Local Recurrence following Radiological Complete Response in Patients Treated with Subsegmental Balloon-Occluded Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
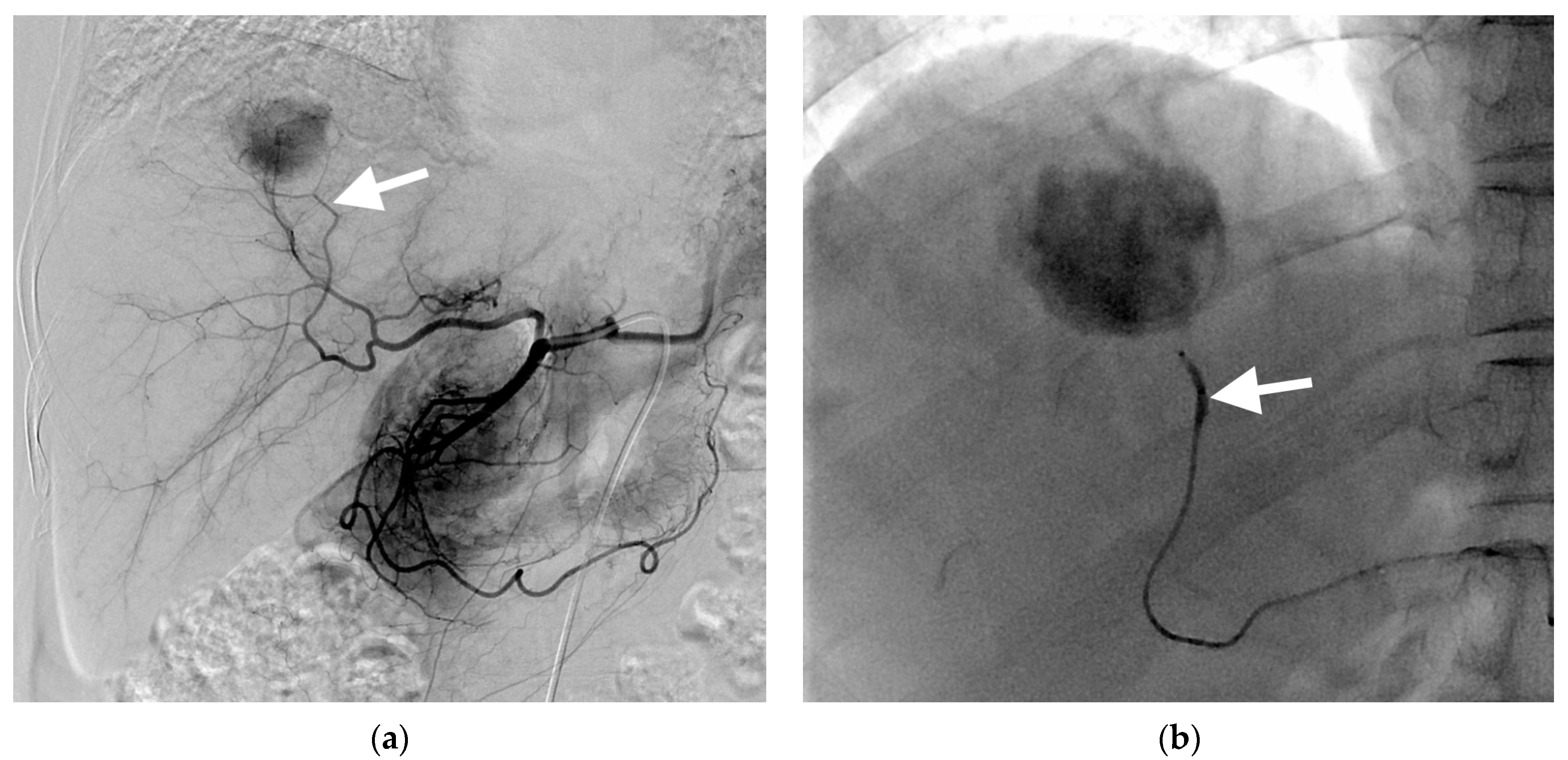
3.1. B-TACE Procedure and Complications
3.2. Follow-Up
3.3. Local Recurrence
3.4. Overall Survival and Post-Recurrence Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á. BCLC strategy for prognosis prediction and treatment recommendation. The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Gores, G.Y.; Mazzaferro, V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut 2014, 63, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Burra, P.; Frigo, A.C.; Trevisani, F.; Farinati, F.; Spolverato, G.; Volk, M.; Giannini, E.G.; Ciccarese, F.; Piscaglia, F.; et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver stages: A multicentre study. J. Hepatol. 2015, 62, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Kim, S.U.; Kim, K.A.; Chung, Y.E.; Kim, M.; Park, M.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, M.D.; et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J. Hepatol. 2015, 62, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S.; Yamashiro, M.; Hashimoto, M.; Hashimoto, N.; Ikuno, M.; Okumura, K.; Yoshida, M.; Matsui, O. Comparison of local control in transcatheter arterial chemoembolization of hepatocellular carcinoma ≤6 cm with or without intraprocedural monitoring of the embolized area using cone-beam computed tomography. Cardiovasc. Intervent. Radiol. 2014, 37, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Bannangkoon, K.; Hongsakul, K.; Tubtawee, T.; Piratvisuth, T. Safety margin of embolized area can reduce local recurrene of hepatocellular carcinoma after superselective transarterial chemoembolization. Clin. Mol. Hepatol. 2019, 25, 74–85. [Google Scholar] [CrossRef]
- Miyayama, S.; Yamashiro, M.; Ikeda, R.; Matsumoto, J.; Takeuchi, K.; Sakuragawa, N.; Ueda, T.; Sanada, T.; Notsumata, K.; Terada, T. Efficacy of superselective conventional transarterial chemoembolization using guidance software for hepatocellular carcinoma within three lesions smaller than 3 cm. Cancer 2021, 13, 6370. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.O.; Kim, E.B.; Jeong, S.W.; Jang, J.Y.; Lee, S.H.; Kim, S.G.; Cha, S.W.; Kim, Y.S.; Cho, Y.D.; Kim, H.S.; et al. Predictive factors for complete response and recurrence after transarterial chemoembolization in hepatocellular carcinoma. Gut Liver 2017, 11, 409–416. [Google Scholar] [CrossRef]
- Jin, Y.J.; Chung, Y.H.; Kim, J.A.; Park, W.; Lee, D.; Shim, J.H.; Lee, D.; Kim, K.M.; Lim, Y.S.; Lee, H.C.; et al. Predisposing factors of hepatocellular carcinoma recurrence following complete remission in response to transarterial chemoembolization. Dig. Dis. Sci. 2013, 58, 1758–1765. [Google Scholar] [CrossRef]
- Bargellini, I.; Bozzi, E.; Campani, D.; Carrai, P.; Simone, P.D.; Pollina, L.; Cioni, R.; Filipponi, F.; Bartolozzi, C. Modified RECIST to assess tumor response after transarterial chemoemolization of hepatocellular carcinoma: CT-pathologic correlation in 178 liver explants. Eur. J. Radiol. 2013, 82, e212–e218. [Google Scholar] [CrossRef]
- Dioguardi Burgio, M.; Ronot, M.; Bruno, O.; Francoz, C.; Paradis, V.; Castera, L.; Durand, F.; Soubrane, O.; Vilgrain, V. Correlation of tumor response on computed tomography with pathological necrosis in hepatocellular carcinoma treated by chemoembolization before liver transplantation. Liver Transpl. 2016, 22, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Gwon, D.I.; Chu, H.H.; Kim, J.W.; Kim, J.H.; Ko, G.Y. Correlation between tumor response on CT and necrosis in hepatocellular carcinoma treated by conventional transcatheter arterial chemoembolization: Threshold value of intratumoral Lipiodol accumulation to predict tumor necrosis. Abdom. Radiol. 2020, 46, 3729–3737. [Google Scholar]
- Irie, T.; Kuramochi, M.; Takahashi, N. Improved accumulation of lipiodol under balloon-occluded transarterial chemoembolization (B-TACE) for hepatocellular carcinoma: Measurement of blood pressure at the embolized artery before and after balloon inflation. Jpn. J. Interent Radiol. 2011, 26, 49–54. [Google Scholar]
- Irie, T.; Kuramochi, M.; Takahashi, N. Dense accumulation of Lipiodol emulsion in hepatocellular carcinoma nodule during selective balloon-occluded transarterial chemoembolization: Measurement of balloon-occluded arterial stump pressure. Cardiovasc. Intervent. Radiol. 2013, 36, 706–713. [Google Scholar] [CrossRef]
- Kim, P.H.; Gwon, D.I.; Kim, J.W.; Chu, H.H.; Kim, J.H. The safety and efficacy of balloon-occluded transcatheter arterial chemoembolization for hepatocellular carcinoma refractory to conventional transcatheter arterial chemoembolization. Eur. Radiol. 2020, 30, 5650–5662. [Google Scholar] [CrossRef] [PubMed]
- Golfieri, R.; Bezzi, M.; Verset, G.; Fucilli, F.; Mosconi, C.; Cappelli, A.; Paccapelo, A.; Lucatelli, P.; Magand, N.; Rode, A.; et al. Retrospective European multicentric evaluation of selective transarterial chemoembolisation with and without balloon-occlusion in patients with hepatocellular carcinoma: A propensity score matched analysis. Cardiovasc. Intervent. Radiol. 2021, 44, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Lucatelli, P.; De Rubeis, G.; Rocco, B.; Basilico, F.; Cannavale, A.; Abbatecola, A.; Nardis, P.G.; Corona, M.; Brozzetti, S.; Catalano, C.; et al. Balloon occluded TACE (B-TACE) vs DEM-TACE for HCC: A single cnter retrospective case control study. BMC Gastroenterol. 2021, 21, 51. [Google Scholar]
- Golfieri, R.; Bezzi, M.; Verset, G.; Fucilli, F.; Mosconi, C.; Cappelli, A.; Lucatelli, P.; Magand, N.; Rode, A.; De Baere, T. Balloon-occluded transarterial chemoembolization: In which size range dose it perform best? A comparison of its efficacy versus conventional transarterial chemoembolization, using propensity score matching. Liver Cancer 2021, 10, 522–534. [Google Scholar] [CrossRef]
- Shirono, T.; Iwamoto, H.; Niizeki, T.; Shimose, S.; Kajiwara, A.; Suzuki, H.; Kamachi, N.; Noda, Y.; Okamura, S.; Nakano, M.; et al. Durable complete response is achieved by balloon-occluded transcatheter arterial chemoembolization for hepatocellular carcinoma. Hepatol. Commun. 2022, 6, 2594–2604. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.H.; Gwon, D.I.; Kim, G.H.; Kim, J.H.; Ko, G.Y.; Shin, J.H.; Ko, H.K.; Yoon, H.K. Balloon-occluded transarterial chemoembolization versus conventional transarterial chemoembolization for the treatment of single hepatocellular carcinoma: A propensity score matching analysis. Eur. Radiol. 2023, 33, 2655–2664. [Google Scholar] [CrossRef]
- Lucatelli, P.; Rocco, B.; Basilico, F.; Ciaglia, S.; Damato, E.; Mosconi, C.; Argirò, R.; Catalano, C. Microballoon interventions for liver tumors: Review of literature and future perspectives. J. Clin. Med. 2022, 11, 5334. [Google Scholar] [CrossRef]
- Sacks, D.; McClenny, T.E.; Cardella, J.F.; Lewis, C.A. Society of Interventional Radiology clinical practice guidelines. J. Vasc. Interv. Radiol. 2003, 14, S199–S202. [Google Scholar] [CrossRef] [PubMed]
- Takayasu, K.; Arii, S.; Matsuo, N.; Yoshikawa, M.; Ryu, M.; Takasaki, K.; Sato, M.; Yamanaka, N.; Shimamura, Y.; Ohto, M. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am. J. Roentgenol. 2000, 175, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Imaeda, T.; Yamawaki, Y.; Seki, M.; Goto, H.; Iinuma, G.; Kanematsu, M.; Mochizuki, R.; Doi, H.; Saji, S.; Shimokawa, K. Lipiodol retention and massive necrosis after lipiodol-chemoembolization of hepatocellular carcinoma: Correlation between computed tomography and histopathology. Cardiovasc. Intervent. Radiol. 1993, 16, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Kwan, S.W.; Fidelman, N.; Ma, E.; Eerlan, R.K., Jr.; Yao, F.Y. Imaging predictors of the response to transarterial chemoembolization in patients with hepatocellular carcinoma: A radiological-pathological correlation. Liver Transpl. 2012, 18, 727–736. [Google Scholar] [CrossRef]
- Choi, B.I.; Kim, H.C.; Han, J.K.; Park, J.H.; Kim, Y.I.; Kim, S.T.; Lee, H.S.; Kim, C.Y.; Han, M.C. Therapeutic effect of transcatheter oily chemoembolization therapy for encapsulated nodular hepatocellular carcinoma: CT and pathologic findings. Radiology 1992, 182, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Okada, S.; Ueno, H.; Ikeda, M.; Shimada, K.; Yamamoto, J.; Kosuge, T.; Yamasaki, S.; Fukushima, N.; Sakamoto, M. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer 2002, 95, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Kai, S.; Iwashita, Y.; Hirano, S.; Ohta, M.; Kitano, S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer 2005, 103, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S.; Matsui, O.; Yamashiro, M.; Ryu, Y.; Kaito, K.; Ozaki, K.; Takeda, T.; Yoneda, N.; Notsumata, K.; Toya, D.; et al. Ultraselective transcatheter arterial chemoembolization with a 2-F tip microcatheter for small hepatocellular carcinomas: Relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J. Vasc. Interv. Radiol. 2007, 18, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, S.; Yamaguchi, T.; Hongo, O.; Iwamoto, H.; Sanefuji, H. Excellent outcomes with angiographic subsegmentectomy in the treatment of typical hepatocellular carcinoma: A retrospective study of local recurrence and long-term survival rates in 120 patients with hepatocellular carcinoma. Cancer 2010, 116, 393–399. [Google Scholar] [CrossRef]
- Mayama, S. Ultraselective conventional transarterial chemoembolization: When and how? Clin. Mol. Hepatol. 2019, 25, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Facciorusso, A.; Sacco, R.; Bartalena, L.; Mosconi, C.; Cea, U.V.; Cappelli, A.; Antonino, M.; Modestino, F.; Brandi, N.; et al. TRANS-TACE: Prognostic role of the transient hypertransaminasemia after conventional chemoembolization for hepatocellular carcinoma. J. Pers. Med. 2021, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Del Prete, V.; Antonion, M.; Crucinio, N.; Neve, V.; De Leo, A.; Carr, B.I.; Barone, M. Post-recurrence survival in hepatocellular carcinoma after percutaneous radiofrequency ablation. Dig. Liver Dis. 2014, 46, 1014–1019. [Google Scholar] [CrossRef] [PubMed]






| Characteristics | Value |
|---|---|
| Age (years) | 63.5 ± 10 (39–82) |
| Sex, male | 44 (73.3%) |
| Hepatitis B virus infection | 41 (68.3%) |
| Hepatitis C virus infection | 3 (5%) |
| Alcohol | 16 (26.7%) |
| BCLC stage A | 50 (83.3%) |
| BCLC stage B | 10 (16.7%) |
| Child–Pugh class A | 57 (95%) |
| Child–Pugh class B | 3 (5%) |
| MELD | 7.9 ± 1.3 (6–11) |
| Aspartate aminotransferase (U/L) | 36 ± 18 (12–119) |
| Alanine aminotransferase (U/L) | 33 ± 25 (12–165) |
| Total bilirubin (mg/dL) | 0.8 ± 0.4 (0.2–1.8) |
| α-Fetoprotein (ng/mL) 1 | 829 ± 3313 (2.3–8899.6) |
| PIVKA-II (mAU/mL) | 41 ± 193 (1.7–1425.4) |
| HCC size (mm) | 31 ± 10.6 (10–50) |
| Single HCC | 49 (81.7%) |
| Two HCCs | 10 (16.7%) |
| Three HCCs | 1 (1.7%) |
| No previous treatment | 46 (76.7%) |
| Previous surgical resection | 9 (15%) |
| Previous RFA | 5 (8.3%) |
| Factors | Univariate Analyses | Multiple Analyses | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Male sex | 0.637 (0.235–1.724) | 0.375 | ||
| Age ≥ 65 years | 0.243 (0.074–0.804) | 0.021 | 0.124 (0.037–0.412) | <0.001 |
| Viral liver cirrhosis | 1.791 (0.455–7.048) | 0.404 | ||
| Child–Pugh B | Inestimable * | 0.600 | ||
| MELD ≥ 8 | 1.604 (0.600–4.291) | 0.347 | ||
| α-Fetoprotein > 830 ng/dL | 0.554 (0.091–3.364) | 0.521 | ||
| Previous treatments | 0.321(0.048–2.148) | 0.241 | ||
| HCC size > 3 cm | 0.475 (0.155–1.454) | 0.192 | 0.416 (0.138–1.256) | 0.120 |
| Multiple HCCs | 1.153 (0.447–2.975) | 0.768 | ||
| Peripheral location | 0.319 (0.101–1.003) | 0.051 | 0.112 (0.046–0.272) | <0.001 |
| Oily subsegmentectomy | Inestimable † | 0.001 ‡ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwon, D.I.; Kim, G.H.; Chu, H.H.; Kim, J.H.; Ko, G.-Y.; Yoon, H.-K. Local Recurrence following Radiological Complete Response in Patients Treated with Subsegmental Balloon-Occluded Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma. Cancers 2023, 15, 4991. https://doi.org/10.3390/cancers15204991
Gwon DI, Kim GH, Chu HH, Kim JH, Ko G-Y, Yoon H-K. Local Recurrence following Radiological Complete Response in Patients Treated with Subsegmental Balloon-Occluded Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma. Cancers. 2023; 15(20):4991. https://doi.org/10.3390/cancers15204991
Chicago/Turabian StyleGwon, Dong Il, Gun Ha Kim, Hee Ho Chu, Jin Hyoung Kim, Gi-Young Ko, and Hyun-Ki Yoon. 2023. "Local Recurrence following Radiological Complete Response in Patients Treated with Subsegmental Balloon-Occluded Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma" Cancers 15, no. 20: 4991. https://doi.org/10.3390/cancers15204991





