; Pollyea, D.A.; Potluri, J.; Chyla, B.; Hogdal, L.; Busman, T.; McKeegan, E.; Salem, A.H.; Zhu, M.; Ricker, J.L.; et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016, 6, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Maiti, A.; Rausch, C.R.; Pemmaraju, N.; Naqvi, K.; Daver, N.G.; Kadia, T.M.; Borthakur, G.; Ohanian, M.; Alvarado, Y.; et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: A single-centre, phase 2 trial. Lancet Haematol. 2020, 7, e724–e736. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Dohner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Strickland, S.A., Jr.; Hou, J.Z.; Fiedler, W.; Lin, T.L.; Walter, R.B.; Enjeti, A.; Tiong, I.S.; Savona, M.; Lee, S.; et al. Venetoclax Combined with Low-Dose Cytarabine for Previously Untreated Patients with Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J. Clin. Oncol. 2019, 37, 1277–1284. [Google Scholar] [CrossRef]
- Wei, A.H.; Montesinos, P.; Ivanov, V.; DiNardo, C.D.; Novak, J.; Laribi, K.; Kim, I.; Stevens, D.A.; Fiedler, W.; Pagoni, M.; et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: A phase 3 randomized placebo-controlled trial. Blood 2020, 135, 2137–2145. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Lachowiez, C.A.; Takahashi, K.; Loghavi, S.; Xiao, L.; Kadia, T.; Daver, N.; Adeoti, M.; Short, N.J.; Sasaki, K.; et al. Venetoclax Combined with FLAG-IDA Induction and Consolidation in Newly Diagnosed and Relapsed or Refractory Acute Myeloid Leukemia. J. Clin. Oncol. 2021, 39, 2768–2778. [Google Scholar] [CrossRef]
- Maiti, A.; DiNardo, C.D.; Daver, N.G.; Rausch, C.R.; Ravandi, F.; Kadia, T.M.; Pemmaraju, N.; Borthakur, G.; Bose, P.; Issa, G.C.; et al. Triplet therapy with venetoclax, FLT3 inhibitor and decitabine for FLT3-mutated acute myeloid leukemia. Blood Cancer J. 2021, 11, 25. [Google Scholar] [CrossRef]
- Kumar, S.; Kaufman, J.L.; Gasparetto, C.; Mikhael, J.; Vij, R.; Pegourie, B.; Benboubker, L.; Facon, T.; Amiot, M.; Moreau, P.; et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 2017, 130, 2401–2409. [Google Scholar] [CrossRef]
- Moreau, P.; Chanan-Khan, A.; Roberts, A.W.; Agarwal, A.B.; Facon, T.; Kumar, S.; Touzeau, C.; Punnoose, E.A.; Cordero, J.; Munasinghe, W.; et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood 2017, 130, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Harrison, S.J.; Cavo, M.; de la Rubia, J.; Popat, R.; Gasparetto, C.; Hungria, V.; Salwender, H.; Suzuki, K.; Kim, I.; et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1630–1642. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, C.; Bowles, K.M.; Abdallah, A.O.; Morris, L.; Mander, G.; Coppola, S.; Wang, J.; Ross, J.A.; Bueno, O.F.; Arriola, E.; et al. A Phase II Study of Venetoclax in Combination with Pomalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2021, 21, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.J.; Davies, F.E.; Monohan, G.P.; Kovacsovics, T.; Burwick, N.; Jakubowiak, A.; Kaufman, J.L.; Hong, W.J.; Dail, M.; Salem, A.H.; et al. Phase 2 study of venetoclax plus carfilzomib and dexamethasone in patients with relapsed/refractory multiple myeloma. Blood Adv. 2021, 5, 3748–3759. [Google Scholar] [CrossRef]
- Bahlis, N.J.; Baz, R.; Harrison, S.J.; Quach, H.; Ho, S.J.; Vangsted, A.J.; Plesner, T.; Moreau, P.; Gibbs, S.D.; Coppola, S.; et al. Phase I Study of Venetoclax Plus Daratumumab and Dexamethasone, with or without Bortezomib, in Patients with Relapsed or Refractory Multiple Myeloma with and without t(11;14). J. Clin. Oncol. 2021, 39, 3602–3612. [Google Scholar] [CrossRef]
- Davids, M.S.; Roberts, A.W.; Seymour, J.F.; Pagel, J.M.; Kahl, B.S.; Wierda, W.G.; Puvvada, S.; Kipps, T.J.; Anderson, M.A.; Salem, A.H.; et al. Phase I First-in-Human Study of Venetoclax in Patients with Relapsed or Refractory Non-Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Anderson, M.A.; Pott, C.; Agarwal, R.; Handunnetti, S.; Hicks, R.J.; Burbury, K.; Turner, G.; Di Iulio, J.; Bressel, M.; et al. Ibrutinib plus Venetoclax for the Treatment of Mantle-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Le Gouill, S.; Morschhauser, F.; Chiron, D.; Bouabdallah, K.; Cartron, G.; Casasnovas, O.; Bodet-Milin, C.; Ragot, S.; Bossard, C.; Nadal, N.; et al. Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: A phase 1/2 trial. Blood 2021, 137, 877–887. [Google Scholar] [CrossRef]
- Zelenetz, A.D.; Salles, G.; Mason, K.D.; Casulo, C.; Le Gouill, S.; Sehn, L.H.; Tilly, H.; Cartron, G.; Chamuleau, M.E.D.; Goy, A.; et al. Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: Results from the CAVALLI phase 1b trial. Blood 2019, 133, 1964–1976. [Google Scholar] [CrossRef]
- Morschhauser, F.; Feugier, P.; Flinn, I.W.; Gasiorowski, R.; Greil, R.; Illes, A.; Johnson, N.A.; Larouche, J.F.; Lugtenburg, P.J.; Patti, C.; et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood 2021, 137, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.L.; Flinn, I.W.; Yuen, S.L.S.; Topp, M.S.; Rusconi, C.; Fleury, I.; Le Du, K.; Arthur, C.; Pro, B.; Gritti, G.; et al. Venetoclax-rituximab with or without bendamustine vs bendamustine-rituximab in relapsed/refractory follicular lymphoma. Blood 2020, 136, 2628–2637. [Google Scholar]
- Pullarkat, V.A.; Lacayo, N.J.; Jabbour, E.; Rubnitz, J.E.; Bajel, A.; Laetsch, T.W.; Leonard, J.; Colace, S.I.; Khaw, S.L.; Fleming, S.A.; et al. Venetoclax and Navitoclax in Combination with Chemotherapy in Patients with Relapsed or Refractory Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma. Cancer Discov. 2021, 11, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Kadia, T.M.; Reville, P.K.; Borthakur, G.; Yilmaz, M.; Kornblau, S.; Alvarado, Y.; Dinardo, C.D.; Daver, N.; Jain, N.; Pemmaraju, N.; et al. Venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: A cohort from a single-centre, single-arm, phase 2 trial. Lancet Haematol. 2021, 8, e552–e561. [Google Scholar] [CrossRef]
- Garcia, J.S.; Kim, H.T.; Murdock, H.M.; Cutler, C.S.; Brock, J.; Gooptu, M.; Ho, V.T.; Koreth, J.; Nikiforow, S.; Romee, R.; et al. Adding venetoclax to fludarabine/busulfan RIC transplant for high-risk MDS and AML is feasible, safe, and active. Blood Adv. 2021, 5, 5536–5545. [Google Scholar] [CrossRef] [PubMed]
- King, A.L.O.; Mirza, F.N.; Lewis, J.M.; Carlson, K.R.; Huntington, S.; Foss, F.M.; Girardi, M. B-cell lymphoma 2 inhibitor venetoclax treatment of a patient with cutaneous T-cell lymphoma. JAAD Case Rep. 2021, 8, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Cyrenne, B.M.; Lewis, J.M.; Weed, J.G.; Carlson, K.R.; Mirza, F.N.; Foss, F.M.; Girardi, M. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood 2017, 130, 2073–2083. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, H. Progress in understanding the mechanisms of resistance to BCL-2 inhibitors. Exp. Hematol. Oncol. 2022, 11, 31. [Google Scholar] [CrossRef]
- Blombery, P.; Anderson, M.A.; Gong, J.N.; Thijssen, R.; Birkinshaw, R.W.; Thompson, E.R.; Teh, C.E.; Nguyen, T.; Xu, Z.; Flensburg, C.; et al. Acquisition of the Recurrent Gly101Val Mutation in BCL2 Confers Resistance to Venetoclax in Patients with Progressive Chronic Lymphocytic Leukemia. Cancer Discov. 2019, 9, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Birkinshaw, R.W.; Gong, J.N.; Luo, C.S.; Lio, D.; White, C.A.; Anderson, M.A.; Blombery, P.; Lessene, G.; Majewski, I.J.; Thijssen, R.; et al. Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nat. Commun. 2019, 10, 2385. [Google Scholar] [CrossRef]
- Tausch, E.; Close, W.; Dolnik, A.; Bloehdorn, J.; Chyla, B.; Bullinger, L.; Dohner, H.; Mertens, D.; Stilgenbauer, S. Venetoclax resistance and acquired BCL2 mutations in chronic lymphocytic leukemia. Haematologica 2019, 104, e434–e437. [Google Scholar] [CrossRef]
- Blombery, P.; Thompson, E.R.; Nguyen, T.; Birkinshaw, R.W.; Gong, J.N.; Chen, X.; McBean, M.; Thijssen, R.; Conway, T.; Anderson, M.A.; et al. Multiple BCL2 mutations cooccurring with Gly101Val emerge in chronic lymphocytic leukemia progression on venetoclax. Blood 2020, 135, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Thangavadivel, S.; Byrd, J.C. Gly101Val BCL2 Mutation: One Step Closer to Understanding Venetoclax Resistance in CLL. Cancer Discov. 2019, 9, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Lucas, F.; Larkin, K.; Gregory, C.T.; Orwick, S.; Doong, T.J.; Lozanski, A.; Lozanski, G.; Misra, S.; Ngankeu, A.; Ozer, H.G.; et al. Novel BCL2 mutations in venetoclax-resistant, ibrutinib-resistant CLL patients with BTK/PLCG2 mutations. Blood 2020, 135, 2192–2195. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, J.; Wang, H.; Wang, Y.; Zhang, Y.; Qian, P.; Lou, Y.; Jin, J.; Zhu, H. Not BCL2 mutation but dominant mutation conversation contributed to acquired venetoclax resistance in acute myeloid leukemia. Biomark. Res. 2021, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Kanagal-Shamanna, R.; Navsaria, L.; Ok, C.Y.; Zhang, S.; Nomie, K.; Han, G.; Hao, D.; Hill, H.A.; Jiang, C.; et al. Efficacy of venetoclax in high risk relapsed mantle cell lymphoma (MCL)—Outcomes and mutation profile from venetoclax resistant MCL patients. Am. J. Hematol. 2020, 95, 623–629. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Rausch, C.R.; Benton, C.; Kadia, T.; Jain, N.; Pemmaraju, N.; Daver, N.; Covert, W.; Marx, K.R.; Mace, M.; et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am. J. Hematol. 2018, 93, 401–407. [Google Scholar] [CrossRef]
- Wang, Y.W.; Tsai, C.H.; Lin, C.C.; Tien, F.M.; Chen, Y.W.; Lin, H.Y.; Yao, M.; Lin, Y.C.; Lin, C.T.; Cheng, C.L.; et al. Cytogenetics and mutations could predict outcome in relapsed and refractory acute myeloid leukemia patients receiving BCL-2 inhibitor venetoclax. Ann. Hematol. 2020, 99, 501–511. [Google Scholar] [CrossRef]
- Cherry, E.M.; Abbott, D.; Amaya, M.; McMahon, C.; Schwartz, M.; Rosser, J.; Sato, A.; Schowinsky, J.; Inguva, A.; Minhajuddin, M.; et al. Venetoclax and azacitidine compared with induction chemotherapy for newly diagnosed patients with acute myeloid leukemia. Blood Adv. 2021, 5, 5565–5573. [Google Scholar] [CrossRef]
- Chow, S.; Tang, K.; Al-Abri, M.; Hall, V.; Tremblay-Lemay, R.; Rashedi, I.; Tsui, H.; Chan, S.M. RUNX1 mutations correlate with response to venetoclax combination therapies in relapsed/refractory acute myeloid leukemia. Leuk. Res. 2021, 111, 106735. [Google Scholar] [CrossRef]
- Wang, J.; Ye, X.; Fan, C.; Zhou, J.; Luo, S.; Jin, J.; Chen, D.; Zheng, Y.; Wu, C.; Zhu, X.; et al. Leukemia cutis with IDH1, DNMT3A and NRAS mutations conferring resistance to venetoclax plus 5-azacytidine in refractory AML. Biomark. Res. 2020, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Mill, C.P.; Fiskus, W.; DiNardo, C.D.; Birdwell, C.; Davis, J.A.; Kadia, T.M.; Takahashi, K.; Short, N.; Daver, N.; Ohanian, M.; et al. Effective therapy for AML with RUNX1 mutation by cotreatment with inhibitors of protein translation and BCL2. Blood 2022, 139, 907–921. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Tiong, I.S.; Quaglieri, A.; MacRaild, S.; Loghavi, S.; Brown, F.C.; Thijssen, R.; Pomilio, G.; Ivey, A.; Salmon, J.M.; et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 2020, 135, 791–803. [Google Scholar] [CrossRef]
- Stahl, M.; Menghrajani, K.; Derkach, A.; Chan, A.; Xiao, W.; Glass, J.; King, A.C.; Daniyan, A.F.; Famulare, C.; Cuello, B.M.; et al. Clinical and molecular predictors of response and survival following venetoclax therapy in relapsed/refractory AML. Blood Adv. 2021, 5, 1552–1564. [Google Scholar] [CrossRef]
- Fleischmann, M.; Scholl, S.; Frietsch, J.J.; Hilgendorf, I.; Schrenk, K.; Hammersen, J.; Prims, F.; Thiede, C.; Hochhaus, A.; Schnetzke, U. Clinical experience with venetoclax in patients with newly diagnosed, relapsed, or refractory acute myeloid leukemia. J. Cancer Res. Clin. Oncol. 2022, 148, 3191–3202. [Google Scholar] [CrossRef]
- Bisaillon, R.; Moison, C.; Thiollier, C.; Krosl, J.; Bordeleau, M.E.; Lehnertz, B.; Lavallee, V.P.; MacRae, T.; Mayotte, N.; Labelle, C.; et al. Genetic characterization of ABT-199 sensitivity in human AML. Leukemia 2020, 34, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Tiong, I.S.; Dillon, R.; Ivey, A.; Teh, T.C.; Nguyen, P.; Cummings, N.; Taussig, D.C.; Latif, A.L.; Potter, N.E.; Runglall, M.; et al. Venetoclax induces rapid elimination of NPM1 mutant measurable residual disease in combination with low-intensity chemotherapy in acute myeloid leukaemia. Br. J. Haematol. 2021, 192, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.A.; Loghavi, S.; Kadia, T.M.; Daver, N.; Borthakur, G.; Pemmaraju, N.; Naqvi, K.; Alvarado, Y.; Yilmaz, M.; Short, N.; et al. Outcomes of older patients with NPM1-mutated AML: Current treatments and the promise of venetoclax-based regimens. Blood Adv. 2020, 4, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.H.; Qian, J.J.; Sun, W.J.; You, L.S.; Wang, Q.Q.; Naranmandura, H.; Jin, J. Venetoclax and arsenic showed synergistic anti-leukemia activity in vitro and in vivo for acute myeloid leukemia with the NPM1 mutation. Am. J. Hematol. 2020, 95, E55–E57. [Google Scholar] [CrossRef]
- Chan, S.M.; Thomas, D.; Corces-Zimmerman, M.R.; Xavy, S.; Rastogi, S.; Hong, W.J.; Zhao, F.; Medeiros, B.C.; Tyvoll, D.A.; Majeti, R. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat. Med. 2015, 21, 178–184. [Google Scholar] [CrossRef]
- Pollyea, D.A.; DiNardo, C.D.; Arellano, M.L.; Pigneux, A.; Fiedler, W.; Konopleva, M.; Rizzieri, D.A.; Smith, B.D.; Shinagawa, A.; Lemoli, R.M.; et al. Impact of Venetoclax and Azacitidine in Treatment-Naive Patients with Acute Myeloid Leukemia and IDH1/2 Mutations. Clin. Cancer Res. 2022, 28, 2753–2761. [Google Scholar] [CrossRef]
- Buege, M.J.; DiPippo, A.J.; DiNardo, C.D. Evolving Treatment Strategies for Elderly Leukemia Patients with IDH Mutations. Cancers 2018, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Jasra, S.; Kazemi, M.; Shah, N.; Chen, J.; Fehn, K.; Wang, Y.; Mantzaris, I.; Kornblum, N.; Sica, A.; Bachier, L.; et al. Case report of combination therapy with Azacytidine, Enasidenib and Venetoclax in primary refractory AML. Exp. Hematol. Oncol. 2021, 10, 1. [Google Scholar] [CrossRef]
- Cathelin, S.; Sharon, D.; Subedi, A.; Cojocari, D.; Phillips, D.C.; Leverson, J.D.; MacBeth, K.J.; Nicolay, B.; Narayanaswamy, R.; Ronseaux, S.; et al. Enasidenib-induced differentiation promotes sensitivity to venetoclax in IDH2-mutated acute myeloid leukemia. Leukemia 2022, 36, 869–872. [Google Scholar] [CrossRef]
- Venugopal, S.; Maiti, A.; DiNardo, C.D.; Loghavi, S.; Daver, N.G.; Kadia, T.M.; Rausch, C.R.; Alvarado, Y.; Ohanian, M.; Sasaki, K.; et al. Decitabine and venetoclax for IDH1/2-mutated acute myeloid leukemia. Am. J. Hematol. 2021, 96, E154–E157. [Google Scholar] [CrossRef]
- Venugopal, S.; Takahashi, K.; Daver, N.; Maiti, A.; Borthakur, G.; Loghavi, S.; Short, N.J.; Ohanian, M.; Masarova, L.; Issa, G.; et al. Efficacy and safety of enasidenib and azacitidine combination in patients with IDH2 mutated acute myeloid leukemia and not eligible for intensive chemotherapy. Blood Cancer J. 2022, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Aldoss, I.; Yang, D.; Pillai, R.; Sanchez, J.F.; Mei, M.; Aribi, A.; Ali, H.; Sandhu, K.; Al Malki, M.M.; Salhotra, A.; et al. Association of leukemia genetics with response to venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Am. J. Hematol. 2019, 94, E253–E255. [Google Scholar] [CrossRef]
- Nechiporuk, T.; Kurtz, S.E.; Nikolova, O.; Liu, T.; Jones, C.L.; D’Alessandro, A.; Culp-Hill, R.; d’Almeida, A.; Joshi, S.K.; Rosenberg, M.; et al. The TP53 Apoptotic Network Is a Primary Mediator of Resistance to BCL2 Inhibition in AML Cells. Cancer Discov. 2019, 9, 910–925. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W.; Ma, S.; Kipps, T.J.; Coutre, S.E.; Davids, M.S.; Eichhorst, B.; Hallek, M.; Byrd, J.C.; Humphrey, K.; Zhou, L.; et al. Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood 2019, 134, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Tausch, E.; Schneider, C.; Robrecht, S.; Zhang, C.; Dolnik, A.; Bloehdorn, J.; Bahlo, J.; Al-Sawaf, O.; Ritgen, M.; Fink, A.M.; et al. Prognostic and predictive impact of genetic markers in patients with CLL treated with obinutuzumab and venetoclax. Blood 2020, 135, 2402–2412. [Google Scholar] [CrossRef]
- Kim, K.; Maiti, A.; Loghavi, S.; Pourebrahim, R.; Kadia, T.M.; Rausch, C.R.; Furudate, K.; Daver, N.G.; Alvarado, Y.; Ohanian, M.; et al. Outcomes of TP53-mutant acute myeloid leukemia with decitabine and venetoclax. Cancer 2021, 127, 3772–3781. [Google Scholar] [CrossRef]
- Venugopal, S.; Shoukier, M.; Konopleva, M.; Dinardo, C.D.; Ravandi, F.; Short, N.J.; Andreeff, M.; Borthakur, G.; Daver, N.; Pemmaraju, N.; et al. Outcomes in patients with newly diagnosed TP53-mutated acute myeloid leukemia with or without venetoclax-based therapy. Cancer 2021, 127, 3541–3551. [Google Scholar] [CrossRef] [PubMed]
- Aldoss, I.; Zhang, J.; Pillai, R.; Shouse, G.; Sanchez, J.F.; Mei, M.; Nakamura, R.; Stein, A.S.; Forman, S.J.; Marcucci, G.; et al. Venetoclax and hypomethylating agents in TP53-mutated acute myeloid leukaemia. Br. J. Haematol. 2019, 187, e45–e48. [Google Scholar] [CrossRef]
- Chyla, B.; Daver, N.; Doyle, K.; McKeegan, E.; Huang, X.; Ruvolo, V.; Wang, Z.; Chen, K.; Souers, A.; Leverson, J.; et al. Genetic Biomarkers of Sensitivity and Resistance to Venetoclax Monotherapy in Patients with Relapsed Acute Myeloid Leukemia. Am. J. Hematol. 2018, 93, E202–E205. [Google Scholar] [CrossRef]
- Konopleva, M.; Thirman, M.J.; Pratz, K.W.; Garcia, J.S.; Recher, C.; Pullarkat, V.; Kantarjian, H.M.; DiNardo, C.D.; Dail, M.; Duan, Y.; et al. Impact of F LT3 Mutation on Outcomes after Venetoclax and Azacitidine for Patients with Treatment-Naive Acute Myeloid Leukemia. Clin. Cancer Res. 2022, 28, 2744–2752. [Google Scholar] [CrossRef] [PubMed]
- Aldoss, I.; Zhang, J.; Mei, M.; Al Malki, M.M.; Arslan, S.; Ngo, D.; Aribi, A.; Ali, H.; Sandhu, K.; Salhotra, A.; et al. Venetoclax and hypomethylating agents in FLT3-mutated acute myeloid leukemia. Am. J. Hematol. 2020, 95, 1193–1199. [Google Scholar] [CrossRef]
- Brinton, L.T.; Zhang, P.; Williams, K.; Canfield, D.; Orwick, S.; Sher, S.; Wasmuth, R.; Beaver, L.; Cempre, C.; Skinner, J.; et al. Synergistic effect of BCL2 and FLT3 co-inhibition in acute myeloid leukemia. J. Hematol. Oncol. 2020, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Singh Mali, R.; Zhang, Q.; DeFilippis, R.A.; Cavazos, A.; Kuruvilla, V.M.; Raman, J.; Mody, V.; Choo, E.F.; Dail, M.; Shah, N.P.; et al. Venetoclax combines synergistically with FLT3 inhibition to effectively target leukemic cells in FLT3-ITD+ acute myeloid leukemia models. Haematologica 2021, 106, 1034–1046. [Google Scholar] [CrossRef]
- Zhang, L.S.; Wang, J.; Xu, M.Z.; Wu, T.M.; Huang, S.M.; Cao, H.Y.; Sun, A.N.; Liu, S.B.; Xue, S.L. Rapid and Efficient Response to Gilteritinib and Venetoclax-Based Therapy in Two AML Patients with FLT3-ITD Mutation Unresponsive to Venetoclax Plus Azacitidine. OncoTargets Ther. 2022, 15, 159–164. [Google Scholar] [CrossRef]
- Post, S.M.; Ma, H.; Malaney, P.; Zhang, X.; Aitken, M.J.L.; Mak, P.Y.; Ruvolo, V.R.; Yasuhiro, T.; Kozaki, R.; Chan, L.E.; et al. AXL/MERTK inhibitor ONO-7475 potently synergizes with venetoclax and overcomes venetoclax resistance to kill FLT3-ITD acute myeloid leukemia. Haematologica 2021, 107, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Gangat, N.; Guglielmelli, P.; Szuber, N.; Begna, K.H.; Patnaik, M.M.; Litzow, M.R.; Al-Kali, A.; Foran, J.M.; Palmer, J.M.; Alkhateeb, H.; et al. Venetoclax with azacitidine or decitabine in blast-phase myeloproliferative neoplasm: A multicenter series of 32 consecutive cases. Am. J. Hematol. 2021, 96, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Morsia, E.; McCullough, K.; Joshi, M.; Cook, J.; Alkhateeb, H.B.; Al-Kali, A.; Begna, K.; Elliott, M.; Hogan, W.; Litzow, M.; et al. Venetoclax and hypomethylating agents in acute myeloid leukemia: Mayo Clinic series on 86 patients. Am. J. Hematol. 2020, 95, 1511–1521. [Google Scholar] [CrossRef]
- Pan, W.; Zhao, X.; Shi, W.; Jiang, Z.; Xiao, H. Venetoclax induced complete remission in extramedullary relapse of AML co-harboring NPM1, TET2, and NRAS mutations after haploidentical hematopoietic stem cell transplantation. Leuk. Lymphoma 2020, 61, 2756–2759. [Google Scholar] [CrossRef]
- Vaillant, F.; Merino, D.; Lee, L.; Breslin, K.; Pal, B.; Ritchie, M.E.; Smyth, G.K.; Christie, M.; Phillipson, L.J.; Burns, C.J.; et al. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell 2013, 24, 120–129. [Google Scholar] [CrossRef]
- Merino, D.; Lok, S.W.; Visvader, J.E.; Lindeman, G.J. Targeting BCL-2 to enhance vulnerability to therapy in estrogen receptor-positive breast cancer. Oncogene 2016, 35, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Muttiah, C.; Travers, A.; Whittle, J.R.; Dawson, S.-J.; Yeo, B.; Visvader, J.E.; Oakman, C.; Lindeman, G.J. Abstract OT-27-01: PALVEN: A phase 1b study of palbociclib, letrozole and venetoclax in estrogen receptor, BCL2-positive metastatic breast cancer. Cancer Res. 2021, 81, OT-27-01. [Google Scholar] [CrossRef]
- Lindeman, G.J.; Bowen, R.; Jerzak, K.J.; Song, X.; Decker, T.; Boyle, F.M.; McCune, S.L.; Armstrong, A.; Shannon, C.M.; Bertelli, G.; et al. Results from VERONICA: A randomized, phase II study of second-/third-line venetoclax (VEN) + fulvestrant (F) versus F alone in estrogen receptor (ER)-positive, HER2-negative, locally advanced, or metastatic breast cancer (LA/MBC). J. Clin. Oncol. 2021, 39, 1004. [Google Scholar] [CrossRef]
- Whittle, J.R.; Vaillant, F.; Surgenor, E.; Policheni, A.N.; Giner, G.; Capaldo, B.D.; Chen, H.R.; Liu, H.K.; Dekkers, J.F.; Sachs, N.; et al. Dual Targeting of CDK4/6 and BCL2 Pathways Augments Tumor Response in Estrogen Receptor-Positive Breast Cancer. Clin. Cancer Res. 2020, 26, 4120–4134. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, Y.; Desgrosellier, J.S. Combined Bcl-2/Src inhibition synergize to deplete stem-like breast cancer cells. Cancer Lett. 2019, 457, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.; Vander Steen, T.; Espinoza, I.; Venkatapoorna, C.M.K.; Hu, Z.; Silva, F.M.; Regan, K.; Cuyas, E.; Meng, X.W.; Verdura, S.; et al. Fatty acid synthase (FASN) regulates the mitochondrial priming of cancer cells. Cell Death Dis. 2021, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Haikala, H.M.; Anttila, J.M.; Marques, E.; Raatikainen, T.; Ilander, M.; Hakanen, H.; Ala-Hongisto, H.; Savelius, M.; Balboa, D.; Von Eyss, B.; et al. Pharmacological reactivation of MYC-dependent apoptosis induces susceptibility to anti-PD-1 immunotherapy. Nat. Commun. 2019, 10, 620. [Google Scholar] [CrossRef]
- Alhoshani, A.; Alatawi, F.O.; Al-Anazi, F.E.; Attafi, I.M.; Zeidan, A.; Agouni, A.; El Gamal, H.M.; Shamoon, L.S.; Khalaf, S.; Korashy, H.M. BCL-2 Inhibitor Venetoclax Induces Autophagy-Associated Cell Death, Cell Cycle Arrest, and Apoptosis in Human Breast Cancer Cells. OncoTargets Ther. 2020, 13, 13357–13370. [Google Scholar] [CrossRef] [PubMed]
- Seveno, C.; Loussouarn, D.; Brechet, S.; Campone, M.; Juin, P.; Barille-Nion, S. gamma-Secretase inhibition promotes cell death, Noxa upregulation, and sensitization to BH3 mimetic ABT-737 in human breast cancer cells. Breast Cancer Res. 2012, 14, R96. [Google Scholar] [CrossRef]
- Aka, Y.; Karakas, B.; Acikbas, U.; Basaga, H.; Gul, O.; Kutuk, O. Kinome-wide RNAi screening for mediators of ABT-199 resistance in breast cancer cells identifies Wee1 as a novel therapeutic target. Int. J. Biochem. Cell Biol. 2021, 137, 106028. [Google Scholar] [CrossRef]
- Booth, L.; Roberts, J.L.; Avogadri-Connors, F.; Cutler, R.E., Jr.; Lalani, A.S.; Poklepovic, A.; Dent, P. The irreversible ERBB1/2/4 inhibitor neratinib interacts with the BCL-2 inhibitor venetoclax to kill mammary cancer cells. Cancer Biol. Ther. 2018, 19, 239–247. [Google Scholar] [CrossRef]
- Liu, D.; Qin, X.; Sun, Z.; Hou, S.; Lv, Q. Low DEDD expression in breast cancer cells indicates higher sensitivity to the Bcl-2-specific inhibitor ABT-199. Biochem. Biophys. Res. Commun. 2020, 525, 549–556. [Google Scholar] [CrossRef]
- Crump, L.S.; Wyatt, G.L.; Rutherford, T.R.; Richer, J.K.; Porter, W.W.; Lyons, T.R. Hormonal Regulation of Semaphorin 7a in ER+ Breast Cancer Drives Therapeutic Resistance. Cancer Res. 2021, 81, 187–198. [Google Scholar] [CrossRef]
- Young, A.; Bu, W.; Jiang, W.; Ku, A.; Kapali, J.; Dhamne, S.; Qin, L.; Hilsenbeck, S.G.; Du, Y.N.; Li, Y. Targeting the Pro-survival Protein BCL-2 to Prevent Breast Cancer. Cancer Prev. Res. 2022, 15, 3–10. [Google Scholar] [CrossRef]
- Goodwin, C.M.; Rossanese, O.W.; Olejniczak, E.T.; Fesik, S.W. Myeloid cell leukemia-1 is an important apoptotic survival factor in triple-negative breast cancer. Cell Death Differ. 2015, 22, 2098–2106. [Google Scholar] [CrossRef]
- Lucantoni, F.; Lindner, A.U.; O’Donovan, N.; Dussmann, H.; Prehn, J.H.M. Systems modeling accurately predicts responses to genotoxic agents and their synergism with BCL-2 inhibitors in triple negative breast cancer cells. Cell Death Dis. 2018, 9, 42. [Google Scholar] [CrossRef]
- Lochmann, T.L.; Floros, K.V.; Naseri, M.; Powell, K.M.; Cook, W.; March, R.J.; Stein, G.T.; Greninger, P.; Maves, Y.K.; Saunders, L.R.; et al. Venetoclax Is Effective in Small-Cell Lung Cancers with High BCL-2 Expression. Clin. Cancer Res. 2018, 24, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.T.; Lin, X.; Faivre, E.J.; Yang, Z.; Huang, X.; Wilcox, D.M.; Bellin, R.J.; Jin, S.; Tahir, S.K.; Mitten, M.; et al. Vulnerability of Small-Cell Lung Cancer to Apoptosis Induced by the Combination of BET Bromodomain Proteins and BCL2 Inhibitors. Mol. Cancer Ther. 2017, 16, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Yamauchi, A.; Jeng, P.S.; Kim, K.; Chen, H.C.; Han, S.; Ganesan, Y.T.; Ishizawa, K.; Jebiwott, S.; Dong, Y.; Pietanza, M.C.; et al. Targeting the differential addiction to anti-apoptotic BCL-2 family for cancer therapy. Nat. Commun. 2017, 8, 16078. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Z.; Shilo, K.; Amann, J.M.; Shulman, A.; Hojjat-Farsangi, M.; Mellstedt, H.; Schultz, J.; Croce, C.M.; Carbone, D.P. Predicting ROR1/BCL2 combination targeted therapy of small cell carcinoma of the lung. Cell Death Dis. 2021, 12, 577. [Google Scholar] [CrossRef]
- Munkhbaatar, E.; Dietzen, M.; Agrawal, D.; Anton, M.; Jesinghaus, M.; Boxberg, M.; Pfarr, N.; Bidola, P.; Uhrig, S.; Hockendorf, U.; et al. MCL-1 gains occur with high frequency in lung adenocarcinoma and can be targeted therapeutically. Nat. Commun. 2020, 11, 4527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, L.; Zhang, Q.; Yan, Y.; Jiang, H.; Hu, R.; Zhou, X.; Liu, X.; Feng, J.; Lin, N. The Ibr-7 derivative of ibrutinib exhibits enhanced cytotoxicity against non-small cell lung cancer cells via targeting of mTORC1/S6 signaling. Mol. Oncol. 2019, 13, 946–958. [Google Scholar] [CrossRef]
- Kim, M.J.; Chen, G.; Sica, G.L.; Deng, X. Epigenetic modulation of FBW7/Mcl-1 pathway for lung cancer therapy. Cancer Biol. Ther. 2021, 22, 55–65. [Google Scholar] [CrossRef]
- Liu, Z.; Shah, N.; Marshall, K.L.; Sprowls, S.A.; Saralkar, P.; Mohammad, A.; Blethen, K.E.; Arsiwala, T.A.; Fladeland, R.; Lockman, P.R.; et al. Overcoming the acquired resistance to gefitinib in lung cancer brain metastasis in vitro and in vivo. Arch. Toxicol. 2021, 95, 3575–3587. [Google Scholar] [CrossRef]
- Potter, D.S.; Du, R.; Bhola, P.; Bueno, R.; Letai, A. Dynamic BH3 profiling identifies active BH3 mimetic combinations in non-small cell lung cancer. Cell Death Dis. 2021, 12, 741. [Google Scholar] [CrossRef]
- Park, D.; Anisuzzaman, A.S.M.; Magis, A.T.; Chen, G.; Xie, M.; Zhang, G.; Behera, M.; Sica, G.L.; Ramalingam, S.S.; Owonikoko, T.K.; et al. Discovery of Small Molecule Bak Activator for Lung Cancer Therapy. Theranostics 2021, 11, 8500–8516. [Google Scholar] [CrossRef]
- Tan, Y.; Li, X.; Tian, Z.; Chen, S.; Zou, J.; Lian, G.; Chen, S.; Huang, K.; Chen, Y. TIMP1 down-regulation enhances gemcitabine sensitivity and reverses chemoresistance in pancreatic cancer. Biochem. Pharmacol. 2021, 189, 114085. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, H.; Xue, R.; Tang, W.; Zhang, S. BH3 Mimetic ABT-199 Enhances the Sensitivity of Gemcitabine in Pancreatic Cancer in vitro and in vivo. Dig. Dis. Sci. 2018, 63, 3367–3375. [Google Scholar] [CrossRef]
- Jakubowska, M.A.; Kerkhofs, M.; Martines, C.; Efremov, D.G.; Gerasimenko, J.V.; Gerasimenko, O.V.; Petersen, O.H.; Bultynck, G.; Vervliet, T.; Ferdek, P.E. ABT-199 (Venetoclax), a BH3-mimetic Bcl-2 inhibitor, does not cause Ca(2+)-signalling dysregulation or toxicity in pancreatic acinar cells. Br. J. Pharmacol. 2019, 176, 4402–4415. [Google Scholar] [CrossRef]
- King, H.M.; Rana, S.; Kubica, S.P.; Mallareddy, J.R.; Kizhake, S.; Ezell, E.L.; Zahid, M.; Naldrett, M.J.; Alvarez, S.; Law, H.C.; et al. Aminopyrazole based CDK9 PROTAC sensitizes pancreatic cancer cells to venetoclax. Bioorg. Med. Chem. Lett. 2021, 43, 128061. [Google Scholar] [CrossRef]
- Morimoto, Y.; Takada, K.; Takeuchi, O.; Watanabe, K.; Hirohara, M.; Hamamoto, T.; Masuda, Y. Bcl-2/Bcl-xL inhibitor navitoclax increases the antitumor effect of Chk1 inhibitor prexasertib by inducing apoptosis in pancreatic cancer cells via inhibition of Bcl-xL but not Bcl-2. Mol. Cell. Biochem. 2020, 472, 187–198. [Google Scholar] [CrossRef]
- Kour, S.; Rana, S.; Contreras, J.I.; King, H.M.; Robb, C.M.; Sonawane, Y.A.; Bendjennat, M.; Crawford, A.J.; Barger, C.J.; Kizhake, S.; et al. CDK5 Inhibitor Downregulates Mcl-1 and Sensitizes Pancreatic Cancer Cell Lines to Navitoclax. Mol. Pharmacol. 2019, 96, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Lafontaine, J.; Cardin, G.B.; Malaquin, N.; Boisvert, J.S.; Rodier, F.; Wong, P. Senolytic Targeting of Bcl-2 Anti-Apoptotic Family Increases Cell Death in Irradiated Sarcoma Cells. Cancers 2021, 13, 386. [Google Scholar] [CrossRef] [PubMed]
- Muenchow, A.; Weller, S.; Hinterleitner, C.; Malenke, E.; Bugl, S.; Wirths, S.; Muller, M.R.; Schulze-Osthoff, K.; Aulitzky, W.E.; Kopp, H.G.; et al. The BCL-2 selective inhibitor ABT-199 sensitizes soft tissue sarcomas to proteasome inhibition by a concerted mechanism requiring BAX and NOXA. Cell Death Dis. 2020, 11, 701. [Google Scholar] [CrossRef]
- Heinicke, U.; Haydn, T.; Kehr, S.; Vogler, M.; Fulda, S. BCL-2 selective inhibitor ABT-199 primes rhabdomyosarcoma cells to histone deacetylase inhibitor-induced apoptosis. Oncogene 2018, 37, 5325–5339. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, C.K., Jr.; Floros, K.V.; Jacob, S.; Coon, C.M.; Puchalapalli, M.; Hu, B.; Harada, H.; Dozmorov, M.G.; Koblinski, J.E.; Smith, S.C.; et al. Unmasking BCL-2 Addiction in Synovial Sarcoma by Overcoming Low NOXA. Cancers 2021, 13, 2310. [Google Scholar] [CrossRef] [PubMed]
- Heisey, D.A.R.; Lochmann, T.L.; Floros, K.V.; Coon, C.M.; Powell, K.M.; Jacob, S.; Calbert, M.L.; Ghotra, M.S.; Stein, G.T.; Maves, Y.K.; et al. The Ewing Family of Tumors Relies on BCL-2 and BCL-XL to Escape PARP Inhibitor Toxicity. Clin. Cancer Res. 2019, 25, 1664–1675. [Google Scholar] [CrossRef]
- Hoda, M.A.; Pirker, C.; Dong, Y.; Schelch, K.; Heffeter, P.; Kryeziu, K.; van Schoonhoven, S.; Klikovits, T.; Laszlo, V.; Rozsas, A.; et al. Trabectedin Is Active against Malignant Pleural Mesothelioma Cell and Xenograft Models and Synergizes with Chemotherapy and Bcl-2 Inhibition In Vitro. Mol. Cancer Ther. 2016, 15, 2357–2369. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Fan, X.; Song, J.; Wu, H.; Han, J.; Lu, L.; Weng, X.; Nie, G. ABT-199-mediated inhibition of Bcl-2 as a potential therapeutic strategy for nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 2018, 503, 1214–1220. [Google Scholar] [CrossRef]
- Yin, L.; Zeng, Y.; Zeng, R.; Chen, Y.; Wang, T.L.; Rodabaugh, K.J.; Yu, F.; Natarajan, A.; Karpf, A.R.; Dong, J. Protein kinase RNA-activated controls mitotic progression and determines paclitaxel chemosensitivity through B-cell lymphoma 2 in ovarian cancer. Oncogene 2021, 40, 6772–6785. [Google Scholar] [CrossRef]
- Perimenis, P.; Galaris, A.; Voulgari, A.; Prassa, M.; Pintzas, A. IAP antagonists Birinapant and AT-406 efficiently synergise with either TRAIL, BRAF, or BCL-2 inhibitors to sensitise BRAFV600E colorectal tumour cells to apoptosis. BMC Cancer 2016, 16, 624. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, A.C.; Jarzabek, M.A.; Lindner, A.U.; Carberry, S.; Conroy, E.; Miller, I.S.; Connor, K.; Shiels, L.; Zanella, E.R.; Lucantoni, F.; et al. Implementing Systems Modelling and Molecular Imaging to Predict the Efficacy of BCL-2 Inhibition in Colorectal Cancer Patient-Derived Xenograft Models. Cancers 2020, 12, 2978. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Du, J.; Zhao, Y.; Gao, Y.; Li, Y.; Zhao, K.; Lu, N. A novel kinase inhibitor, LZT-106, downregulates Mcl-1 and sensitizes colorectal cancer cells to BH3 mimetic ABT-199 by targeting CDK9 and GSK-3beta signaling. Cancer Lett. 2021, 498, 31–41. [Google Scholar] [CrossRef]
- Huang, Q.; He, S.; Zhan, D. Osimertinib is a dual inhibitor of hepatocellular carcinoma and angiogenesis in an EGFR-independent manner, and synergizes with venetoclax. J. Cancer Res. Clin. Oncol. 2023, 149, 10727–10735. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Dobrikov, M.; Keir, S.T.; Gromeier, M.; Pastan, I.H.; Reisfeld, R.; Bigner, D.D.; Chandramohan, V. Synergistic antitumor effects of 9.2.27-PE38KDEL and ABT-737 in primary and metastatic brain tumors. PLoS ONE 2019, 14, e0210608. [Google Scholar] [CrossRef]
- Adamova, B.; Rihova, K.; Pokludova, J.; Benes, P.; Smarda, J.; Navratilova, J. Synergistic cytotoxicity of perifosine and ABT-737 to colon cancer cells. J. Cell. Mol. Med. 2023, 27, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; You, Z.; Jia, J.; Lin, S.; Han, S.; Liu, A.; Long, H.; Wang, S. Curcumin enhances the antitumor effect of ABT-737 via activation of the ROS-ASK1-JNK pathway in hepatocellular carcinoma cells. Mol. Med. Rep. 2016, 13, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, G.; Zhao, W.; Lin, L.; Ye, T. Norcantharidin combined with ABT-737 for hepatocellular carcinoma: Therapeutic effects and molecular mechanisms. World J. Gastroenterol. 2016, 22, 3962–3968. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Dong, X.; Huang, D.C.S.; Xu, P.; Zhao, Q.; Chen, B. Current Advances and Future Strategies for BCL-2 Inhibitors: Potent Weapons against Cancers. Cancers 2023, 15, 4957. https://doi.org/10.3390/cancers15204957
Xu J, Dong X, Huang DCS, Xu P, Zhao Q, Chen B. Current Advances and Future Strategies for BCL-2 Inhibitors: Potent Weapons against Cancers. Cancers. 2023; 15(20):4957. https://doi.org/10.3390/cancers15204957
Chicago/Turabian StyleXu, Jiaxuan, Xiaoqing Dong, David C. S. Huang, Peipei Xu, Quan Zhao, and Bing Chen. 2023. "Current Advances and Future Strategies for BCL-2 Inhibitors: Potent Weapons against Cancers" Cancers 15, no. 20: 4957. https://doi.org/10.3390/cancers15204957
APA StyleXu, J., Dong, X., Huang, D. C. S., Xu, P., Zhao, Q., & Chen, B. (2023). Current Advances and Future Strategies for BCL-2 Inhibitors: Potent Weapons against Cancers. Cancers, 15(20), 4957. https://doi.org/10.3390/cancers15204957





