A Phase II Study of Osimertinib in Patients with Advanced-Stage Non-Small Cell Lung Cancer following Prior Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor (EGFR TKI) Therapy with EGFR and T790M Mutations Detected in Plasma Circulating Tumour DNA (PLASMA Study)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Patients
2.2. Trial Design, Treatment and Assessments
2.3. Trial Endpoints
2.4. Trial Oversight
2.5. Cell-Free DNA Extract, Library Construction and Targeted Panel Next-Generation Sequencing
2.6. Sequence Data Processing and Mutation Calling
2.7. Statistical Analysis
3. Results
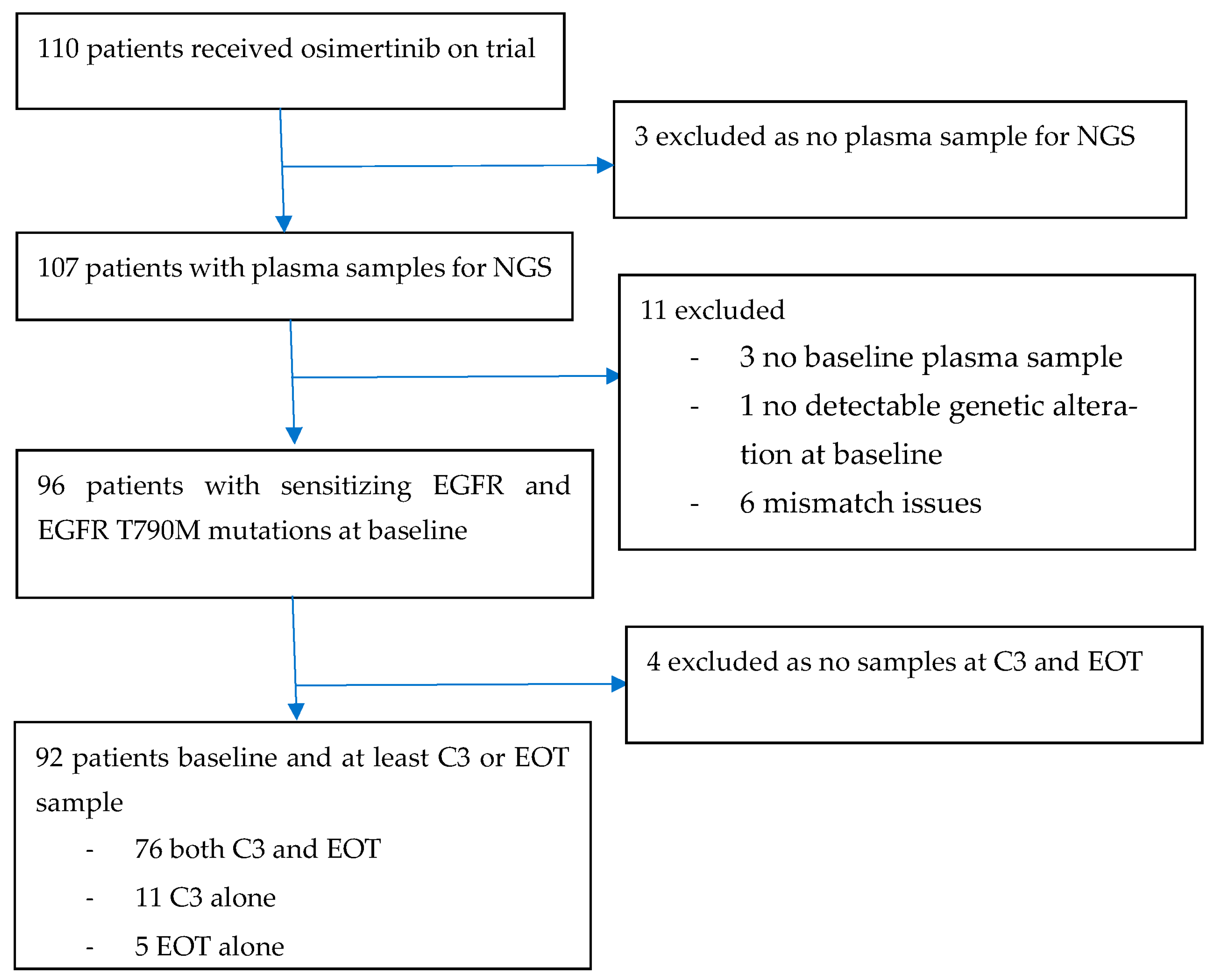
3.1. Patient Characteristics
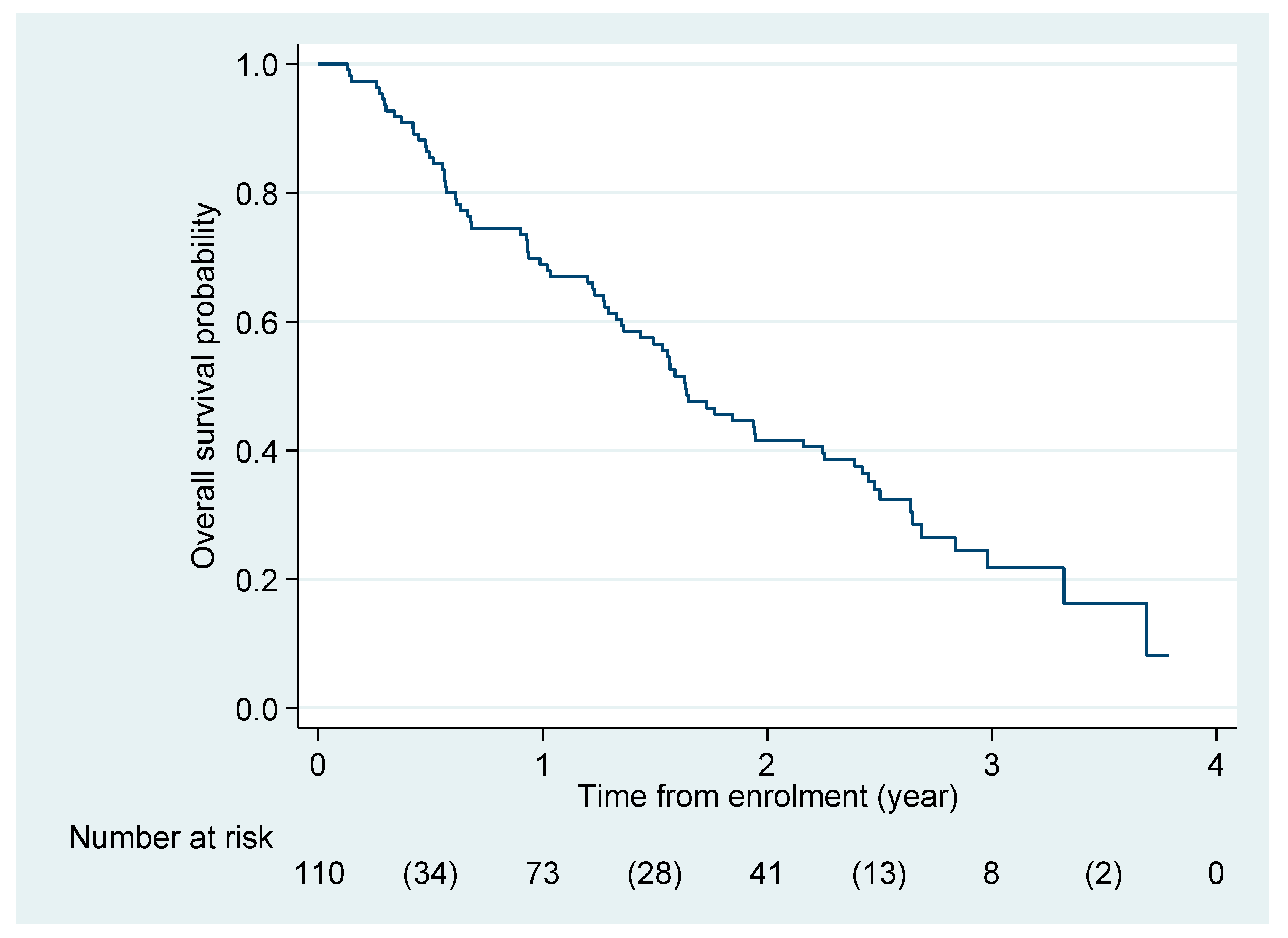
3.2. Efficacy
3.3. Adverse Events and Dosing Adjustments
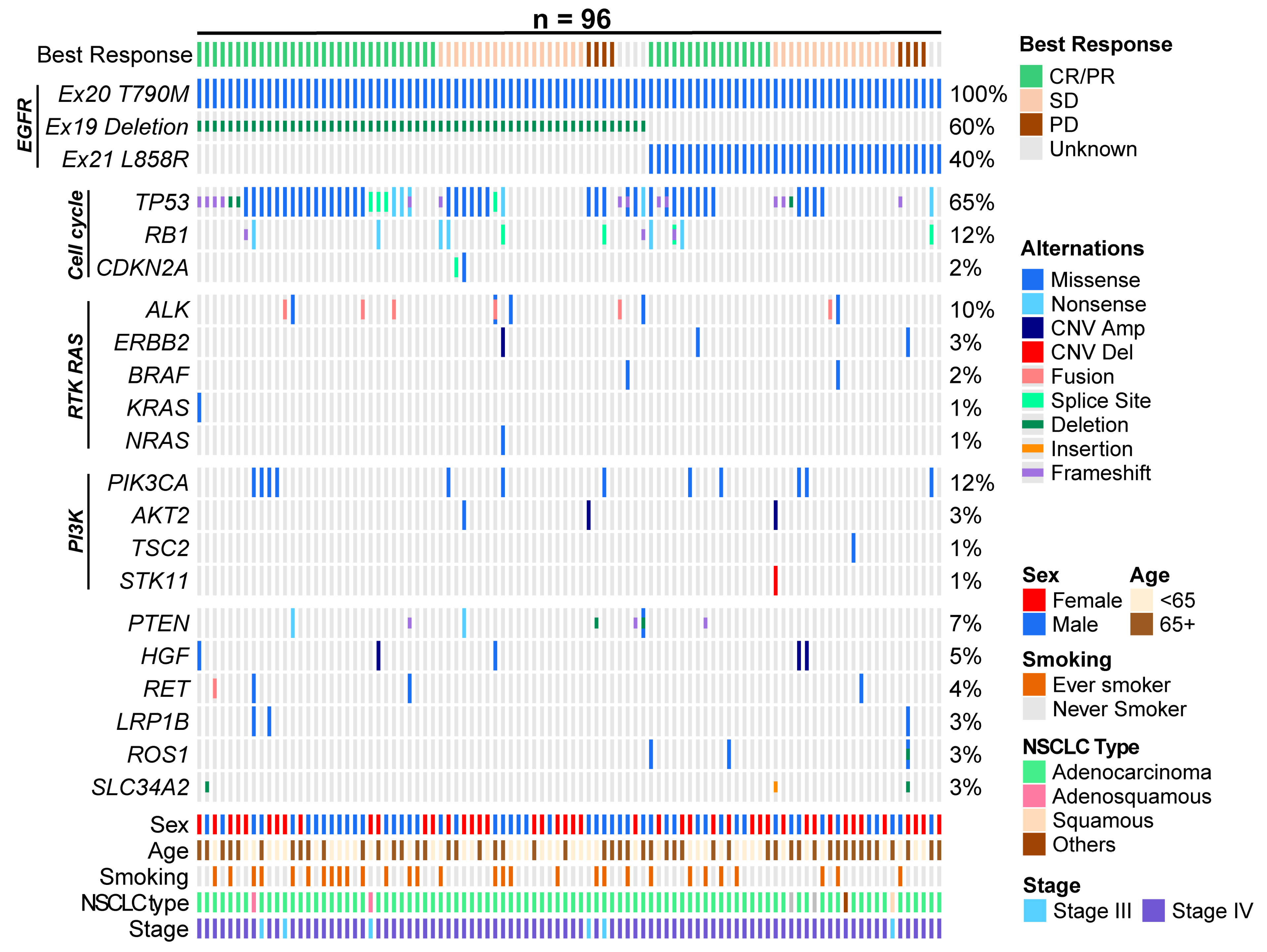
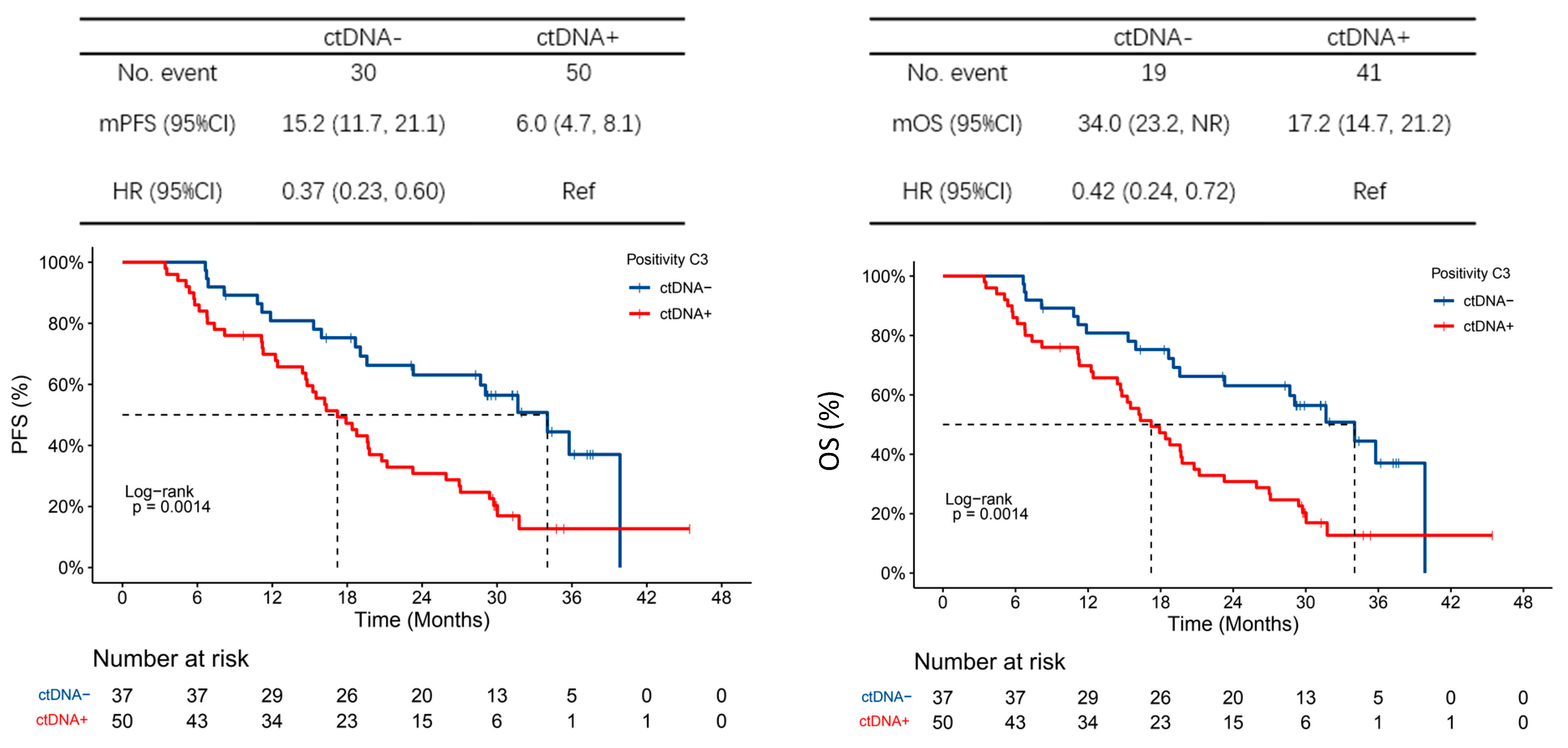
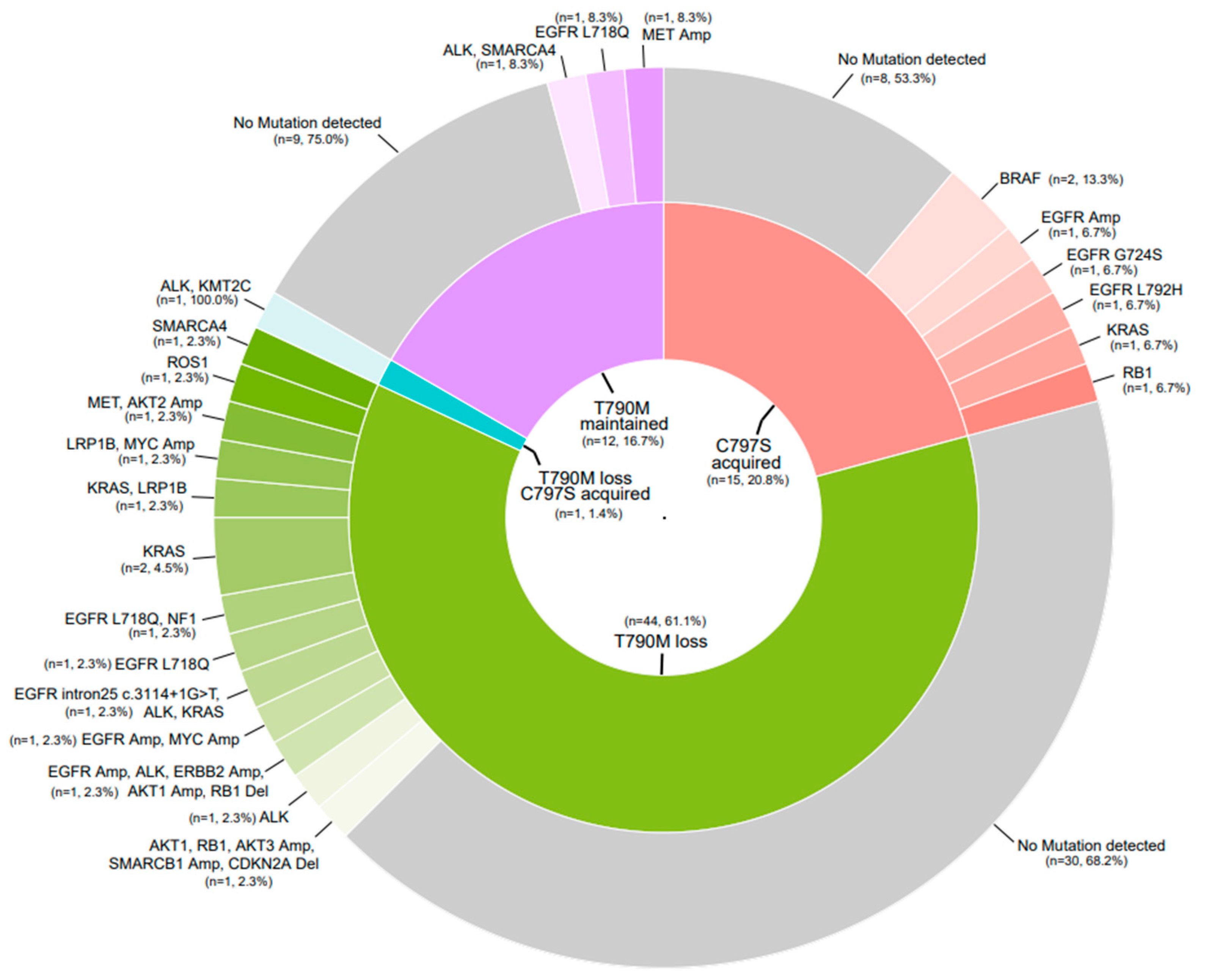
3.4. Exploratory Endpoints
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Eng. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Planchard, D.; Lu, S.; Sun, H.; Yamamoto, N.; Kim, D.-W.; Tan, D.S.W.; Yang, J.C.H.; Azrif, M.; Mitsudomi, T.; et al. Pan-Aisan adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: A CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann. Oncol. 2019, 30, 1710219. [Google Scholar]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.M.E.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Eng. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Chouaid, C.; Dujon, C.; Do, P.; MMonnet, I.; Madroszyk, A.; Le Caer, H.; Auliac, J.B.; Berard, H.; Thomas, P.; Lena, H.; et al. Feasibility and clinical impact of re-biopsy in advanced non-small-cell lung cancer: A prospective multicenter study in a real-world setting (GPEC study 12-01). Lung Cancer 2014, 86, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, A.; Patil, V.; Norohna, V.; Joshi, A.; Choughale, A.; Rajeev, K.; Mahajan, A.; Janu, A.; Goud, S.; More, S.; et al. Rebiopsy Post Progression in EGFR Mutated Lung Cancer. J. Thorac. Oncol. 2016, 12, P3.02b-094. [Google Scholar]
- Suda, K.; Murakami, I.; Obata, K.; Sakai, K.; Fujino, T.; Koga, T.; Ohara, S.; Hamada, A.; Soh, J.; Nishio, K.; et al. Spatial heterogeneity of acquired resistance mechanisms to 1st/2nd generation EGFR tyrosine kinase inhibitors in lung cancer. Lung Cancer 2020, 148, 100–104. [Google Scholar] [CrossRef]
- Takahama, T.; Azuma, K.; Shimokawa, M.; Takeda, M.; Ishii, H.; Kato, T.; Saito, H.; Daga, H.; Tsuboguchi, Y.; Okamoto, I.; et al. Plasma screening for the T790M mutation of EGFR and phase 2 study of osimertinib efficacy in plasma T790M-positive non-small cell lung cancer: West Japan Oncology Group 8815/LPS study. Cancer 2020, 126, 1940–1948. [Google Scholar] [CrossRef]
- Taylor, S.C.; Laperriere, G.; Germain, H. Droplet Digital PCR versus qPCR for gene expression analysis with low abundant targets: From variable nonsense to publication quality data. Sci. Rep. 2017, 25, 2409. [Google Scholar] [CrossRef]
- Li, J.Y.-C.; Ho, J.C.-M.; Wong, K.-H. T790M mutant copy number quantified via ddPCR predicts outcome after osimertinib treatment in lung cancer. Oncotarget 2018, 9, 27929–27939. [Google Scholar] [CrossRef]
- Thress, K.S.; Brant, R.; Carr, T.H.; Dearden, S.; Jenkins, S.; Brown, H.; Hammett, T.; Mireille, C.; Barrett, J.C. EGFR mutation detection in ctDNA from NSCLC patient plamsa: A cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer 2015, 90, 509–515. [Google Scholar] [CrossRef]
- Shu, Y.; Wu, X.; Tong, X.; Wang, X.; Chang, Z.; Mao, Y.; Chen, X.; Sun, J.; Wang, Z.; Hong, Z.; et al. Circulating tumor DNA mutation profiling by targeted next generation sequencing provides guidance for personalized treatments in multiple cancer types. Sci. Rep. 2017, 7, 583. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.; del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Zhang, Q.; Larson, D.E.; Shen, D.; McLellan, M.D.; Lin, L.; Miller, C.A.; Mardis, E.R.; Ding, L.; Wilson, R.K. VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012, 22, 568–576. [Google Scholar] [CrossRef]
- Wang, H.; Ou, Q.; Li, D.; Qin, T.; Bao, H.; Hou, X.; Wang, K.; Wang, F.; Deng, Q.; Liang, J.; et al. Genes associated with increased brain metastasis risk in non–small cell lung cancer: Comprehensive genomic profiling of 61 resected brain metastases versus primary non–small cell lung cancer (Guangdong Association Study of Thoracic Oncology 1036). Cancer 2019, 125, 3535–3544. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Newman, A.M.; Bratman, S.V.; Stehr, H.; Lee, L.J.; Liu, C.L.; Diehn, M.; Alizadeh, A.A. FACTERA: A practical method for the discovery of genomic rearrangements at breakpoint resolution. Bioinformatics 2014, 30, 3390–3393. [Google Scholar] [CrossRef]
- Amarasinghe, K.C.; Li, J.; Hunter, S.M.; Ryland, G.L.; Cowin, P.A.; Campbell, I.G.; Halgamuge, S.K. Inferring copy number and genotype in tumour exome data. BMC Genom. 2014, 15, 732. [Google Scholar] [CrossRef] [PubMed]
- Papadimitrakopoulou, V.A.; Mok, T.S.; Han, J.-Y.; Ahn, M.-J.; Delmonte, A.; Ramalingam, S.S.; Kim, S.W.; Shepherd, F.A.; Laskin, J.; He, Y.; et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann. Oncol. 2020, 31, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Brevet, M.; Johnson, M.L.; Azzoli, C.G.; Ladanyi, M. Detection of EGFR mutations in plasma DNA from lung cancer patients by mass. Lung Cancer 2011, 73, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Y.; Wu, X.; He, C.; Liu, Q.; Wang, F. Comprehensive analysis of EGFR T790M detection by ddPCR and ARMS-PCR and the effect of mutant abundance on the efficacy of osimertinib in NSCLC patients. J. Thorac. Dis. 2019, 11, 3004–3014. [Google Scholar] [CrossRef]
- Lee, C.K.; Chan, A.K.; Park, K.; Leung, L.; Lam, K.C.; Yeung, S.W.; Lo, D.Y.; Mok, T.S. Droplet digital PCR: A novel detection method of activating epidermal growth factor receptor (EGFR) mutations in plasma of patients with advanced stage non-small cell lung cancer (NSCLC). J. Thorac. Oncol. 2013, 8, S487–S488. [Google Scholar]
- Qiu, M.; Wang, J.; Xu, Y. Circulating Tumor DNA Is Effective for the Detection of EGFR Mutation in Non–Small Cell Lung Cancer: A Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2014, 24, 206–212. [Google Scholar] [CrossRef]
- Offin, M.; Chan, J.M.; Tenet, M.; Rizvi, H.A.; Shen, R.; Riely, G.J.; Rekhtman, N.; Daneshbod, Y.; Quintanal-Villalonga, A.; Penson, A.; et al. Concurrent RB1 and TP53 alterations define a subset of EGFR-mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J. Thorac. Oncol. 2019, 14, 1784–1793. [Google Scholar] [CrossRef]
- Chevallier, M.; Tsantoulis, P.; Addeo, A.; Friedlaender, A. Influence of Concurrent Mutations on Overall Survival in EGFR-mutated Non-small Cell Lung Cancer. Cancer Genom. Proteom. 2020, 17, 597–603. [Google Scholar] [CrossRef]
- Jiang, Z.; Hao, Y.; Ding, X.; Zhang, Z.; Liu, P.; Wei, X.; Xi, J. The effects and mechanisms of SLC34A2 on tumorigenicity in human non-small cell lung cancer stem cells. Tumour Biol. 2016, 37, 10383–10392. [Google Scholar] [CrossRef]
- Chmmielecki, J.; Mok, T.; Wu, Y.-L.; Han, J.-Y.; Ahn, M.M.-J.; Ramalingam, S.S.; John, T.; Okamoto, I.; Yang, J.C.H.; Shepherd, F.A.; et al. Analysis of acquired resistance mechanisms to Osimertinib in patients with EGFR-mutated advanced non-small cell lung cancer from the AURA3 trial. Nat. Commun. 2023, 14, 1071. [Google Scholar] [CrossRef]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-L.; Jiang, Y.; Chiang, C.-L.; Karwowska, S.; Yerram, R.; Sharma, R.; Scudder, S.; Chiu, C.-H.; Tsai, C.-M.; Palma, J.F.; et al. Efficacy of liquid biopsy for disease monitoring and early prediction of tumor progression in EGFR mutation-positive non-small cell lung cancer. PLoS ONE 2022, 17, e0267362. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, F.V.; Volkov, N.M.; Zhabina, A.S.; Stepanova, M.L.; Rysev, N.A.; Klimenko, V.V.; Myslik, A.V.; Artemieva, E.V.; Egorenkov, V.V.; Abduloeva, N.H.; et al. Monitoring of the presence of EGFR-mutated DNA during EGFR-targeted therapy may assist in the prediction of treatment outcome. Cancer Treat. Res. Commun. 2022, 31, 100524. [Google Scholar] [CrossRef]
- Wu, T.-H.; Hsiue, E.H.-C.; Yang, J.C.-H. Opportunities of circulating tumor DNA in lung cancer. Cancer Treat. Rev. 2019, 78, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Takahama, T.; Shimokawa, M.; Azuma, K.; Takeda, M.; Kato, T.; Daga, H.; Isamu, O.; Akamatsu, H.; Shunsuke, T.; et al. Predicting osimertinib-treatment outcomes through EGFR mutant-fraction monitoring in the circulating tumor DNA of EGFR T790M-positive patients with non-small cell lung cancer (WJOG8815L). Mol. Oncol. 2021, 15, 126–137. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | All Patients (n = 110) |
|---|---|
| Median age (range), years | 65.8 (40.7–93.7) |
| Gender (%) | |
| Male | 54 (49.1) |
| Female | 56 (50.9) |
| Region of participation (%) | |
| Hong Kong | 12 (10.9) |
| Korea | 22 (20.0) |
| Singapore | 30 (27.3) |
| Taiwan | 15 (13.6) |
| Thailand | 31 (28.2) |
| Smoking status (%) | |
| Never smoker | 78 (70.9) |
| Ex-smoker | 26 (23.6) |
| Current smoker | 6 (5.5) |
| ECOG performance status (%) | |
| 0 | 25 (22.7) |
| 1 | 75 (68.2) |
| 2 | 10 (9.1) |
| CNS metastasis (%) | 36 (33.3) |
| Metastasis (%) | 104 (94.6) |
| Clinical staging at enrolment (%) | |
| Stage III | 6 (5.5) |
| Stage IV | 104 (94.6) |
| Prior therapy (%) | |
| Chemotherapy | 30 (27.3) |
| Radiotherapy | 54 (49.1) |
| Surgery | 38 (34.6) |
| Prior TKI (%) | |
| Afatinib | 20 (18.2) |
| Erlotinib | 30 (27.2) |
| Gefitinib | 60 (54.6) |
| EGFR mutation status (%) | |
| Exon 19 deletion | 66 (60.0) |
| L858R | 44 (40.0) |
| Best Response | All Patients (n = 110) | CNS Metastasis (n = 36) |
|---|---|---|
| CR | 1 (0.9) | 0 (0) |
| PR | 55 (50.0) | 22 (61.1) |
| SD | 37 (33.6) | 10 (27.8) |
| PD | 10 (9.1) | 3 (8.3) |
| Not evaluable | 7 (6.4) | 1 (2.8) |
| ORR (95% CI) | ||
| Intention to treat (ITT) | 50.9 (41.2–60.2) | 61.1 (43.5–76.9) |
| Modified intention to treat (mITT) | 54.4 (44.3–64.2) | 62.9 (44.9–78.5) |
| Median DoR (95% CI), months | 7.2 (3.6–11.0) | 3.6 (1.9–13.1) |
| DCR (95% CI) | ||
| ITT | 84.5 (76.4–90.7) | 88.9 (73.9–96.9) |
| mITT | 90.3 (82.9–95.2) | 91.4 (76.9–98.2) |
| TRAE | All Grades (n = 110) | Grade 3 |
|---|---|---|
| Paronychia | 25 (22.7%) | 0 |
| Dry skin | 23 (20.9%) | 0 |
| Rash | 15 (13.6%) | 0 |
| Diarrhoea | 14 (12.7%) | 0 |
| Pruritis | 11 (10.0%) | 1 (0.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ang, Y.L.E.; Zhao, X.; Reungwetwattana, T.; Cho, B.-C.; Liao, B.-C.; Yeung, R.; Loong, H.H.; Kim, D.-W.; Yang, J.C.-H.; Lim, S.M.; et al. A Phase II Study of Osimertinib in Patients with Advanced-Stage Non-Small Cell Lung Cancer following Prior Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor (EGFR TKI) Therapy with EGFR and T790M Mutations Detected in Plasma Circulating Tumour DNA (PLASMA Study). Cancers 2023, 15, 4999. https://doi.org/10.3390/cancers15204999
Ang YLE, Zhao X, Reungwetwattana T, Cho B-C, Liao B-C, Yeung R, Loong HH, Kim D-W, Yang JC-H, Lim SM, et al. A Phase II Study of Osimertinib in Patients with Advanced-Stage Non-Small Cell Lung Cancer following Prior Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor (EGFR TKI) Therapy with EGFR and T790M Mutations Detected in Plasma Circulating Tumour DNA (PLASMA Study). Cancers. 2023; 15(20):4999. https://doi.org/10.3390/cancers15204999
Chicago/Turabian StyleAng, Yvonne L. E., Xiaotian Zhao, Thanyanan Reungwetwattana, Byoung-Chul Cho, Bin-Chi Liao, Rebecca Yeung, Herbert H. Loong, Dong-Wan Kim, James Chih-Hsin Yang, Sun Min Lim, and et al. 2023. "A Phase II Study of Osimertinib in Patients with Advanced-Stage Non-Small Cell Lung Cancer following Prior Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor (EGFR TKI) Therapy with EGFR and T790M Mutations Detected in Plasma Circulating Tumour DNA (PLASMA Study)" Cancers 15, no. 20: 4999. https://doi.org/10.3390/cancers15204999
APA StyleAng, Y. L. E., Zhao, X., Reungwetwattana, T., Cho, B.-C., Liao, B.-C., Yeung, R., Loong, H. H., Kim, D.-W., Yang, J. C.-H., Lim, S. M., Ahn, M.-J., Lee, S.-H., Suwatanapongched, T., Kongchauy, K., Ou, Q., Yu, R., Tai, B. C., Goh, B. C., Mok, T. S. K., & Soo, R. A. (2023). A Phase II Study of Osimertinib in Patients with Advanced-Stage Non-Small Cell Lung Cancer following Prior Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor (EGFR TKI) Therapy with EGFR and T790M Mutations Detected in Plasma Circulating Tumour DNA (PLASMA Study). Cancers, 15(20), 4999. https://doi.org/10.3390/cancers15204999





