Proton Therapy for Advanced Juvenile Nasopharyngeal Angiofibroma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Proton Treatment Planning and Delivery
2.3. Follow-Up and Outcome Analysis
2.4. Photon Treatment Planning and Comparative Evaluation of Treatment Plans
2.5. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
3.2. Treatment Outcomes
3.3. Treatment Plan Comparisons
3.3.1. CTV Coverage
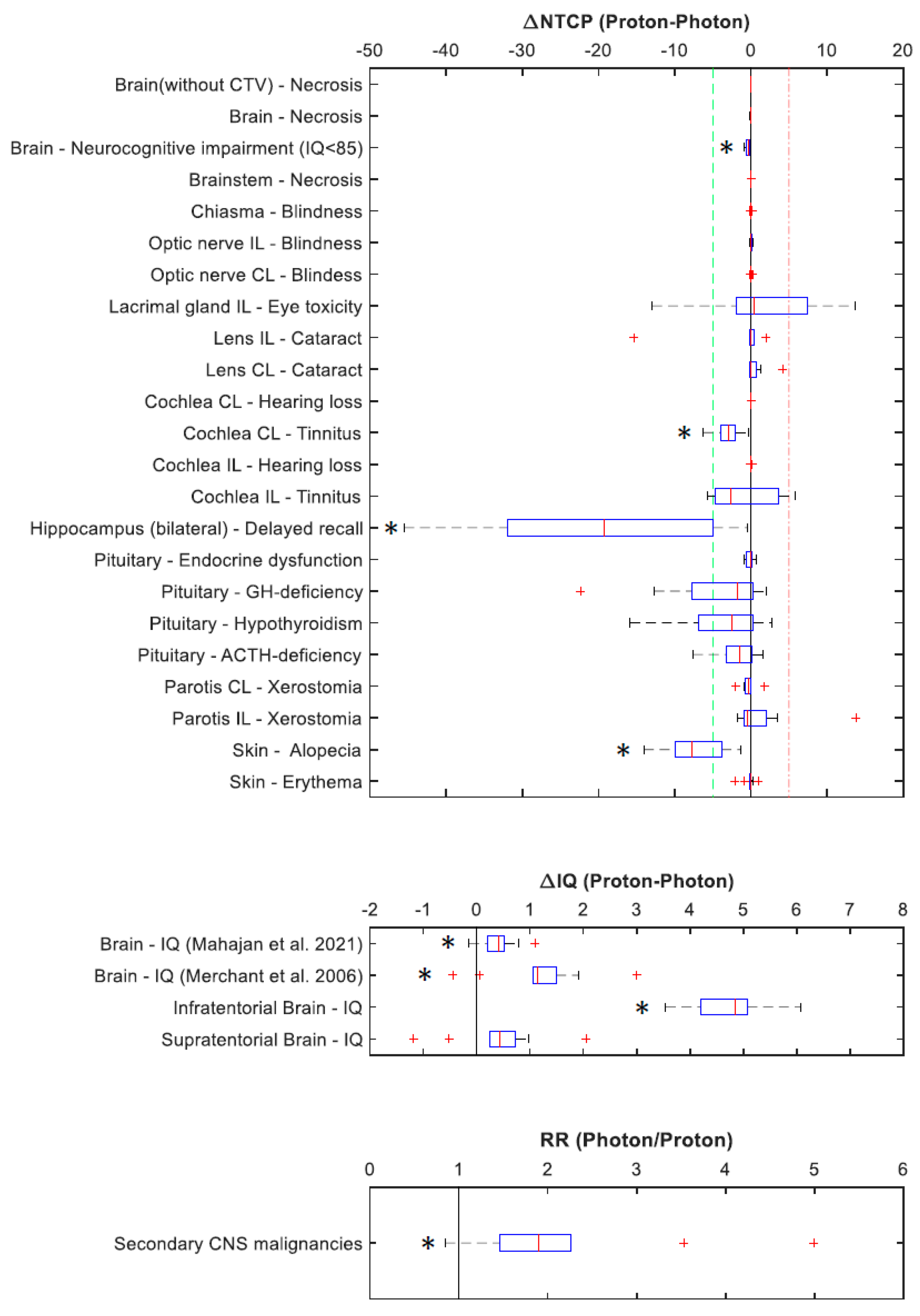
3.3.2. Sparing of OAR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Organ at risk | Complication | Formula | Parameters | Publication |
|---|---|---|---|---|
| Brain | IQ estimation [Wechsler Intelligence Scale] | time = time since radiotherapy (set at 5 years) age = age of the patient at the time of radiotherapy Dmean = average dose | Merchant 2006 [27] | |
| IQ estimation at a long follow-up time | age = age of the patient at the time of radiotherapy Dmean = average dose in 2 Gy equivalent dose using an α/β = 3 Gy | Mahajan 2021 [28] | ||
| Neurocognitive impairment estimation at a long follow-up time (IQ < 85) | a = 3.39 n = 1/a TD50 = 33.5 Gy m = 0.28 α/β = 3 Gy vi, corresponding to a volume fraction of a structure receiving a dose Di in 2 Gy equivalent dose using an α/β = 3 Gy | Mahajan 2021 [28] | ||
| Necrosis (5 year post-radiotherapy) | Dmax = minimum dose received in 0.03 cm3, in 2 Gy equivalent dose using an α/β = 3 Gy TD50 = 98.6 Gy m = 0.24 α/β = 3 Gy | Mahajan 2021 [28] | ||
| Infratentorial brain | IQ estimation (Wechsler Intelligence Scale) | time = time since radiotherapy (set at 5 years) age = age of the patient at the time of radiotherapy Dmean = average dose | Merchant 2006 [27] | |
| Supratentorial brain | IQ estimation (Wechsler Intelligence Scale) | time = time since radiotherapy (set at 5 years) age = age of the patient at the time of radiotherapy Dmean = average dose | Merchant 2006 [27] | |
| Brain (without CTV) | Necrosis (5 year post-radiotherapy) | Dmax = minimum dose received in 0.03 cm3, in 2 Gy equivalent dose using an α/β = 0.96 Gy TD50 = 109.0 Gy γ = 2.8 | Bender 2012 [32] | |
| Brainstem | Necrosis (5 year post-radiotherapy) | n = 0.112 TD50 = 65 Gy m = 0.144 α/β = 2.32 Gy vi, corresponding to a volume fraction of a structure receiving a dose Di in 2 Gy equivalent dose using an α/β = 2.32 Gy | Dell’Oro 2021 [31] | |
| Chiasma | Blindness (5 year post-radiotherapy) | a = 4.0 n = 1/a TD50 = 65.0 Gy m = 0.14 vi, corresponding to a volume fraction of a structure receiving a dose Di | Burman 1991 [33] | |
| Optic nerve (IL/CL) | ||||
| Cochlea (IL/CL) | Tinnitus (grade ≥ 2, 1–2 years post-radiotherapy) | n = 1.0 TD50 = 46.52 Gy m = 0.35 α/β = 3.25 Gy vi, corresponding to a volume fraction of a structure receiving a dose Di in 2 Gy equivalent dose using an α/β = 3.25 Gy | Dell’Oro 2021 [31] adapted from Lee 2015 [61] and De Marzi 2015 [62] | |
| Hearing loss (grade ≥ 1–2, 2 years post-radiotherapy) | n = 1.0 TD50 = 55.57 Gy m = 0.14 α/β = 3.25 Gy vi, corresponding to a volume fraction of a structure receiving a dose Di in 2 Gy equivalent dose using an α/β = 3.25 Gy | |||
| Hippocampus bilateral | Delayed recall (Wechsler Memory scale III Word Lists, 1.5 years post-radiotherapy) | D40% = minimum dose received in 40% if the structure volume, in 2 Gy equivalent dose using an α/β = 2.0 Gy TD50 = 14.88 Gy m = 0.54 | Gondi 2012 [29] | |
| Pituitary | Endocrine dysfunction (grade ≥ 1–2, 2 years post-radiotherapy) | n = 0.25 TD50 = 60.6 Gy m = 0.15 α/β = 2.5 Gy vi, corresponding to a volume fraction of a structure receiving a dose Di in 2 Gy equivalent dose using an α/β = 2.5 Gy | Dell’Orro 2021 [6] adapted from De Marzi 2015 [62] | |
| GH-deficiency (5 year post-radiotherapy) | TD50 = 27.2 Gy γ = 0.5 D2% = minimum dose received in 2% of the structure, in 2 Gy equivalent dose using an α/β = 3 Gy | Wheeler 2023 [30] | ||
| Hypothyroidism (5 year post-radiotherapy) | TD50 = 39.2 Gy γ = 0.75 D2% = minimum dose received in 2% of the structure, in 2 Gy equivalent dose using an α/β = 3 Gy | |||
| ACTH-deficiency (5 year post-radiotherapy) | TD50 = 58.0 Gy γ = 0.74 D2% = minimum dose received in 2% of the structure, in 2 Gy equivalent dose using an α/β = 3 Gy | |||
| Lacrimal gland IL | Ocular toxicity (grade ≥ 2, acute toxicity) | Dmax = minimum dose received in 0.03 cm3 of the structure β0 = −5.174 β1 = 0.205 Gy−1 | Batth 2013 [34] | |
| Lens (IL/CL) | Cataract (5 year post-radiotherapy) | a = 3.33 n = 1/a TD50 = 18.0 Gy m = 0.27 vi, corresponding to a volume fraction of a structure receiving a dose Di | Burman 1991 [33] | |
| Parotis (IL/CL) | Xerostomia (1 year post-radiotherapy) | n = 1.0 TD50 = 39.9 Gy m = 0.4 vi, corresponding to a volume fraction of a structure receiving a dose Di | Houweling 2010 [36] | |
| Skin | Alopecia (grade ≥ 2, acute toxicity) | D5% = minimum dose received in 5% of the volume structure β0 = −1.33 β1 = 0.08 Gy−1 | Dutz 2019 [35] | |
| Erythema (grade ≥ 2, acute toxicity) | V35 Gy = volume of the structure in cm3 receiving a minimum dose of 35 Gy β0 = −1.54 β1 = 0.06 cm−3 |
References
- Lund, V.J.; Stammberger, H.; Nicolai, P.; Castelnuovo, P.; Beal, T.; Beham, A.; Bernal-Sprekelsen, M.; Braun, H.; Cappabianca, P.; Carrau, R.; et al. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol. Suppl. 2010, 22, 1–143. [Google Scholar]
- Li, W.; Ni, Y.; Lu, H.; Hu, L.; Wang, D. Current perspectives on the origin theory of juvenile nasopharyngeal angiofibroma. Discov. Med. 2019, 27, 245–254. [Google Scholar]
- Sennes, L.U.; Butugan, O.; Sanchez, T.G.; Bento, R.F.; Tsuji, D.H. Juvenile nasopharyngeal angiofibroma: The routes of invasion. Rhinology 2003, 41, 235–240. [Google Scholar]
- Chakraborty, S.; Ghoshal, S.; Patil, V.M.; Oinam, A.S.; Sharma, S.C. Conformal radiotherapy in the treatment of advanced juvenile nasopharyngeal angiofibroma with intracranial extension: An institutional experience. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1398–1404. [Google Scholar] [CrossRef]
- Safadi, A.; Schreiber, A.; Fliss, D.M.; Nicolai, P. Juvenile Angiofibroma: Current Management Strategies. J. Neurol. Surg. B Skull Base 2018, 79, 21–30. [Google Scholar] [CrossRef]
- Mallick, S.; Benson, R.; Bhasker, S.; Mohanti, B.K. Long-term treatment outcomes of juvenile nasopharyngeal angiofibroma treated with radiotherapy. Acta Otorhinolaryngol. Ital. 2015, 35, 75–79. [Google Scholar]
- Lopez, F.; Triantafyllou, A.; Snyderman, C.H.; Hunt, J.L.; Suarez, C.; Lund, V.J.; Strojan, P.; Saba, N.F.; Nixon, I.J.; Devaney, K.O.; et al. Nasal juvenile angiofibroma: Current perspectives with emphasis on management. Head Neck 2017, 39, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Uetz, S.; Crosby, D.L. Current Management of Juvenile Nasopharyngeal Angiofibroma. Curr. Treat. Options Allergy 2020, 7, 335–346. [Google Scholar] [CrossRef]
- Beham, A.; Beham-Schmid, C.; Regauer, S.; Aubock, L.; Stammberger, H. Nasopharyngeal angiofibroma: True neoplasm or vascular malformation? Adv. Anat. Pathol. 2000, 7, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.A.; Mendenhall, W.M.; Amdur, R.J.; Stringer, S.P.; Cassisi, N.J. Long-term results of radiation therapy for juvenile nasopharyngeal angiofibroma. Am. J. Otolaryngol. 2001, 22, 172–175. [Google Scholar] [CrossRef]
- Nicolai, P.; Schreiber, A.; Bolzoni Villaret, A. Juvenile angiofibroma: Evolution of management. Int. J. Pediatr. 2012, 2012, 412545. [Google Scholar] [CrossRef]
- Cohen-Cohen, S.; Scheitler, K.M.; Choby, G.; Janus, J.; Moore, E.J.; Kasperbauer, J.L.; Cloft, H.J.; Link, M.; Gompel, J.J.V. Contemporary Surgical Management of Juvenile Nasopharyngeal Angiofibroma. J. Neurol. Surg. B Skull Base 2022, 83, e266–e273. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.H.; Bradley, P.J. Management dilemmas in the treatment and follow-up of advanced juvenile nasopharyngeal angiofibroma. ORL J. Otorhinolaryngol. Relat. Spec. 2006, 68, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Rupa, V.; Mani, S.E.; Backianathan, S.; Rajshekhar, V. Management and Outcome in Patients with Advanced Juvenile Nasopharyngeal Angiofibroma. J. Neurol. Surg. B Skull Base 2018, 79, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Chen, P.; Safa, A.; Juillard, G.; Calcaterra, T.C. The role of radiation in the treatment of advanced juvenile angiofibroma. Laryngoscope 2002, 112, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.-I.; Barrera, R.M.; Sharma, C. Multisession CyberKnife Radiosurgery for the Treatment of Advanced-Stage Intracranial Extended Juvenile Nasopharyngeal Angiofibroma. Cureus 2011, 3, e39. [Google Scholar] [CrossRef]
- Deguchi, K.; Fukuiwa, T.; Saito, K.; Kurono, Y. Application of cyberknife for the treatment of juvenile nasopharyngeal angiofibroma: A case report. Auris Nasus Larynx 2002, 29, 395–400. [Google Scholar] [CrossRef]
- Park, C.K.; Kim, D.G.; Paek, S.H.; Chung, H.T.; Jung, H.W. Recurrent juvenile nasopharyngeal angiofibroma treated with gamma knife surgery. J. Korean Med. Sci. 2006, 21, 773–777. [Google Scholar] [CrossRef]
- Mitin, T.; Zietman, A.L. Promise and pitfalls of heavy-particle therapy. J. Clin. Oncol. 2014, 32, 2855–2863. [Google Scholar] [CrossRef]
- Greenberger, B.A.; Yock, T.I. The role of proton therapy in pediatric malignancies: Recent advances and future directions. Semin. Oncol. 2020, 47, 8–22. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Constine, L.S.; Deasy, J.O.; Eisbruch, A.; Jackson, A.; Marks, L.B.; Ten Haken, R.K.; Yorke, E.D. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): An introduction to the scientific issues. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Eekers, D.B.; In‘t Ven, L.; Roelofs, E.; Postma, A.; Alapetite, C.; Burnet, N.G.; Calugaru, V.; Compter, I.; Coremans, I.E.M.; Hoyer, M.; et al. The EPTN consensus-based atlas for CT- and MR-based contouring in neuro-oncology. Radiother. Oncol. 2018, 128, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Chera, B.S.; Amdur, R.J.; Patel, P.; Mendenhall, W.M. A radiation oncologist’s guide to contouring the hippocampus. Am. J. Clin. Oncol. 2009, 32, 20–22. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Doyon, J.; McDonald, D.; Holmes, C.; Lavoie, K.; Hurwitz, A.S.; Kabani, N.; Toga, A.; Evans, A.; Petrides, M. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage 1999, 10, 233–260. [Google Scholar] [CrossRef] [PubMed]
- Kataria, T.; Sharma, K.; Subramani, V.; Karrthick, K.P.; Bisht, S.S. Homogeneity Index: An objective tool for assessment of conformal radiation treatments. J. Med. Phys. 2012, 37, 207–213. [Google Scholar] [CrossRef]
- D’Souza, W.D.; Rosen, I.I. Nontumor integral dose variation in conventional radiotherapy treatment planning. Med. Phys. 2003, 30, 2065–2071. [Google Scholar] [CrossRef]
- Merchant, T.E.; Kiehna, E.N.; Li, C.; Shukla, H.; Sengupta, S.; Xiong, X.; Gajjar, A.; Mulhern, R.K. Modeling radiation dosimetry to predict cognitive outcomes in pediatric patients with CNS embryonal tumors including medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 210–221. [Google Scholar] [CrossRef]
- Mahajan, A.; Stavinoha, P.L.; Rongthong, W.; Brodin, N.P.; McGovern, S.L.; El Naqa, I.; Palmer, J.D.; Vennarini, S.; Indelicato, D.J.; Aridgides, P.; et al. Neurocognitive Effects and Necrosis in Childhood Cancer Survivors Treated with Radiation Therapy: A PENTEC Comprehensive Review. Int. J. Radiat. Oncol. Biol. Phys. 2021. [Google Scholar] [CrossRef]
- Gondi, V.; Hermann, B.P.; Mehta, M.P.; Tome, W.A. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 348–354. [Google Scholar] [CrossRef]
- Wheeler, G.; Grassberger, C.; Samers, J.; Dwyer, M.; Wiltshire, K.; Daly, P.; Alvarez, B.; Campbell, B.A.; Kerr, A.J.; Kron, T.; et al. Central Endocrine Complications Among Childhood Cancer Survivors Treated with Radiation Therapy: A PENTEC Comprehensive Review. Int. J. Radiat. Oncol. Biol. Phys. 2023. [Google Scholar] [CrossRef]
- Dell’Oro, M.; Wilson, P.; Short, M.; Hua, C.H.; Merchant, T.E.; Bezak, E. Normal tissue complication probability modeling to guide individual treatment planning in pediatric cranial proton and photon radiotherapy. Med. Phys. 2022, 49, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Bender, E.T. Brain necrosis after fractionated radiation therapy: Is the halftime for repair longer than we thought? Med. Phys. 2012, 39, 7055–7061. [Google Scholar] [CrossRef]
- Burman, C.; Kutcher, G.J.; Emami, B.; Goitein, M. Fitting of normal tissue tolerance data to an analytic function. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 123–135. [Google Scholar] [CrossRef]
- Batth, S.S.; Sreeraman, R.; Dienes, E.; Beckett, L.A.; Daly, M.E.; Cui, J.; Mathai, M.; Purdy, J.A.; Chen, A.M. Clinical-dosimetric relationship between lacrimal gland dose and ocular toxicity after intensity-modulated radiotherapy for sinonasal tumours. Br. J. Radiol. 2013, 86, 20130459. [Google Scholar] [CrossRef] [PubMed]
- Dutz, A.; Luhr, A.; Agolli, L.; Troost, E.G.C.; Krause, M.; Baumann, M.; Vermeren, X.; Geismar, D.; Schapira, E.F.; Bussiere, M.; et al. Development and validation of NTCP models for acute side-effects resulting from proton beam therapy of brain tumours. Radiother. Oncol. 2019, 130, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Houweling, A.C.; Philippens, M.E.; Dijkema, T.; Roesink, J.M.; Terhaard, C.H.; Schilstra, C.; Ten Haken, R.K.; Eisbruch, A.; Raaijmakers, C.P. A comparison of dose-response models for the parotid gland in a large group of head-and-neck cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1259–1265. [Google Scholar] [CrossRef]
- Moteabbed, M.; Yock, T.I.; Paganetti, H. The risk of radiation-induced second cancers in the high to medium dose region: A comparison between passive and scanned proton therapy, IMRT and VMAT for pediatric patients with brain tumors. Phys. Med. Biol. 2014, 59, 2883–2899. [Google Scholar] [CrossRef]
- Schneider, U.; Kaser-Hotz, B. Radiation risk estimates after radiotherapy: Application of the organ equivalent dose concept to plateau dose-response relationships. Radiat. Environ. Biophys. 2005, 44, 235–239. [Google Scholar] [CrossRef]
- Alshaikh, N.A.; Eleftheriadou, A. Juvenile nasopharyngeal angiofibroma staging: An overview. Ear Nose Throat J. 2015, 94, E12–E22. [Google Scholar] [CrossRef]
- Kuppersmith, R.B.; Teh, B.S.; Donovan, D.T.; Mai, W.Y.; Chiu, J.K.; Woo, S.Y.; Butler, E.B. The use of intensity modulated radiotherapy for the treatment of extensive and recurrent juvenile angiofibroma. Int. J. Pediatr. Otorhinolaryngol. 2000, 52, 261–268. [Google Scholar] [CrossRef]
- McGahan, R.A.; Durrance, F.Y.; Parke, R.B., Jr.; Easley, J.D.; Chou, J.L. The treatment of advanced juvenile nasopharyngeal angiofibroma. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Amdur, R.J.; Yeung, A.R.; Fitzgerald, B.M.; Mancuso, A.A.; Werning, J.W.; Mendenhall, W.M. Radiotherapy for juvenile nasopharyngeal angiofibroma. Pract. Radiat. Oncol. 2011, 1, 271–278. [Google Scholar] [CrossRef] [PubMed]
- McAfee, W.J.; Morris, C.G.; Amdur, R.J.; Werning, J.W.; Mendenhall, W.M. Definitive radiotherapy for juvenile nasopharyngeal angiofibroma. Am. J. Clin. Oncol. 2006, 29, 168–170. [Google Scholar] [CrossRef]
- Paganetti, H.; Blakely, E.; Carabe-Fernandez, A.; Carlson, D.J.; Das, I.J.; Dong, L.; Grosshans, D.; Held, K.D.; Mohan, R.; Moiseenko, V.; et al. Report of the AAPM TG-256 on the relative biological effectiveness of proton beams in radiation therapy. Med. Phys. 2019, 46, e53–e78. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.J.; Blend, R.; Keane, T.; Fitzpatrick, P.; Beale, F.; Clark, R.; Garrett, P.; Harwood, A.; Payne, D.; Rider, W. Primary radiation therapy for juvenile nasopharyngeal angiofibroma. Laryngoscope 1984, 94, 1599–1605. [Google Scholar] [CrossRef]
- Lim, J.; Talsania, M.; Azar, M. Spontaneous Cerebrospinal Fluid Leak after Initiation of Dopamine Agonist Therapy in Macroprolactinomas: Two Case Reports and a Literature Review. AACE Clin. Case Rep. 2020, 6, e90–e93. [Google Scholar] [CrossRef]
- Hongmei, Y.; Zhe, W.; Jing, W.; Daokui, W.; Peicheng, C.; Yongjie, L. Delayed cerebrospinal fluid rhinorrhea after gamma knife surgery in a patient with a growth hormone-secreting adenoma. J. Clin. Neurosci. 2012, 19, 900–902. [Google Scholar] [CrossRef]
- Panda, S.; Phalak, M.; Thakar, A.; Dharanipathy, S. Cerebrospinal Fluid Leak in Juvenile Nasopharyngeal Angiofibroma-Rare Sequelae of Flutamide-Induced Tumor Shrinkage. World Neurosurg. 2018, 120, 78–81. [Google Scholar] [CrossRef]
- Heckl, S.; Aschoff, A.; Kunze, S. Radiation-induced cavernous hemangiomas of the brain: A late effect predominantly in children. Cancer 2002, 94, 3285–3291. [Google Scholar] [CrossRef]
- Becker, L.; Gebauer, J.; Kuchler, J.; Staackmann, C.; Schacht, H.; Lauten, M.; Jensen-Kondering, U.; Schramm, P.; Langer, T.; Neumann, A. Are radiation-induced cavernomas clinically relevant findings? Results from long-term follow-up with brain magnetic resonance imaging of childhood cancer survivors. Radiol. Oncol. 2021, 55, 274–283. [Google Scholar] [CrossRef]
- Trybula, S.J.; Youngblood, M.W.; Kemeny, H.R.; Clark, J.R.; Karras, C.L.; Hartsell, W.F.; Tomita, T. Radiation Induced Cavernomas in the Treatment of Pediatric Medulloblastoma: Comparative Study Between Proton and Photon Radiation Therapy. Front. Oncol. 2021, 11, 760691. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.G.; Jabbour, P.; Yadla, S.; Awad, I.A. Emerging clinical imaging techniques for cerebral cavernous malformations: A systematic review. Neurosurg. Focus. 2010, 29, E6. [Google Scholar] [CrossRef] [PubMed]
- Awad, I.A.; Polster, S.P. Cavernous angiomas: Deconstructing a neurosurgical disease. J. Neurosurg. 2019, 131, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dutz, A.; Luhr, A.; Troost, E.G.C.; Agolli, L.; Butof, R.; Valentini, C.; Baumann, M.; Vermeren, X.; Geismar, D.; Timmermann, B.; et al. Identification of patient benefit from proton beam therapy in brain tumour patients based on dosimetric and NTCP analyses. Radiother. Oncol. 2021, 160, 69–77. [Google Scholar] [CrossRef]
- Adeberg, S.; Harrabi, S.B.; Bougatf, N.; Verma, V.; Windisch, P.; Bernhardt, D.; Combs, S.E.; Herfarth, K.; Debus, J.; Rieken, S. Dosimetric Comparison of Proton Radiation Therapy, Volumetric Modulated Arc Therapy, and Three-Dimensional Conformal Radiotherapy Based on Intracranial Tumor Location. Cancers 2018, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Haldbo-Classen, L.; Amidi, A.; Lukacova, S.; Wu, L.M.; Oettingen, G.V.; Lassen-Ramshad, Y.; Zachariae, R.; Kallehauge, J.F.; Hoyer, M. Cognitive impairment following radiation to hippocampus and other brain structures in adults with primary brain tumours. Radiother. Oncol. 2020, 148, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Kiehna, E.N.; Li, C.; Xiong, X.; Mulhern, R.K. Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 1546–1554. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The cerebellum and cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef]
- Merchant, T.E.; Sharma, S.; Xiong, X.; Wu, S.; Conklin, H. Effect of cerebellum radiation dosimetry on cognitive outcomes in children with infratentorial ependymoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 547–553. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Stovall, M.; Robison, L.L. Long-term effects of radiation exposure among adult survivors of childhood cancer: Results from the childhood cancer survivor study. Radiat. Res. 2010, 174, 840–850. [Google Scholar] [CrossRef]
- Lee, T.F.; Yeh, S.A.; Chao, P.J.; Chang, L.; Chiu, C.L.; Ting, H.M.; Wang, H.Y.; Huang, Y.J. Normal tissue complication probability modeling for cochlea constraints to avoid causing tinnitus after head-and-neck intensity-modulated radiation therapy. Radiat. Oncol. 2015, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- De Marzi, L.; Feuvret, L.; Boule, T.; Habrand, J.L.; Martin, F.; Calugaru, V.; Fournier-Bidoz, N.; Ferrand, R.; Mazal, A. Use of gEUD for predicting ear and pituitary gland damage following proton and photon radiation therapy. Br. J. Radiol. 2015, 88, 20140413. [Google Scholar] [CrossRef] [PubMed]

| Patient | Age at the Time of RT | GTV (cm3) | Post-RT Tumor Volume (cm3) | Tumor Volume Reduction (%) |
|---|---|---|---|---|
| 1 | 13 | 28 | - | - |
| 2 | 14 | 159 | 29 | 81.8 |
| 3 | 19 | 8 | 0 | 100.0 |
| 4 | 15 | 29 | 0 | 100.0 |
| 5 | 21 | 34 | 18 | 47.1 |
| 6 | 12 | 166 | 49 | 70.5 |
| 7 | 20 | 89 | - | - |
| 8 | 12 | 35 | 0 | 100.0 |
| 9 | 12 | 67 | 0 | 100.0 |
| 10 | 14 | 7 | 0 | 100.0 |
| Proton | Photon | Δabs (Proton-Photon) | Δrel (Proton-Photon) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | p-Value | ||
| CTV | D0.03 cm³ | 105.5 | ± | 2.3 | 104.4 | ± | 0.7 | 1.1 | ± | 2.2 | 1.0 | ± | 2.1 | 0.193 |
| D2% | 102.9 | ± | 0.7 | 103.1 | ± | 0.4 | −0.2 | ± | 0.7 | −0.2 | ± | 0.7 | 0.263 | |
| D95% | 97.1 | ± | 1.8 | 97.3 | ± | 0.6 | −0.2 | ± | 0.8 | −0.2 | ± | 1.9 | 0.769 | |
| V95% | 97.8 | ± | 4.4 | 99.6 | ± | 0.5 | −1.8 | ± | 4.3 | −1.8 | ± | 4.3 | 0.250 | |
| V107% | 0.1 | ± | 0.2 | 0.0 | ± | 0.0 | 0.1 | ± | 0.2 | - | ± | - | - | |
| HI | 5.1 | ± | 1.8 | 5.4 | ± | 0.8 | −0.3 | ± | 1.8 | −5.6 | ± | 30.5 | 0.695 | |
| CI | 0.58 | ± | 0.18 | 0.56 | ± | 0.16 | 0.03 | ± | 0.06 | 4.8 | ± | 9.0 | 0.287 | |
| Proton | Photon | Δabs (Proton-Photon) | Δrel (Proton-Photon) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | p-Value | ||
| Brain (without CTV) | D0.03 cm³ | 45.4 | ± | 0.9 | 45.9 | ± | 0.6 | −0.4 | ± | 0.6 | −1.0 | ± | 1.4 | 0.006 |
| D2% | 29.7 | ± | 10.0 | 32.9 | ± | 7.0 | −3.2 | ± | 3.8 | −12.0 | ± | 14.6 | 0.004 | |
| D50% | 0.1 | ± | 0.1 | 1.4 | ± | 1.3 | −1.4 | ± | 1.3 | −93.3 | ± | 12.0 | 0.002 | |
| Dmean | 3.0 | ± | 1.4 | 5.1 | ± | 1.6 | −2.1 | ± | 1.7 | −39.1 | ± | 29.3 | 0.006 | |
| V10 Gy | 11.4 | ± | 6.7 | 17.6 | ± | 6.3 | −6.1 | ± | 7.9 | −31.3 | ± | 43.9 | 0.049 | |
| V15 Gy | 7.2 | ± | 4.0 | 10.0 | ± | 4.1 | −2.8 | ± | 3.0 | −28.3 | ± | 29.1 | 0.020 | |
| V20 Gy | 4.7 | ± | 2.6 | 6.7 | ± | 3.2 | −2.0 | ± | 1.4 | −30.9 | ± | 18.9 | 0.004 | |
| V35 Gy | 1.7 | ± | 1.2 | 2.1 | ± | 1.4 | −0.3 | ± | 0.2 | −20.0 | ± | 14.7 | 0.002 | |
| ID | 4924.2 | ± | 2432.6 | 8294.5 | ± | 2969.2 | −3370.3 | ± | 2755.4 | |||||
| Supratentorial Brain | D2% | 31.5 | ± | 11.7 | 34.8 | ± | 8.5 | −3.3 | ± | 3.9 | −12.0 | ± | 14.9 | 0.027 |
| D50% | 0.1 | ± | 0.1 | 0.9 | ± | 0.5 | −0.9 | ± | 0.6 | −88.2 | ± | 24.0 | 0.002 | |
| V20 Gy | 5.2 | ± | 3.1 | 7.1 | ± | 3.6 | −1.8 | ± | 1.6 | −27.5 | ± | 21.7 | ||
| Infratentorial Brain | D0.03 cm³ | 43.1 | ± | 6.8 | 44.1 | ± | 5.5 | −1.0 | ± | 1.6 | −2.8 | ± | 4.9 | 0.035 |
| D50% | 0.1 | ± | 0.0 | 10.1 | ± | 1.7 | −10.0 | ± | 1.7 | −99.3 | ± | 0.4 | 0.002 | |
| Dmean | 2.8 | ± | 1.4 | 11.4 | ± | 2.1 | −8.7 | ± | 1.3 | −76.7 | ± | 8.9 | 0.002 | |
| V10 Gy | 8.4 | ± | 4.4 | 52.2 | ± | 17.2 | −43.8 | ± | 15.6 | −83.6 | ± | 6.3 | 0.002 | |
| V15 Gy | 6.4 | ± | 3.9 | 17.9 | ± | 7.7 | −11.4 | ± | 4.7 | −65.2 | ± | 12.2 | 0.002 | |
| V20 Gy | 5.1 | ± | 3.3 | 7.8 | ± | 4.9 | −2.7 | ± | 2.0 | −38.6 | ± | 21.7 | 0.002 | |
| ID | 679.0 | ± | 353.5 | 2770.8 | ± | 653.4 | −2091.8 | ± | 383.0 | |||||
| Cerebellum | D0.03 cm³ | 13.7 | ± | 10.6 | 26.3 | ± | 7.4 | −12.6 | ± | 5.3 | −53.5 | ± | 28.6 | 0.002 |
| D2% | 3.1 | ± | 2.9 | 18.8 | ± | 4.1 | −15.8 | ± | 2.3 | −85.4 | ± | 10.9 | 0.002 | |
| D50% | 0.0 | ± | 0.0 | 9.6 | ± | 1.6 | −9.6 | ± | 1.6 | −99.7 | ± | 0.2 | 0.002 | |
| Dmean | 0.3 | ± | 0.2 | 10.0 | ± | 1.8 | −9.7 | ± | 1.6 | −97.3 | ± | 1.8 | 0.002 | |
| V10 Gy | 0.4 | ± | 0.6 | 45.9 | ± | 19.9 | −45.5 | ± | 19.6 | −99.4 | ± | 1.0 | 0.002 | |
| V15 Gy | 0.2 | ± | 0.3 | 8.6 | ± | 6.3 | −8.5 | ± | 6.1 | −98.6 | ± | 1.8 | 0.002 | |
| V20 Gy | 0.1 | ± | 0.1 | 1.9 | ± | 2.3 | −1.8 | ± | 2.3 | −66.6 | ± | 46.2 | 0.016 | |
| ID | 43.6 | ± | 31.2 | 1522.7 | ± | 312.4 | −1479.1 | ± | 295.7 | |||||
| Cerebellum anterior | D0.03 cm³ | 9.2 | ± | 9.3 | 20.8 | ± | 6.9 | −11.5 | ± | 5.8 | −61.4 | ± | 30.1 | 0.002 |
| D2% | 3.7 | ± | 4.2 | 15.8 | ± | 4.8 | −12.1 | ± | 3.5 | −79.4 | ± | 17.9 | 0.002 | |
| D50% | 0.1 | ± | 0.1 | 7.1 | ± | 2.5 | −7.0 | ± | 2.5 | −98.9 | ± | 0.9 | 0.002 | |
| Dmean | 0.4 | ± | 0.4 | 7.5 | ± | 2.4 | −7.1 | ± | 2.2 | −95.2 | ± | 4.0 | 0.002 | |
| V10 Gy | 0.5 | ± | 1.2 | 25.7 | ± | 23.1 | −25.2 | ± | 22.7 | −98.5 | ± | 2.5 | 0.002 | |
| V15 Gy | 0.2 | ± | 0.6 | 3.2 | ± | 3.2 | −2.9 | ± | 2.9 | −70.9 | ± | 40.5 | 0.016 | |
| V20 Gy | 0.1 | ± | 0.3 | 1.0 | ± | 1.6 | −0.9 | ± | 1.4 | −53.1 | ± | 46.7 | 0.031 | |
| ID | 6.2 | ± | 5.2 | 128.1 | ± | 39.3 | −121.9 | ± | 38.2 | |||||
| Cerebellum posterior | D0.03 cm³ | 12.1 | ± | 9.5 | 26.0 | ± | 7.4 | −13.9 | ± | 4.2 | −58.5 | ± | 25.1 | 0.002 |
| D2% | 3.0 | ± | 3.1 | 19.0 | ± | 4.2 | −16.0 | ± | 2.2 | −86.0 | ± | 11.3 | 0.002 | |
| D50% | 0.0 | ± | 0.0 | 9.9 | ± | 1.6 | −9.8 | ± | 1.6 | −99.8 | ± | 0.2 | 0.002 | |
| Dmean | 0.3 | ± | 0.2 | 10.3 | ± | 1.7 | −10.0 | ± | 1.5 | −97.5 | ± | 1.9 | 0.002 | |
| V10 Gy | 0.3 | ± | 0.7 | 48.4 | ± | 19.8 | −48.0 | ± | 19.5 | −99.4 | ± | 1.0 | 0.002 | |
| V15 Gy | 0.1 | ± | 0.3 | 9.3 | ± | 6.9 | −9.2 | ± | 6.7 | −98.8 | ± | 1.9 | 0.002 | |
| V20 Gy | 0.1 | ± | 0.1 | 2.0 | ± | 2.5 | −2.0 | ± | 2.4 | −67.2 | ± | 46.6 | 0.016 | |
| ID | 37.2 | ± | 28.3 | 1395.7 | ± | 285.2 | −1358.6 | ± | 271.9 | |||||
| Hippocampus (bilateral) | D0.03 cm³ | 30.2 | ± | 11.1 | 34.5 | ± | 6.2 | −4.3 | ± | 5.4 | −15.3 | ± | 20.5 | 0.049 |
| D2% | 27.7 | ± | 11.3 | 32.0 | ± | 7.0 | −4.3 | ± | 5.1 | −16.8 | ± | 21.7 | 0.049 | |
| D40% | 4.3 | ± | 5.5 | 13.7 | ± | 8.1 | −9.3 | ± | 5.1 | −70.4 | ± | 24.3 | 0.002 | |
| D50% | 2.3 | ± | 3.6 | 10.3 | ± | 6.9 | −8.0 | ± | 4.8 | −80.0 | ± | 17.1 | 0.002 | |
| Dmean | 6.0 | ± | 3.9 | 12.2 | ± | 5.4 | −6.2 | ± | 3.6 | −49.8 | ± | 27.1 | 0.004 | |
| ID | 24.5 | ± | 14.5 | 51.7 | ± | 24.7 | −27.3 | ± | 18.3 | |||||
| Hippocampus CL | D0.03 cm³ | 20.1 | ± | 15.5 | 28.9 | ± | 11.0 | −8.9 | ± | 7.5 | −39.5 | ± | 31.3 | 0.006 |
| D2% | 19.1 | ± | 15.0 | 28.2 | ± | 11.0 | −9.1 | ± | 7.3 | −41.0 | ± | 31.1 | 0.006 | |
| D50% | 1.5 | ± | 2.3 | 9.6 | ± | 6.3 | −8.1 | ± | 4.9 | −86.5 | ± | 12.6 | 0.002 | |
| Dmean | 4.0 | ± | 4.0 | 11.4 | ± | 5.6 | −7.4 | ± | 3.7 | −69.6 | ± | 20.0 | 0.002 | |
| ID | 7.9 | ± | 7.3 | 24.1 | ± | 13.2 | −16.2 | ± | 10.2 | |||||
| Hippocampus IL | D0.03 cm³ | 29.3 | ± | 11.8 | 33.4 | ± | 6.7 | −4.1 | ± | 5.5 | −15.7 | ± | 22.4 | 0.049 |
| D2% | 28.6 | ± | 11.9 | 32.7 | ± | 6.9 | −4.1 | ± | 5.4 | −16.3 | ± | 23.0 | 0.049 | |
| D50% | 5.5 | ± | 6.2 | 11.5 | ± | 7.6 | −6.0 | ± | 6.4 | −35.0 | ± | 112.2 | 0.037 | |
| Dmean | 8.1 | ± | 4.9 | 13.2 | ± | 5.3 | −5.0 | ± | 4.1 | −38.2 | ± | 37.6 | 0.010 | |
| ID | 16.6 | ± | 9.3 | 27.7 | ± | 12.3 | −11.1 | ± | 9.1 | |||||
| Cochlea CL | D0.03 cm³ | 8.3 | ± | 7.0 | 22.1 | ± | 4.7 | −13.8 | ± | 6.3 | −63.2 | ± | 29.0 | 0.002 |
| D2% | 9.8 | ± | 7.4 | 23.7 | ± | 4.9 | −14.0 | ± | 6.6 | −59.6 | ± | 28.1 | 0.002 | |
| Dmean | 7.5 | ± | 6.4 | 21.1 | ± | 4.5 | −13.6 | ± | 5.8 | −65.3 | ± | 28.1 | 0.002 | |
| ID | 0.9 | ± | 0.8 | 2.6 | ± | 1.1 | −1.7 | ± | 0.9 | |||||
| Skin | D2% | 20.9 | ± | 6.9 | 23.2 | ± | 6.2 | −2.3 | ± | 2.7 | −10.7 | ± | 12.2 | 0.049 |
| D5% | 14.2 | ± | 5.3 | 18.0 | ± | 4.3 | −3.8 | ± | 2.0 | −23.2 | ± | 13.7 | 0.002 | |
| Dmean | 2.3 | ± | 1.1 | 4.4 | ± | 1.4 | −2.1 | ± | 0.5 | −50.0 | ± | 9.1 | 0.002 | |
| V10 Gy * | 52.4 | ± | 30.9 | 95.8 | ± | 44.1 | −43.3 | ± | 17.8 | −47.4 | ± | 12.3 | 0.002 | |
| V15 Gy * | 29.5 | ± | 27.0 | 45.3 | ± | 27.9 | −15.9 | ± | 7.4 | −42.3 | ± | 18.6 | 0.002 | |
| V20 Gy * | 14.2 | ± | 13.0 | 21.0 | ± | 16.6 | −6.8 | ± | 6.5 | −28.7 | ± | 50.4 | 0.004 | |
| ID | 1294.0 | ± | 625.4 | 2498.2 | ± | 876.4 | −1204.3 | ± | 311.8 | |||||
| Proton | Photon | Δabs (Proton-Photon) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ at Risk | Complication | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | p-Value |
| Infratentorial Brain | IQ * | 112.4 | ± | 4.1 | 107.6 | ± | 4.3 | 4.7 | ± | 0.7 | 0.002 |
| Cochlea CL | Tinnitus | 0.8 | ± | 0.7 | 3.7 | ± | 2.1 | −2.9 | ± | 1.8 | 0.002 |
| Cochlea IL | Tinnitus | 7.6 | ± | 7.7 | 8.8 | ± | 4.6 | −1.1 | ± | 4.2 | 0.432 |
| Hippocampus (bilateral) | Delayed recall | 8.5 | ± | 10.7 | 28.3 | ± | 24.1 | −19.9 | ± | 15.6 | 0.002 |
| Pituitary | GH-deficiency | 62.6 | ± | 20.1 | 67.2 | ± | 13.1 | −4.6 | ± | 7.7 | 0.131 |
| Pituitary | Hypothyroidism | 43.9 | ± | 18.6 | 47.4 | ± | 13.7 | −3.5 | ± | 5.6 | 0.131 |
| Pituitary | ACTH-deficiency | 25.0 | ± | 9.2 | 26.7 | ± | 6.9 | −1.7 | ± | 2.7 | 0.131 |
| Lacrimal gland IL | Ocular toxicity | 18.7 | ± | 28.4 | 17.2 | ± | 24.7 | 1.5 | ± | 7.5 | 0.557 |
| Parotis CL | Xerostomia | 2.2 | ± | 1.6 | 2.5 | ± | 1.0 | −0.3 | ± | 0.9 | 0.084 |
| Parotis IL | Xerostomia | 5.9 | ± | 7.9 | 4.4 | ± | 3.8 | 1.5 | ± | 4.6 | 0.695 |
| Skin | Alopecia | 45.3 | ± | 10.3 | 52.7 | ± | 8.4 | −7.4 | ± | 3.9 | 0.002 |
| RR (Photon/Proton) | |||||||||||
| Mean | ± | SD | p-Value | ||||||||
| Brain | Secondary malignancies | 2.1 | ± | 1.2 | 0.006 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoeltgen, L.; Tessonnier, T.; Meixner, E.; Hoegen, P.; Kim, J.-Y.; Deng, M.; Seidensaal, K.; Held, T.; Herfarth, K.; Debus, J.; et al. Proton Therapy for Advanced Juvenile Nasopharyngeal Angiofibroma. Cancers 2023, 15, 5022. https://doi.org/10.3390/cancers15205022
Hoeltgen L, Tessonnier T, Meixner E, Hoegen P, Kim J-Y, Deng M, Seidensaal K, Held T, Herfarth K, Debus J, et al. Proton Therapy for Advanced Juvenile Nasopharyngeal Angiofibroma. Cancers. 2023; 15(20):5022. https://doi.org/10.3390/cancers15205022
Chicago/Turabian StyleHoeltgen, Line, Thomas Tessonnier, Eva Meixner, Philipp Hoegen, Ji-Young Kim, Maximilian Deng, Katharina Seidensaal, Thomas Held, Klaus Herfarth, Juergen Debus, and et al. 2023. "Proton Therapy for Advanced Juvenile Nasopharyngeal Angiofibroma" Cancers 15, no. 20: 5022. https://doi.org/10.3390/cancers15205022
APA StyleHoeltgen, L., Tessonnier, T., Meixner, E., Hoegen, P., Kim, J.-Y., Deng, M., Seidensaal, K., Held, T., Herfarth, K., Debus, J., & Harrabi, S. (2023). Proton Therapy for Advanced Juvenile Nasopharyngeal Angiofibroma. Cancers, 15(20), 5022. https://doi.org/10.3390/cancers15205022







